Short Communication - Der Pharma Chemica ( 2021) Volume 13, Issue 3
Molecular design and theoretical properties of Nitramine based molecules as potential High Energy Materials
Rajasekhar Koorella* and Satheesh MarojuRajasekhar Koorella, Department of Organic Chemistry, Indian Institute of Chemical Technology, Hyderabad, India, Email: krpsacrhem@uohyd.ac.in
Abstract
Six new molecules based on nitramine functionality were designed and predicted the theoretical properties using DFT calculations. All the Six molecules were found to exhibit better or comparable properties than the existing explosives RDX, HMX and useful for explosive applications.
Keywords
High Energy Materials, Design, Theoretical Properties, DFT Calculation, Explosive applications.
Introduction
High energy materials (HEMs) are the compounds which store chemical energy and mainly contains nitro (−NO2) [1-5], azido (−N3) [6-8], nitrate ester (−ONO2) [9,10], nitramino (−NNO2) [11-14] functional groups. Because of high energy materials release large amount of energy upon detonation, they are widely used in the military, industrial and mining applications [15]. Among the functional groups, compounds with nitramino (−NNO2) functionality have been shown as stable and less sensitive explosives towards external stimuli. During the last decades, a large number of high energy materials were discovered with nitramine functionality [16]. Among them, RDX, HMX and CL-20 are well known nitramine class of explosives, widely used in explosive applications. The discovery of new high energy materials with improved performance than the existing is high demanding and challenging area for the chemists. Especially, the molecules with higher density exhibits higher detonation pressure. Hence, designing the molecules with higher density is a key factor before initiating the synthesis. It has been observed that the molecules of cyclic and caged structures possess higher density compared to acyclic molecules due to packed molecular arrangement. Furthermore, the increase in the number of C-N and N-N bonds in the structure increases the energy of molecule resulting in the higher detonation pressure. Density functional theory (DFT) is a widely used quantum mechanical modelling method to investigate the electronic structure of the molecule. In order to meet the requirements of high energy materials, six nitramine based molecules were designed and investigated the physical properties using DFT calculations. All the six molecules were shown better or comparable properties than the existing HEMs. Herein we describe the computational results of designed molecules and possible synthetic route of most promising molecules.
Computational Methods
Gaussian 09 software package was used to optimize the structures and post-optimization, the structures were found to be with no imaginary frequencies. B3LYP, with the basis set 6-311G++ (d, p) was used to compute the structures to an optimal minimum [17]. Material Studio (v.08) was used to perform polymorph calculations to obtain the theoretical maximum density of the structures [18]. DRIEDING was the force field which we have used during the polymorph calculations [19]. MOPAC 16 was used to compute heats of formation (HOF) of the molecules [20] and semi-empirical method PM7 was used to obtain the HOF data. EXPLO5 thermo-kinetic code was used to obtain detonation performance parameters [21]. BKW equation of state was used to calculate the detonation performance parameters with the values of constants α = 0.5; ß = 0.38; κ = 9.32; θ = 4120; ε = 1.00, and the co-volumes set:1 was used to determine the detonation products [22].
Results and Discussions
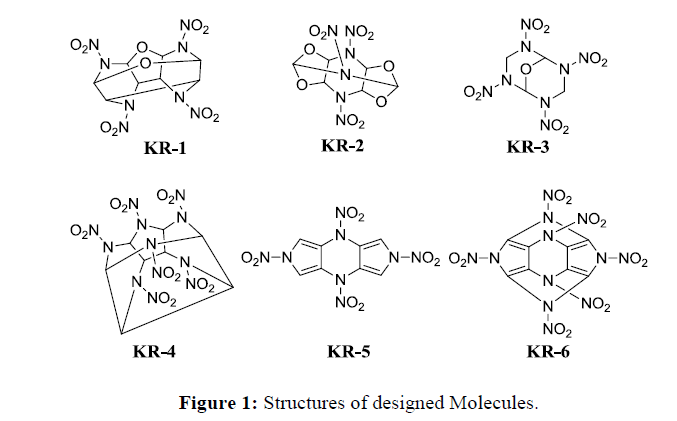
The molecules were designed by considering bicyclic, caged and aromatic five or six membered ring systems with nitramine functionality and named as KR-1 to KR-6. The Molecules KR-1, KR-2 and KR-3 shows ether linkage and KR-2 closely resembles the structure of TEX with additional bridged -NNO2 functionality. By comparing the structure of KR-5 with rest of the molecules, it is observed that bridged and caged structures showed higher density with greater detonation properties compared to planar structures. It is also noted that by increasing the number of - NNO2 groups in the molecules increases the density of molecule thereby increasing detonation properties.
