Review Article - Der Pharma Chemica ( 2022) Volume 14, Issue 10
Mouth Dissolving Tablet
Shantanu Shevkar*, Avinash Dhoble and Santosh WaghmareShantanu Shevkar, Department of Pharmaceutics, Loknete Shri Dadapatil Pharate College of Pharmacy Mandavgan Pharata. Tal- Shirur, Dist-Pune, India, Email: sshevkar01@gmail.com
Received: 30-Sep-2022, Manuscript No. dpc-22-76341; Editor assigned: 03-Oct-2022, Pre QC No. dpc-22-76341; Reviewed: 17-Oct-2022, QC No. dpc-22-76341; Revised: 19-Oct-2022, Manuscript No. dpc-22-76341; Published: 26-Oct-2022, DOI: 10.4172/0975-413X.14.10.10-18
Abstract
The longing of further developed agreeability in orally directed items has provoked the advancement of various definitions with further developed execution and agreeableness. Mouth dissolving tablets (MDTs) have gotten steadily expanding request during the most recent couple of many years, and the field has turned into a quickly developing region in the drug business. The one of a kind property of mouth dissolving tablet is that they are quickly breaking down as well as dissolving and delivery the medication when they interact with spit, subsequently forestall the necessity of water during organization. This article surveys the previous applications and systems of taste covering and furthermore underline on the new turns of events and approaches of sharpness decrease for orally utilized drugs. Aside from the ordinary strategies for creation, this survey likewise gives the definite idea of a few one of kind licenses; innovations created and showcased details of Mouth Dissolving Tablets (MDTs).
Keywords
Mouth dissolving tablets (MDTs); Super disintegrants; Taste masking; Patented technology
INTRODUCTION
Drug Delivery Systems (DDS) are a strategic tool for expanding markets/indications, extending product life cycles and generating opportunities. DDS make a significant contribution to global pharmaceutical sales through market segmentation, and are moving rapidly. Drug delivery systems are becoming increasingly sophisticated as scientists acquire a better understanding of the physicochemical parameters pertinent to their performance. Despite of tremendous advancements in drug delivery, the oral route remains the perfect route for the administration of therapeutic agents because of low cost of therapy, ease of administration, self‐medication, leading to high levels of patient compliance. Tablets and capsules are the most popular dosage forms [1]. But one important drawback of such dosage forms is ‘Dysphagia’ or difficulty in swallowing. This is seen to afflict nearly 35% of the general population. This disorder is also associated with a number of conditions like:
1. Motion sickness
2. Unconsciousness
3. Elderly patients
4. Children
5. Mentally disabled persons
6. Unavailability of water.
Improved patient compliance has achieved enormous demand. Consequently demand for their technologies is also increasing many folds. To develop a chemical entity, a lot of money, hard work and time are required. So focus is rather being laid on the development of new drug delivery systems for already existing drugs, with enhanced efficacy and bioavailability, thus reducing the dose and dosing frequency to minimize the side effects [2]. It is always the aim of a scientist or a dosage form designer to enhance the safety of a drug molecule while maintaining its therapeutic efficacy. Recent advances in Novel Drug Delivery Systems (NDDS) aim for the same by formulating a dosage form, convenient to be administered so as to achieve better patient compliance. Pharmaceutical technologists have put in their best efforts to develop a Mouth Dissolving Drug Delivery System [3], i.e Mouth Dissolving Tablet.
Ideal Properties of MDT
Mouth Dissolving Tablet should
• Not require water or other liquid to swallow [4].
• Easily dissolve or disintegrate in saliva within a few seconds.
• Have a pleasing taste.
• Leave negligible or no residue in the mouth when administered [4].
• Be portable and easy to transport.
• Be able to be manufactured in a simple conventional manner within low cost.
• Be less sensitive to environmental conditions like temperature, humidity [5,6].
Advantage of MDT
• No need of water to swallow the tablet [7].
• Can be easily administered to pediatric, elderly and mentally disabled patients.
• Accurate dosing as compared to liquids.
• Dissolution and absorption of drug is fast, offering rapid onset of action [8].
