Research Article - Der Pharma Chemica ( 2020) Volume 12, Issue 2
Molecular docking and Invitro cytotoxicity activity of styryl compound against the HeLa cancer cell line from the extract of crotolaria medicaginea
Panneerselvam Kalaivani and Mannuthusamy Gopalakrishnan*Mannuthusamy Gopalakrishnan, Professor, Department of Chemistry, Annamalai University, Annamalai Nagar, Chidambaram 608 002, Tamil Nadu, India, Email: mgkrishnan61@gmail.com
Abstract
Background: Stilbene compounds occur in some type of plant species, they preventing many disorder and illness. Recently focus a number of researches in medicine and plant physiology similarly to have emerged as promising molecules that probably have an effect on human health.
Methods: In these review, the aim of present study evaluated the stilbene compound from the methanolic extract of plant, Crotolaria medicaginea. The compound was isolated and evaluated for their molecular docking studies using BIO-VIA Discovery studio and cytotoxicity activity towards human cervical cancer cell line (HeLa) by using MTT colorimetric assay techniques.
Results: The IC50 (half maximal inhibitory concentration) value of the controlled compound was determined. It confirmed a percentage of cell viability of 97% at 35.64 μg/ml. The docking studies reveal that the isolated compound inside 4j96 protein showed a minimum CDOCKER score. The structure had been elucidated on the basis of FT-IR, 1H and 13C NMR spectral studies, the results of these studies have been confirmed the isolated compound.
Conclusion: the present investigation concluded that the extracted compound has shown to be a potent invitro anticancer activity against the human cervical cancer Hela cell line and docking studies.
Keywords
Stilbene, Crotolaria medicaginea, Anticancer, Human cervical cancer cell line, Cytotoxicity, CDOCKER energy
Introduction
Cervical cancer is the most common female genital tract malignancy, and it is the third most common malignant tumor next to breast cancer and colorectal cancer in the global women [1], especially in under developed and developing countries [2]. This cancer slowly develops; ranging from a malignant tumor abnormally selected cervical intraepithelial pathologic processes that will additional develop to invasive cervical cancer [3]. This cancer starts within the cervix, the lower slender a part of the uterus and mostly the cases of cervical cancer are infected by high risk type of Human Papilloma virus (HPV). Primarily the patients were from the generative age group [4].
Stilbenes are natural compounds found in some types of plants. Stilbene derivatives are synthesized comparatively easily, are usually thermally, and chemically stable [5]. Synthesis and bioactivity analysis of changed stilbene derivatives received abundant attention and interest in medicinal chemistry [6]. Stilbene derivatives plays a crucial role in preventing several disease and illness, such as inflammation [7-10], cancer [11,12] and heart diseases [13,14], various reviews have summarized the effects of resveratrol treatment on breast, colorectal, liver, pancreatic and prostate cancer. Many studies found the analysis of respective stilbene derivatives could also contain anticancer activities. Methoxylation has been significantly improving the antitumor potential of compounds [15,16]. The greater the number of methoxy group’s presence, the better the anti-tumor activity [17]. Some experimental evidence shows that, methoxy substituted stilbenes are having good cancer chemo-preventive agent and most of methoxy resveratrol derivatives exhibit potent cytotoxic and pro-apoptotic activity against cancer cells [8-11]. Polymethoxy stilbenes are able to interact with biomembrane models and have a higher bioavailability than resveratrol [18-20]. A various literature survey has been suggested that the position of methoxy group had a significant impact on the biological activities and oral pharmacokinectic profiles of methoxy stilbenes. Pharmacokinetics plays a highly imortant role in drug discovery and development [21]. Recently, the pharmacokinetic
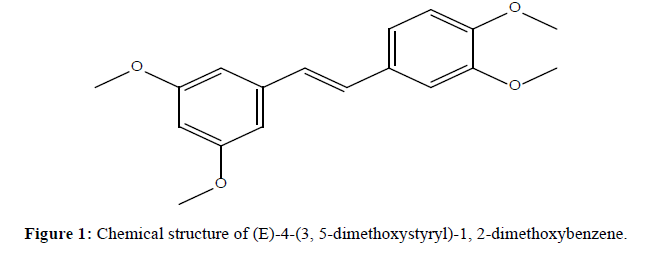
profiles of several methylated resveratrol analogues were reported [22,23]. Our studies suggested that the positions of the methoxy groups of stilbenes were important. In this study, methoxy stilbene derivatives were isolated from the crude extract of the plant Crotolaria medicaginea (Figure 1). The styryl compound was extracted and evaluated for their cytotoxcity activity towards human cervical cancer cell line (HeLa) by using MTT assay techniques. These findings recommended that stilbene derivative is apparently related to a significant reduction in the chance of cervical cancer. A study has reported that substituted stilbene derivative can inhibit the proliferation of HeLa cells have better inhibitory activities against human cervical carcinoma HeLa cells.