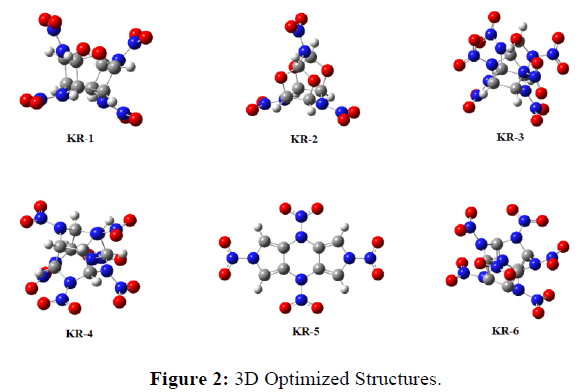
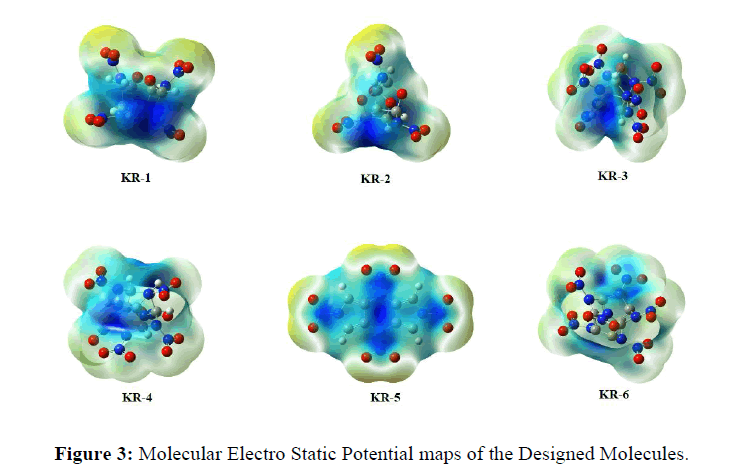
The optimized minima structures suggest that there might be hydrogen bonding between the Oxygen-Hydrogen-Nitrogen atoms, which might add to the stability of the molecules (Figures 1 & 2). We have also studied the molecular electro static potential (MESP) maps of the molecules and suggest that, there is a decent amount of electronic distribution to maintain the structure stable and detonate only when ignited. However, this is just based on a single molecule, the real-time sensitivity is a subject to molecular dynamics study (Figure 3) [23].
The detonation calculations were evaluated, and the results are listed in the Table 1. The potential energetic characteristics of designed molecules have been assessed by comparing with those of known contemporary explosives. All these molecules have found to exhibit density value ranging from 1.85 to 2.10 and detonation velocity from 8.35 to 10.71 Km/s. Among them, KR-6 has found to exhibit highest density of 2.1 g/cc along with the maximum detonation velocity of 10.712 Km/s which is higher than most of the existing high energy materials. In order to compare the accuracy of computational results, benchmark calculations have been performed with experimental properties of known explosive molecules and observed that calculated values seem in agreement with the experimental data [24].