• Bioavailability of drugs is increased as some drugs are absorbed from mouth, pharynx and esophagus through saliva passing down into the stomach [9].
• Advantageous over liquid medication in terms of administration as well as Transportation
• First pass metabolism is reduced, thus offering improved bioavailability and thus reduced dose and side effects.
• Free of risk of suffocation due to physical obstruction when swallowed, thus
MEHANISM OF SUPERDISINTEGRANT [10,11]
Swelling
There are four major mechanisms for tablets disintegration as follows Perhaps the most widely accepted general mechanism of action for tablet disintegration is swelling. Tablets with high porosity show poor disintegration due to lack of adequate swelling force. On the other hand, sufficient swelling force is exerted in the tablet with low porosity. It is worthwhile to note that if the packing fraction is very high, fluid is unable to penetrate in the tablet and disintegration is again slows down.
Porosity and capillary action (wicking)
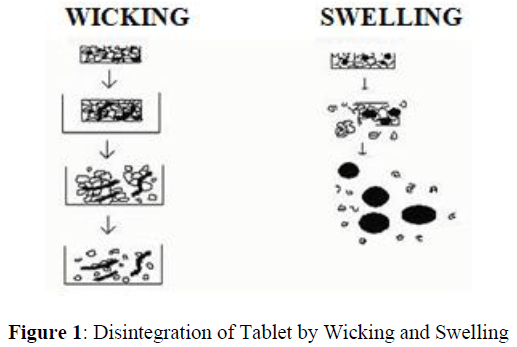
Disintegration by capillary action is always the first step. When we put the tablet into suitable aqueous medium, the medium penetrates into the tablet and replaces the air adsorbed on the particles, which weakens the intermolecular bond and breaks the tablet into fine particles. Water uptake by tablet depends upon hydrophilicity of the drug /excipient and on tableting conditions. For these types of disintegrants maintenance of porous structure and low interfacial tension towards aqueous fluid is necessary which helps in disintegration by creating a hydrophilic network around the drug particles (Figure 1).
Water is pulled by Particles swell and breakup disintegrant and the matrix form physical bonding force.
Due to disintegrating particle
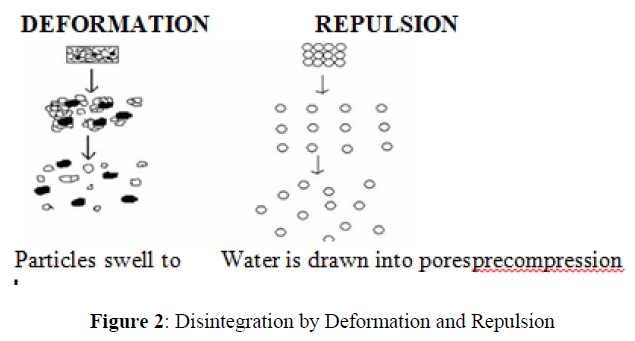
Another mechanism of disintegration attempts to explain the swelling of tablet made with ‘non swellable’ disintegrants. Guyot-Hermann has proposed a particle repulsion theory based on the observation that non swelling particle also cause disintegration of tablets. The electric repulsive forces between particles are the mechanism of disintegration and water is required for it. Researchers found that repulsion is secondary to wicking.
Due to deformation
During tablet compression, disintegrated particles get deformed and these deformed particles get into their normal structure when they come in contact with aqueous media or water. Occasionally, the swelling capacity of starch was improved when granules were extensively deformed during compression. This increase in size of the deformed particles produces a breakup of the tablet. This may be a mechanism of starch and has only recently begun to be studied (Figure 2).
Newer manufacturing technologies used now a day for MDT’s
Freeze drying/Lyophilization MoldingSublimation Spray Dryin
gDirect Compression Mass Extrusion Nanonization
Cotton Candy Process Fast Dissolving Films
Freeze drying/Lyophilization
It is one of the first generation techniques for preparing MDT, in which sublimation of water takes place from the product after freezing. The formulations show enhanced dissolution characteristics due to the appearance of glossy amorphous structure to bulking agents and sometimes to drug. The ideal drug characteristics for this process are relative water insolubility with fine particle size and good aqueous stability in suspensions. Primary problems associated with water-soluble drugs are formation of eutectic mixture, because of freezing point depression and formation of glassy solid on freezing, which might collapse on sublimation. The addition of mannitol or crystal forming materials induces crystallinity and imparts rigidity to amorphous material. The advantage of using freeze-drying process is that pharmaceutical substances can be processed at non elevated temperature, thereby eliminating adverse thermal effects. High cost of equipment and processing limits the use of this process. Other disadvantages include lack of resistance necessary for standard blister packs of the final dosage forms [12, 13].
