Research Article - Der Pharma Chemica ( 2017) Volume 9, Issue 2
Macrocyclic Schiff Base Complexes-Preparation, Characterisation and Biological Activity
Vanita Goel, Sonika and Rajesh Malhotra
Abstract
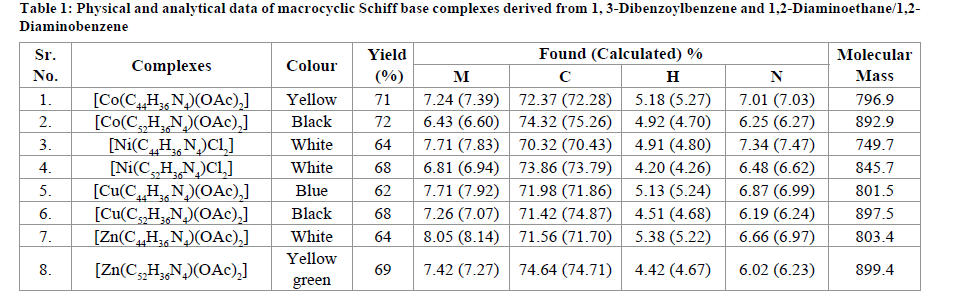
New macrocyclic Schiff base complexes of Transit. Metals was synthesized using 1, 3-Dibenzoylbenzene and 1, 2-Diaminoethane/1,2- Diaminobenzene. The complexes MLX2 [where M=Zn(II), Co(II), Cu(II) and Ni(II); L was a condensation product of 1, 3-Dibenzoylbenzene and 1, 2-Diaminoethane/1, 2-Diaminobenzene; X=Cl-, CH3COO- ] were synthesized using template method. These complexes were characterized by elemental analysis, spectral analysis (IR, NMR), Magnetic moment measurements, ESR studies and electronic measurements. All the complexes were screened for gram positive bacteria (Bacillus subtilis, Staphylococcus aureus), gram negative bacteria (Escherichia coli, Pseudomonas aeruginosa) and phytopathogenic fungi (Candida albicans, Saccharomyces cerevisiae). Minimum inhibitory concentration of the synthesized complexes was also measured and compared with standard antibacterial Ciprofloxacin and antifungal Amphotericin-B.
Keywords
Template synthesis, Gram positive bacteria, Gram negative bacteria, Phytopathogenic fungi, Minimum inhibitory concentration
Introduction
Template reactions have been widely used for the synthesis of macrocyclic complexes, in which transition. metal ions are generally used as the template agent [1]. Synthesis of transitionmetal macrocyclic complexes of Schiff base ligands had gain a growing interest due to simplicity of their synthesis and wide applications in pharmacological field for the use as antimicrobial agents against pathogenic microorganisms [2,3]. Due to demand of new metal based antibacterial and antifungal compounds, coordination chemistry is becoming an emerging area of research. Due to lipophilic nature and penetration of complexes through the lipid membrane, transition metal complexes exhibited good antimicrobial activities. Moreover these complexes find their uses in many industries. Macrocyclic complexes are used as catalysts [4] and anti-corrosion agents [5]. Macrocyclic complexes are of great importance in many biological processes like oxygen transport and photosynthesis [6]. These complexes are widely used as anticancer [7,8] antifungal [9] anti-inflammatory [10] and antidiabetic [11] drugs. Because of similarity with biomolecules, the study of macrocyclic Schiff base complexes had reached to a significant era. We have incorporated metal ions onto cyclic/acyclic systems and synthesized biologically active macrocyclic Schiff base complexes of transition metals [12-15]. In continuation of the same work present complexes have been synthesized and their pharmacological effect has been explored.