Experimental Section
Materials and methods
All the chemicals and solvents were used in this experiment were purchased from sigma-Aldrich. Infra red spectroscopy were recorded on Aglient Pro FT-IR spectrometer using KBr pellet method.1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were obtained in CDCl3 with a Bruker 400 MHz NMR spectrometer using tetramethylsilane (TMS) as a internal standard and the chemical shift value (δ) were reported in parts per million (ppm) scale.
Extraction and isolation
Air-dried and powdered of the plant was extracted with methanol in a soxhlet apparatus for 2 days. The methanolic extract of the plant was further isolated with petroleum ether, benzene, chloroform and ethyl acetate. The ethyl acetate fraction was distilled under reduced pressure to yield a dark colored product. The compound was separated by column chromatography on 100-200 mesh silica gel eluted with a stepwise gradient of using petroleum ether: ethyl acetate. The column fractions were monitored by TLC [24].
Spectral studies of (E)-4-(3,5-dimethoxystyryl)-1,2-dimethoxybenzene
Dark color, Yield: 44%, Fourier transform (FT)-IR (KBr, cm-1); 2835 (-OCH3 Ali), 2999,3068(-CH Str(Ar)),1593(C=C); 1HNMR (400 MHz, DMSO): (δ,ppm), 3.862(s, 6H, -OCH3), 3.815(s, 3H, -OCH3), 3.743 (s, 3H, -OCH3), 6.9 (d, 2H), 6.301-6.932 (m, 6H); 13C-NMR (100MHz, DMSO) (δ,ppm), 161.0, 150.43, 149.05, 139.59, 136.15, 126.60, 120.05, 111.22, 108.79, 104.36, 99.73, 55.38; For C18H20O4: C 71.98 %, H 6.71 %, O 21.31 %.
In vitro anticancer activity
Cell line: HeLa cervical cancer cell line was obtained from the National centre for cell science, Pune. These cells cultures were maintained in Eagles minimum Essential Medium (EMEM) containing 10% fetal bovine serum medium at 37°C in 5% CO2, 95% air and 100% relative humidity. Protection cultures were the passages weekly, and the culture medium was changed twice a week. Cell treatment procedure: The obtained monolayer cell culture were segregated with trypsin-ethylene diamine tetra acetic acid to create viable cells and single cell suspensions were counted by using hemocytometer and medium containing 5% fetal bovine serum, to create a end density of 1 X 105 cells/well. 100 μl/well of cell suspension have been seeded independently in 96-well culture plates at a plating density of 10,000 cells per well and incubated at 37°C in a humidified atmosphere with 5% CO2 and 95% air. After 24 hours, the cells were exposed with different concentrations of the test samples. The sample was first dissolved or distributed in DMSO, and the solution becomes diluted to two times the required last test concentration containing serum free medium. Extra 4 dilutions were making ready to provide a whole of 5 sample concentrations. Following additions, the plates were incubated for an additional 48 hours at 37°C in a humidified atmosphere with 5% CO2 and 95% air. All the assays were performed in manipulate and triplicate was well kept for entire concentrations [24].