Molding
There are two types of molding process i.e. solvent method and heat method. Solvent method involves moistening the powder blend with a hydro-alcoholic solvent followed by compression at low pressures in molded plates to form a wetted mass (compression molding). Air-drying is done to remove the solvent. The tablets manufactured so formed are less compact than compressed tablets and possess a porous structure that hastens dissolution. In the heat molding process a suspension is prepared that contains a drug, agar and sugar (e.g. mannitol or lactose). This suspension is poured in the blister packaging wells, and then agar is solidified at the room temperature to form a jelly and dried at 30ºC under vacuum. The main concern about these molded tablets is their mechanical strength, which can be achieved by using binding agents. The spray congealing of a molten mixture of hydrogenated cottonseed oil, sodium carbonate, lecithin, polyethylene glycol and an active ingredient into a lactose based tablet triturate form was used to prepare the taste masked drug particles. As compared to the lyophi Figure 1llization technique, tablets produced by the molding technique are easier to scale up for industrial scale manufacturing [14].
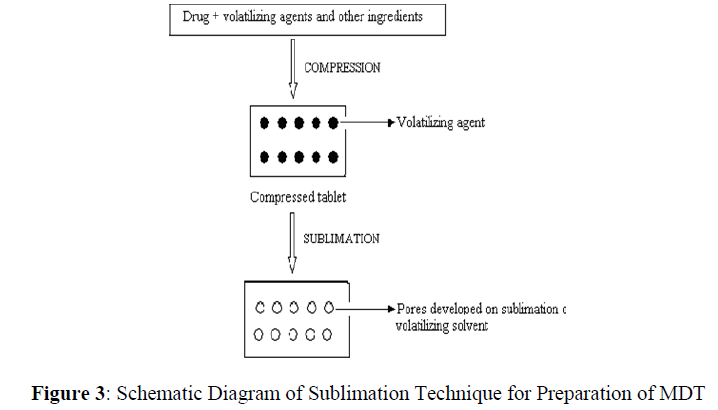
Sublimation
This process involves addition of some inert volatile substances like urea, urethane, naphthalene, camphor, etc to other excipients and the compression of blend into tablet. Removal of volatile material by sublimation creates pores in tablet structure, due to which tablet dissolves when comes in contact with saliva. Additionally several solvents like cyclohexane, benzene etc can also be used as pore forming agents. Mouth dissolving tablets with highly porous structure and good mechanical strength have been developed by this method [15, 16] (Figure 3).
Sprays-Drying
Spray-drying for the production of MDTs. The formulations contained hydrolyzed and non-hydrolyzed gelatin as a supporting agent for the matrix, mannitol as a bulking agent and sodium starch glycolate or croscarmellose as a disintegrant. By adding an acid (e.g., citric acid) or an alkali (e.g., sodium bicarbonate) disintegration and dissolution were further enhanced. The porous powder was obtained by spray drying the above suspension which was compressed into tablets. Tablets manufactured by this method shows disintegration time < 20 sec in an aqueous medium.
Direct compression represents the simplest and most cost effective tablet manufacturing technique. MDT can be prepared by using this technique because of the availability of improved excipients especially super-disintegrants and sugar based excipients.
Super-disintegrates
The rate of disintegration gets affected by the addition of super disintegrants and hence the dissolution. Other ingredients like water-soluble
Excipients and effervescent agents also increase the disintegration.
Sugar based excipients
Type 1 saccharides (lactose and mannitol) exhibit low mouldability but high dissolution rate.
Type 2 saccharides (maltose and maltilol) exhibit high mouldability but low dissolution rate.