Materials and Methods
Synthesis of complexes
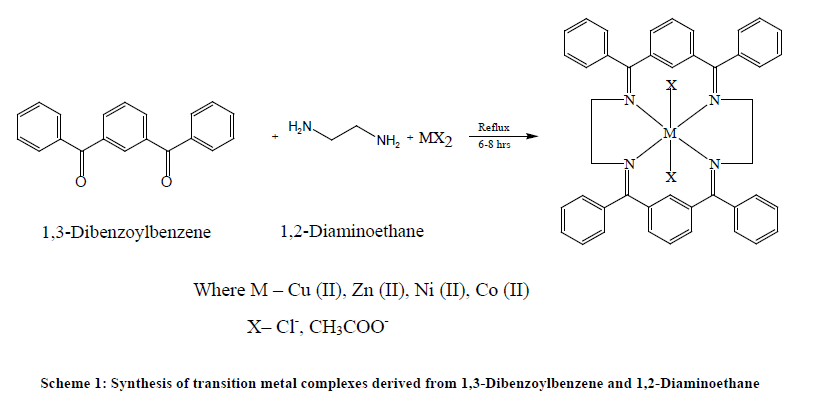
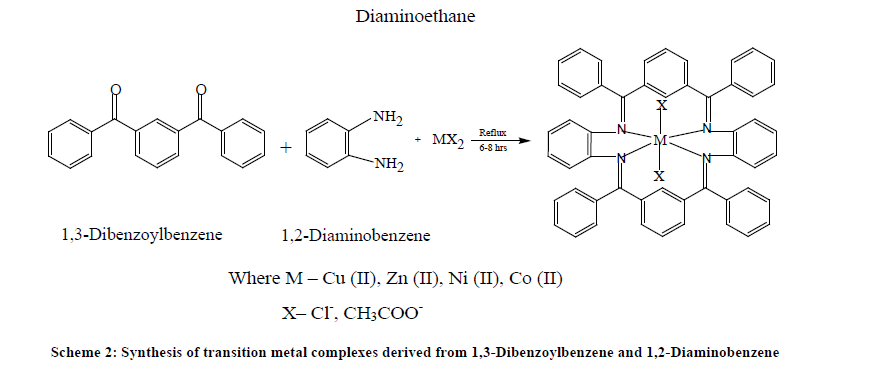
Template synthesis of metal complexes was carried out by mixing the methanolic solution of 1,3-Dibenzoylbenzene and 1,2-Diaminoethane/1,2-Diaminobenzene in the presence of metal salts. To the hot methanolic solutions of 1,2-Diaminoethane/1,2- Diaminobenzene (10 mmol), copper, nickel, cobalt, zinc salts (5 mmol) dissolved in same solvent were added and refluxed for half an hour. 1,3-Dibenoylbenzene (10 mmol) was added to refluxing mixture. The reaction mixture was refluxed for 6-8 hrs and then concentrated to half of its volume by evaporation on water bath. Crystalline solids were obtained on standing the reaction mixture overnight. These crystalline solids were filtered and washed with methanol, acetone and diethyl ether and dried in vacuo. The complexes were insoluble in water and common organic solvents but soluble in Dimethylsulphoxide (DMSO) and Dimethylformamide (DMF).
The purity of complexes was checked by TLC. These complexes were found thermally stable and decomposed only above 300ºC temperature. The complexes were monomeric in nature as indicated by elemental analyses and molecular weight determination. The molar conductance of these complexes determined in DMSO was found to be in the range of 5-15 ohm-1 cm2 mol-1. The low value of molar conductance indicated that these complexes were non electrolytic. Tests for anions were negative in undecomposed complexes confirmed their absence outside the coordination sphere but in decomposed complexes these tests were positive indicating presence of anions inside the coordination sphere.
The template synthesis of complexes derived from 1,3-Dibenzoylbenzene and 1,2-Diaminoethane/1,2-Diaminobenzene in the presence of copper, nickel, zinc and cobalt metal ions may be represented by the following Table 1 and Schemes 1 and 2.
Biological activity
Clinical strains of human pathogenic bacteria such as Escherichia coli (MTCC1652), Pseudomonas aeruginosa (MTCC741), Bacillus subtilis (MTCC121) Staphylococcus aureus (MTCC96) and phytopathogenic fungi Saccharomyces cerevisae (MTCC170), Candida albicans (MTCC3017) were procured from Microbial Type Culture Collection IMTECH, Chandigarh. Bacterial strains were sub cultured on Nutrient Agar (NA) and fungi on Malt Extract Agar (MEA) medium and were incubated aerobically at 37ºC.
Antimicrobial activity
The antimicrobial activity of the complexes was determined against above mentioned four bacterial strains and two yeast strains using the agar well diffusion method [16]. Density of all the microbial cultures were adjusted to 0.5 McFarland standards, which were visually comparable to a microbial suspension of approximately 1.5 × 108 cfu/ml. 20 ml of the Nutrient agar medium (for bacteria) and Malt extract agar medium (for yeast) were poured into each Petri plate. One hundred microlitre (100 μl) inocula of the test microorganisms was spread in each plate and kept for 15 min for adsorption. Solutions of each complex were prepared by dissolving the complex in 20% DMSO. Using sterile cork borer of 8 mm diameter, wells were bored into the seeded agar plates and these were loaded with a 100 μl volume of the solution of each complex. All the plates were incubated at 37ºC for 24 h. Antimicrobial activity of each complex was evaluated by measuring the zone of growth inhibition against the test organisms with a zone reader (Hi Antibiotic zone scale). Ciprofloxacin and Amphotericin-B were used as a positive control. This procedure was performed in three replicate plates for each organism.
Determination of minimum inhibitory concentration (MIC)
Minimum inhibitory concentration of the complexes for each test organism has been determined by following the modified agar well diffusion method [17]. A twofold serial dilution of each complex has been prepared. Each complex has been dissolved in 20% DMSO to achieve a concentration of 100 mg/ml followed by dilution in sterile distilled water (1:1) to achieve a decreasing concentration range of 50 mg/ml to 0.39 mg/ml. A 100 μl volume of each dilution has introduced into wells (in triplicate) in the agar plates already seeded with 100 μl of standardized inoculum (106 cfu/ml) of the test microbial strain. All test plates are incubated aerobically at 37ºC for 24 hrs and observed for the inhibition zones. Zone of inhibition (>8 mm) has been observed in each plate. Concentration of the complex that completely inhibited the growth of the microorganism has been taken as minimum inhibitory concentration.