MTT (3-[4,5-dimethylthiazol-2-yl]2,5-diphenyltetrazolium bromide) assay
MTT(3-[4,5-dimethylthiazol-2-yl]2,5-diphenyltetrazolium bromide) is a colorimetric assay based on a enzymatic reduction of mitochondria succinate dehydrogenase in living cells to reduce yellow colored water soluble tetrazolium salt of MTT into insoluble
purple formazan. The quantity of cell viability is directly proportional to the activity of enzyme formazan produced, and inversely proportional to the cell inhibition. After 48 hrs of incubation, 10 μl of phosphate buffered saline in 15 μl of MTT solution was pipetted out every well and the plates were incubated at 37°C for four hours. The plates were shaken gently at room temperature and then 100 μl of DMSO was added to the each well to dissolve the formazan crystals. The optical density was absorbed at 570 nm by using micro plate reader [25].
Statistical analysis
The experimental results expressed as the mean ± standard deviation (n=3) analyzed by one way analysis of variance ANOVA followed by post hoc Dunnett’s test. The non linear regression graph was plotted between % cell inhibition and log10 concentration and IC50 was determined using the graph pad prism program.
Calculation of IC50 and % inhibition
IC50 value is a concentration that inhibits half of the cells in vitro. The half maximal inhibitory concentration (IC50) of the control compound was calculated. The MTT assay results were expressed as the percent inhibition according to the following formula: % inhibition = [1- (Absorbance of treatments/ Absorbance of DMSO) x 100]
Molecular docking studies
Molecular docking studies were completed to analyze the binding mode between the extracted compound and the FGF Receptor2 the use of CDOCKER docking protocol of discovery Studio 2016 (CHARMm based docker) is molecular dynamics based docking algorithm [26]. The protein selecting for the present investigation is one of the most crucial aspects. The 3D X-ray crystal structure of the FGFR2 (PDB ID: 4j96) were retrieved from Protein Data Bank (PDB) based on good resolution of 2.297 Ǻ. 4j96 protein is selected as target receptor because to its significant role in anticancer studies [27,28]. Thereafter, the protein is once selected, according to energy minimization with the useful resource of using CHARMm force field until the best gradient tolerance is received. The 3D structure of the extracted compound becomes received by Chem Draw Ultra 12.0 and Chem Draw 3D Pro 12.0 Softwares. CDOCKER is a grid-based molecular docking method that employs CHARMm [29].The default parameters have been used within the docking stimulations with CDOCKER [30]. Different poses of protein-ligand complex is acquired after docking approach with their unique CDOCKER energy and CDOCKER interaction scores. The ligand poses were analyzed and interaction of ligand molecule with the 4j96 protein structure was studied on the basis of H-bonding made by the poses to the receptor molecule and Vander Waals forces between the poses and receptor molecule.
Conclusion
In conclusion, the presented study revealed that the methanolic extract from plant crotalaria medicaginea possesses invitro anticancer activity against the human cervical cancer cell line. A study has reported that substituted stilbene compound can inhibit the proliferation of HeLa cells having better inhibitory activities against human cervical carcinoma HeLa cells. It has shown that moderate cytotoxicity activity towards the HeLa cell line at IC50 concentrations. Docking results reported that the extracted ligand is well docked with target protein 4j96, it shown to a minimum CDOCKER scores. The structure of isolated styryl compound was characterized by FT-IR, 1H and 13C NMR spectroscopic techniques.
References
[1] M. Amarnath, R.Y. Deepthi, K. Chandan, et al., J. Med. Chem, 2011, 54, 6751.
[2] Z. Yusmazura, W.Y. Lim and N. H. Nik Fakhuruddin, J. of Biomed. & Clin. Sci., 2017,2 (1): p. 11-19.
[3] M. N. Mohd Rushdan, Current status and future direction of cervical cancer prevention program in Malaysia., 2008.
[4] U. Vadapalli, S. Muvvala, R. Alluri, et al., IJPSR., 2017, 8(11): 4803-4811.