Mass – Extrusion
This technology involves softening of the active blend using the solvent mixture of water soluble polyethylene glycol and methanol. This softened mass is extruded through the extruder or syringe and a cylindrical shaped extrude is obtained which are finally cut into even segments using heated blade to form tablets. Granules of bitter drugs can be coated using this method to mask their taste [19].
Nanonization
A recently developed Nanomelt technology involves reduction in the particle size of drug to nano size by wet-milling technique. Surface adsorption of the nano crystals of the drug is done on selected stabilizers for stabilizing them against agglomeration, which are then incorporated into MDTs. This technique is mainly advantageous for poor water soluble drugs and also for a wide range of doses (up to 200 mg of drug per unit).
Cotton Candy Process
The FLASHDOSE® is a MDDDS manufactured using Shearform™ technology in association with Ceform TI™ technology to eliminate the bitter taste of the medicament *. A matrix known as ‘floss’, with a combination of excipients, either alone or with drugs is prepared by using shear form technology. Like cotton-candy fibers floss is fibrous material made of saccharides such as sucrose, dextrose, lactose and fructose at temperatures ranging between 180–266 °F. However, other polysaccharides such as polymaltodextrins and poly-dextrose can be transformed into fibers at 30–40% lower temperature than sucrose. Due to this modification thermo labile drugs can be safely incorporated into the formulation. This process results in a highly porous product and offer very pleasant mouth feel due to fast solubilization of sugars in presence of saliva. The manufacturing process can be divided into four steps as detailed below:
• Floss blend: - The floss mix is prepared by blending the 80% sucrose in combination with mannitol/dextrose and 1% surfactant. The surfactant maintains the structural integrity of the floss fibers by acting as crystallization enhancer. This process helps in retaining the dispersed drug in the matrix, thereby minimizes the migration out of the mixture.
• Floss processing: - The floss formation machine uses flash heat and flash flow processes to produce matrix from the carrier material. The machine is similar to that used in ‘cotton-candy’ formation which consists of a spinning head and heating elements. In the flash heat process, the heat induces an internal flow condition of the carrier material. This is followed by its exit through the spinning head (2000–3600 rpm) that flings the floss under centrifugal force and draws into long and thin floss fibers, which are usually amorphous in nature [20].
PATENTED TECHNOLOGIES
Zydis technology
Zydis is a unique freeze dried oral solid dosage form that can be administered without water and it dissolves instantly on tongue in less than 3 sec. The drug is physically trapped in a water soluble matrix, and then freeze dried to produce a product that rapidly dissolves. The matrix consists of water soluble saccharides and polymer (gelatin, dextran, alginates) to provide rapid dissolution and to allow sufficient physical strength to withstand handling. Water is used during the process to produce porous units for rapid disintegration. Various gums are used to eliminate sedimentation problem of dispersed drug. Glycine is used to prevent the shrinkage of zydis unit during the process and long term storage. As the zydis dosage form is weak in physical strength, unit is contained in peelable blister pack, which allows removal of product without damaging it. An ideal drug candidate for zydis would be chemically stable and water insoluble and should have small particle size (Less than 50 microns). Water soluble drugsmight form eutectic mixtures and not freeze adequately, hence the dose is limited to 60mg. larger drug particles might present sedimentation problem during processing [21].
Orasolv technology
It is CIMA lab’s first fast dissolving formulation. Tablets are prepared by direct compression at low compression force in order to minimize oral
disintegration and dissolution time. Orasolv technology is an example of slightly effervescent tablet that rapidly dissolve in mouth. The active medicaments are taste masked and dispersed in saliva due to the action of effervescent agents. It provides the pleasant sensation in mouth of the patient. The major disadvantage of Orasolv technology is its low mechanical strength. The tablets produced are soft and friable and need to be packaged in specially designed pack.
Durasolv technology
It is also a patented technology by CIMA lab, producing second generation MDT’s. The tablets prepared by this technology contain drug, fillers, lubricant and tablets prepared by conventional equipment. Durasolv formulations have higher mechanical strength than its predecessors due to application of higher compaction pressure. Durasolv product is so durable that it can be packed in either traditional blister pack or vials. It is one of the appropriate technologies for product requiring low amounts of active ingredients [22].