| Molecule | Density (g/cc) |
HOF (KJ/mol) |
Oxygen Balance (%) | Detonation Pressure (GPa) | Detonation Velocity (Km/s) | |
|---|---|---|---|---|---|---|
| KR-1 | 1.94 | -110.69 | -42.53 | 31.86 | 8.506 | |
| KR-2 | 2.04 | -527.14 | -24.83 | 35.14 | 8.784 | |
| KR-3 | 2.03 | -169.4 | -7.04 | 43.29 | 9.683 | |
| KR-4 | 2.05 | 364.55 | -27.57 | 41.71 | 9.489 | |
| KR-5 | 1.85 | -47.03 | -47.03 | 30.56 | 8.353 | |
| KR-6 | 2.10 | -14.03 | -14.03 | 58.52 | 10.712 | |
| RDX | 1.818a | 1.82b | 91.21 | -21.6 | 36.28 | 9.041 |
| HMX | 1.92a | 1.91b | 153.32 | -21.6 | 39.41 | 9.309 |
| FOX-7 | 1.89a | 1.885b | -42.07 | -21.6 | 35.56 | 8.969 |
| CL-20 | 2.00a | 2.04b | 393.33 | -10.95 | 43.28 | 9.604 |
| TNAA | 1.87a | 1.88b | -134.0 | 30.11 | 24.31 | 7.783 |
a: Theoretical values; b: Experimental values
It is noteworthy that the amount of carbon in the molecules played a key role in determining the detonation performance. Molecules KR-1 and KR- 2, despite having decent density values, fall short to the expected detonation performance due to the amount of carbon atoms they have. Of the designed six molecules, KR-3 and KR-6, as they have reduced carbon content, exhibit brilliant detonation pressure and detonation velocity values. Especially molecule KR-6, has been found the best explosive molecule with its density 2.10 g/cc, detonation velocity 10.712 Km/s and detonation pressure close to 60 GPa.
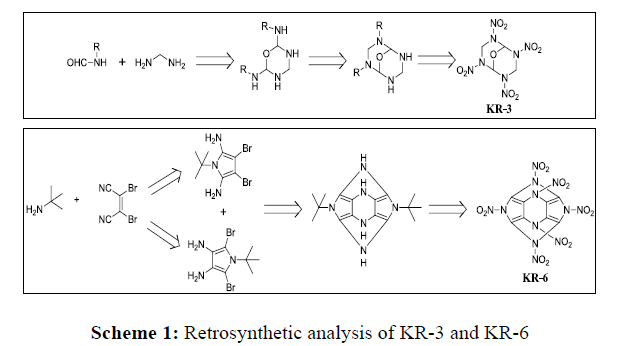
In order to understand the feasibility of synthesis, we have evaluated the possible synthetic route of two promising molecules KR-3 and KR-6. The retrosynthetic analysis of KR-3 and KR-6 indicates that, these molecules can be synthesized from commercially available starting materials methane diamine and (Z)-2,3-Dibromo-2-butene-1,4-dinitrile respectively as shown in Scheme 1.
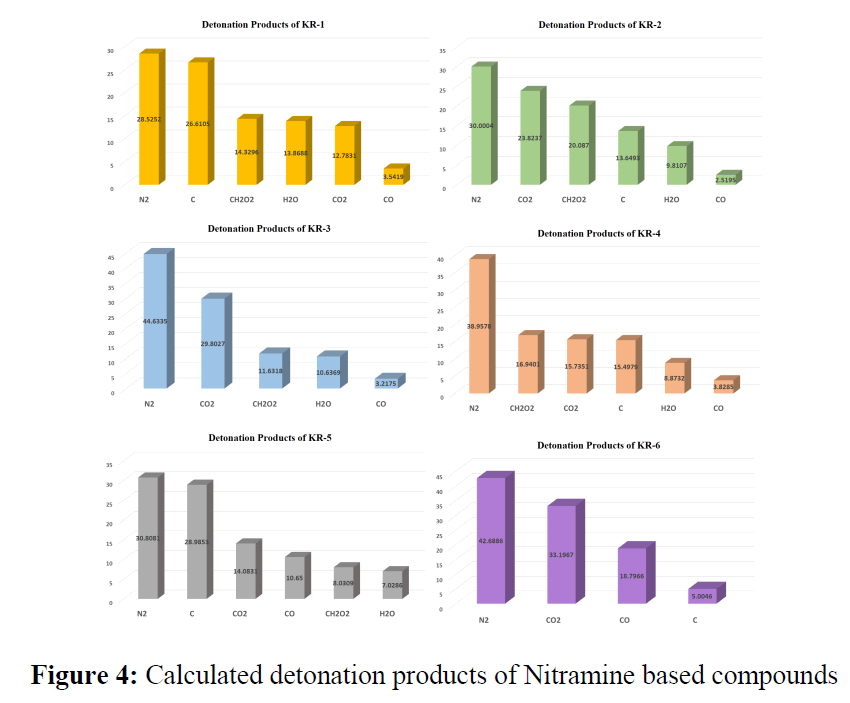
The expected detonation products of designed molecules were calculated and shown in Figure 4. It is observed that in all the cases the major detonated product is Nitrogen and in majority cases, the least detonated product is Carbon monoxide. The other detonated products include water, carbon and carbon dioxide, representing the environmental compatibility of explosives after detonation.