Conclusion
Conductance measurements, elemental analyses and magnetic susceptibilities, as well as IR, NMR and electronic spectral studies confirmed a distorted octahedral geometry for all these complexes. MIC of complexes shows that these complexes are very effective on tested microorganisms. These complexes may be used for the formulation of novel chemotherapeutic agents as the problem of antimicrobial resistance has increased enormously [28]. Because of the partial sharing of its positive charge with a donor group in the chelate ring system, polarity of the metal ion decreases. The lipophilic nature of the central metal ion increases due to chelation which favours its permeation through the lipoid layer of the membrane [29]. So the metal complexes cross the bacterial membrane more effectively thereby activity of the complexes increases. Many other factors, such as dipole moment, conductivity, solubility and the influence of the metal ion may also be responsible for the remarkable antibacterial activities of these complexes.
References
[1] N.E. Borisova, M.D. Reshetova, Y.A. Ustynyuk, Chem. Rev., 2006, 107, 46-79.
[2] M.S. Niasari, M. Bazarganipour, M.R. Ganjali, P. Norouzi, Transit. Met. Chem., 2007, 32, 9.
[3] T. Aboul-Fadl, F.A. Mohammed, E.A. Hassan, Arch Pharmacol. Res., 2003, 26, 10, 778-784.
[4] K.C. Gupta, A.K. Sutar, Coord. Chem. Rev., 2008, 252, 1420-1450.
[5] A.R. Prasad, M.A. Quraishi, Corrosion. Sci., 2010, 52(3), 933.
[6] S. Chandra, K. Gupta, Transit. Met. Chem., 2002, 27, 196-199.
[7] S.M.M. Ali, M. Abul Kalam Azad, M. Jesmin, Asian. Pacif. J. Trop. Biomed., 2012, 2(6), 438-442.
[8] K. Chaubey, S.N. Pandeya, Int. J. Pharm. Tech. Res., 2012, 4(4), 590-598.
[9] G. Kumar, D. Kumar, S. Devi, R. Johari, C.P. Singh, Eur. J. Med. Chem., 2010, 45, 3056.
[10] S.M. Sondhi, N. Singh, A. Kumar, O. Lozach, L. Meijer, Bioorg. Med. Chem., 2006, 14(11), 3758-3765.
[11] V.C. Silveira, J.S. Luz, C.C. Oliveira, I. Graziani, M.R. Ciriolo, A.M.C. Ferreira, J. Inorg. Biochem., 2008, 102, 1090.
[12] R. Malhotra, S. kumar, Jyoti, H.R. Singal, K.S. Dhindsa, Indian. J. Chem., 2000, 39A, 421.
[13] S. Kumar, R. Malhotra, K.S. Dhindsa, Polyhedron., 1993, 11, 1383.
[14] S. Singh, R. Malhotra, A. Hooda, K.S. Dhindsa, Bull. Soc. Chem. Belg., 1996, 105, 108.
[15] V. Goel, Sonika, Neeraj, R. Malhotra, Asian. J. Chem., 2016, 28, 10, 2311.
[16] K.R. Aneja, C. Sharma, R. Joshi, Jund. J. Microbiol., 2011, 4, 175.
[17] A. Nostro, Á.M. P. Germano, V. D'Angelo, A. Marino, M. A. Cannatelli, Lett. Appl. Microbiol., 2000, 30, 384.
[18] Q. Zeng, J. Sun, S. Gou, K. Zhou, J. Fang, H. Chen, Transit. Met. Chem., 1998, 23, 371.
[19] L.K. Gupta, S. Chandra, Transit. Met. Chem., 2006, 31, 368.
[20] C. Lodeiro, R. Bastida, E. Bertolo, A. Macias, A. Rodriguez, Transit. Met. Chem., 2003, 28, 388.
[21] S. Chandra, L.K. Gupta, J. Indian Chem. Soc., 2005, 82, 454.
[22] K. Nakamoto, 1978, Wiley Interscience Publication, NY, USA.
[23] V.B. Rana, P. Singh, D.P. Singh, Polyhedron., 1982, 1, 377.
[24] A.B.P. Lever, Inorg. Electron. Spectrosc., Amsterdam Elsevier, 1984, 2nd edn.
[25] D.P. Singh, K. Kumar, S.S. Dhiman, J. Sharma, J. Enzyme Inhib. Med. Chem., 2009, 24, 795.
[26] V.B. Rana, P. Singh, D.P. Singh, M. P. Teotia, Transit. Met. Chem., 1981, 6, 36.
[27] K. Sharma, Parveen, D. P. Singh, R. Chopra, Der. Pharma. Chemica., 2015, 7, 292.
[28] Z.H. Chohan, H. Pervez, A. Rauf, K.M. Khan, C.T. Supuran, J. Enzyme. Inhib. Med. Chem., 2004, 19, 417.
[29] Z.H. Chohan, A. Scozzafava and C.T. Supuran, J. Enzyme. Inhib. Med. Chem., 2002, 17, 261.