[5] J. Chong, A. Poutaraud and P. Hugueney, Plant Science., 2009, 177(3), 143-155.
[6] B. De Filippis, A. Ammazzalorso, M. Fantacuzzi, et al., ChemMedChem., 2015, 6, 1513-1517.
[7] E. J. Park, H. Y. Min, Y. H. Ahn, et al., Bioorg. Med. Chem. Lett., 2015, 14.
[8] J. J. Heynekamp, W. M. Webwe, L. A. Hunsker, et al., J. Med. Chem., 2006, 49, 7182-7189.
[9] C. J. Lion, C. S. Matthews, M. F. G. Stevens, et al., J Med Chem., 2005, 48, 1292-1295.
[10] L. Botella and C. Nájara, Tetrahedron., 2004,60, 5563-5570.
[11] M. C. Scherzberg, A. Kiehl, A. Zivkovic, et al., Toxicology and Applied Pharmacology., 2015, 287, 67-76.
[12] M. Robeti, D. Pizziranti, D. Simoni, et al., J Med Chem., 2003, 46, 3546-54.
[13]A. Gosslau, M. Chen, C.T. Ho, et al., Br J Cancer., 2005, 92, 513-21.
[14] H. Park, S.E. Aiyar, P. Fan, et al., Cancer Res., 2007, 67, 5717-26.
[15] Z. Ma, O. Molavi, A. Haddadi, et al., Cancer Chemother Pharmacol., 2008, 63(1), 27-35.
[16] Loai Aljerf, Nuha Al Masri, EC Pharmacology and Toxicology., 2018, 6.6, 463-468.
[17] M. Bernier, R. K Paul, K. S S Dossou, et al., Pharmacol Res Perspect. 2013, 1(2).
[18] M. G. Sarpietro, S. Ottimo, M. C. Giuffrida, et al., Int. J. Pharm., 2010, 388, 144-150.
[19] Nunzio Cardullo, Carmela Spatafora, Nicolo` Mussobe, et al., J Nat Prod., 2015, 78, 11, 2675-2683.
[20] M. G. Sarpietro, C. Spatafora, C. Tringali, et al., J. Agric. Food Chem., 2007, 55, 3720-3728.
[21] J. H. Lin and A. Y. Lu, Pharmacol Rev., 1997, 49(4), 403-449.
[22] C. M. Remsberg, J. A.Yanez, Y. Ohgami, et al., Phytother. Res., 2008, 22, 169-179.
[23] I. M. Kapetanovic, M. Muzzio, Z. Huang, et al., Cancer Chemother. Pharmacol., 2011, 68, 593-601.
[24] T. Mosmann J Immunol Methods., 1983, 65(1-2), 55-63.
[25] Z. Tofighi, P. Asgharian, S. Goodarzi, et al., Med. Chem. Res., 2014, 23, 1718-1724.
[26] B. R. Brooks, R. E. Bruccoleri, B. D. Olafson, et al., J of Comput Chem., 1983, 187-217.
[27] Loai Aljerf and Nuha Almasri, J of Progressive Research in Modern Physics and Chemistry., 2018., 3(1), 97-103.
[28] Loai Aljerf, Farouk Dehmchi, Viet Ty Pham, et al., American Research Journal of Chemistry., 2019, 3(1), 1-14.
[29] G. Wu, D. H. Robertson, C. L. Brooks, et al., J. Comput. Chem., 2003, 24, 1549-1562.
[30] Srikanth lingala, Ragunandan Nerella. Sreenivas Enanganti, J of pharmacy and pharmaceutical sciences., 2013, 453-465.
[31] Y. Niu and C. Yan, J Cancer Ther., 2016, 7, 232-8.
[32] M. Ahmed and K. Jamil, Bio Med., 2011, 3, 60-71.
[33] Y. Peng, Z. Z. Fu, C. S. Guo, et al., Iran J Pharm Res., 2015, 14(1), 251-61.
[34] Q. Tian and Y. H. Zang, J BUON., 2015, 20, 1487-96.