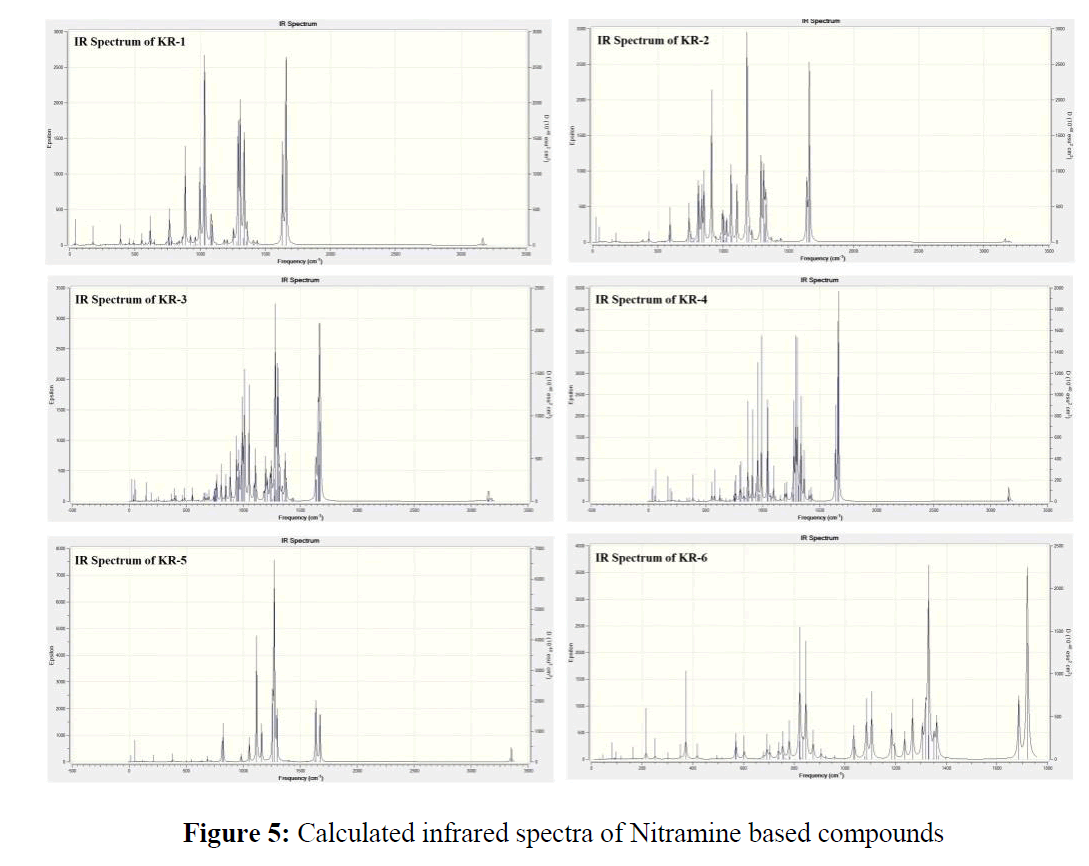
The Infrared spectrum of all the six designed molecules has been predicted by DFT calculations. The IR spectrum of KR-1 to KR-3 shows the N-O symmetric stretching vibration at 1660 cm-1 whereas asymmetric stretch shows at 1310 cm-1 and stretching frequency of C-N is at 1290 cm-1. Since these molecules possess ether linkage, C-O-C stretch observed in the region 950-1150 cm-1. On the other hand KR-5 and KR-6 contains Pyrrole ring system along with nitramine functionality. The IR indicates N-O symmetric stretching vibration at 1670 cm-1 and asymmetric stretch at 1350 cm-1 along with the C-N stretching at 1120 cm-1 (Figure 5).
Conclusions
In conclusion, we have designed six molecules based on nitramine functionality and evaluated the properties for explosive applications. All the designed molecules were found better or comparable explosive properties than the known explosives RDX and HMX. Furthermore, the molecules can easily be synthesized from commercially available starting materials.
Acknowledgements
A.P., S.M. and R.K. thank DRDO for financial assistance.
References
- Wilbrand J. Justus Liebigs Ann Chem. 1863, 128: p. 178−179.
- Akopyan ZA, Struchkov YT and Dashevskii VG. J Struct Chem. 1966, 7: p. 385-392.
- Kolb JR and Rizzo HF. Propellants Explos Pyrotech. 1979, 4: p. 10-16.
- Bellamy AJ. Org Process Res Dev. 2010, 14: p. 632-639.
- Zhang MX, Eaton PE and Gilardi R. Angew Chem Int Ed. 2000, 39: p. 401-404.
- Flanagan JE and Frankel MB. 1,3-Diazido-2-Nitrazapropane U.S. Patent. 1978, 4: p.123.
- Flanagan JE, Frankel MB and Witucki EF. Azido Compounds U.S. Patent. 1979, 4: p. 910 A.
- Nagayama K andOyumi Y. Propellants Explos Pyrotech. 1996, 21: p. 74-78.
- Townend DJ and Warren RC. Polymer. 1985, 26: p. 79-83.
- Yoo CS, Holmes NC, Souers PC et al., J Appl Phys. 2000, 88: p. 70-75.
- Pagoria PF, Lee GS, Mitchell AR et al., Thermochim Acta. 2002, 384: p. 187-204.
- Chakraborty D, Muller RP, Dasgupta S et al., J Phys Chem A. 2000, 104: p. 2261-2272.
- Sewell TD, Menikoff R, Bedrov Dety al., J Chem Phys. 2003, 119: p. 7417-7426.
- Nielsen AT, Chafin AP, Christian SL et al., Tetrahedron. 1998, 54: p. 11793-11812.
- Agrawal JP and Hodgson RD. John Wiley and Sons Ltd.
- Junqing Y, Guixiang W, Xuedong G et al., ACS Omega. 2018, 38: p. 9739-9745.
- Frisch MJ, Trucks GW, Schlegel HB et al., Gaussian Inc., Wallingford CT, 2009.
- Jensen F. Introduction to Computational Chemistry, Second Edition, John Wiley & Sons, LTD. 2007.
- DassaultSystemes BIOVIA, Material Studio, v.08, San Diego: Dassault Systemes.
- Mayo SL, Olafson BD and Goddard III WA. J Phys Chem. 1990, 94: p. 8897-8909.
- James JPS. Computational Chemistry-MOPAC16, Colorado Springs, CO, USA. 2016.
- Suceska M. EXPLO5 program, Zagreb, Croatia, 2005.
- Charles LM. Numerical Modelling of Explosives and Propellants.2008.
- Nair UR, Asthana SN, Subhananda AR et al., Defence Science J. 2010, 60: p. 137-151.