Wowtab technology
Yamanauchi pharmaceutical company patented this technology. ‘wow’ means ‘without water’. The active ingredients may constitute upto 50% w/w of the tablet. In this technique, saccharides of both low and high mouldability are used to prepare the granules. Mouldability is the capacity of a compound to be compressed. Highly mouldable substance has high compressibility and thus shows slow dissolution. The combination of high and low mouldability is used to produce tablets of adequate hardness. Active ingredients are mixed with low mouldability saccharides and then granulated with high mouldabiity saccharides and then compressed into tablet. The Wowtab product dissolves quickly in 15 s or less. Wowtab product can be packed in both into conventional bottle and blister packs [23].
Flashdose Technology
This technology is patented by Fuisz. This system uses the combination of both Shearform and Ceform technologies in order to mask the bitter taste of the drug. A sugar based matrix, called ‘Floss’ is used, which is made up of a combination of excipients (crystalline sugars) alone or in combination with drugs. Nurofen meltlet, a new form of Ibuprofen, as a mouth‐dissolving tablet is the first commercial product prepared by this technology and launched by Biovail Corporation.
Drawbacks
The dosage form can accommodate only up to 600 mg of drug. Tablets produced are highly friable, soft and moisture sensitive. Therefore specialized packing is required.
Flashtab technology
Prographarm labs have a patent over this technology. In this technology, micro granules of the taste‐masked active drug are used. These may be prepared by using conventional techniques like coacervation, microencapsulation, and extrusion spheronisation. All these processes utilize conventional tabletting technology. These taste‐masked micro crystals of active drug, disintegrating agent, a swelling agent and other excipients like soluble diluents etc are compressed to form a multiparticulate tablet that disintegrates rapidly.
Shearform Technology
In this technology, a shear form matrix, ‘Floss’ is prepared. Feedstock prepared with a sugar carrier is subjected to flash heat processing. In this process, sugar is simultaneously subjected to centrifugal force and to a temperature gradient, which causes the temperature of the mass to rise and hence an internal flow condition is created, permitting part of it to move with respect of the mass. The flowing mass comes out through the spinning head that flings the floss. The produced floss is amorphous in nature. So by various techniques, it is further chopped and recrystallized to provide a uniform flow, thus facilitate blending. Then the recrystallized matrix, active drug and other excipients are blended together and finally compressed into tablets. Active drug and other excipients may be blended with the floss before recrystallizing it.
Ceform technology
This technology involves preparation of microspheres of the active drug. Drug material alone or in combination with other pharmaceutical substances, and excipients is placed into a precision engineered rapidly spinning machine. The centrifugal force comes into action, which throws the dry drug blend at high speed through small heated openings. Due to the heat provided by carefully controlled temperature, drug blend liquefies to form a sphere, without affecting the drug stability. The microspheres thus formed are compressed into tablets. As the drug and excipients both can be processed simultaneously, it creates a unique microenvironment in which the materials can be incorporated into the microspheres that can alter the characteristics of the drug, such as enhancing solubility and stability.
Nanocrystal technology
For MDT, Elan's proprietary NanoCrystal technology can enable formulation and improve compound activity and final product characteristics. Decreasing particle size increases the surface area, which leads to an increase in dissolution rate. This can be accomplished predictably and efficiently using NanoCrystal technology. NanoCrystal particles are small particles of drug substance, typically less than 1000 nanometers (nm) in diameter, which are produced by milling the d For fast dissolving tablets, Elan's proprietary NanoCrystal technology can enable formulation and improve compound activity and final product characteristics. Decreasing particle size increases the surface area, which leads to an increase in dissolution rate. This can be accomplished predictably and efficiently using NanoCrystal technology.
NanoCrystal™ Fast dissolving technology provides for:
• Pharmacokinetic benefits of orally administered nanoparticles (<2 microns) in the form of a rapidly disintegrating tablet matrix
• Exceptional durability, enabling use of conventional packaging equipment and formats.
• Wide range of doses (up to 200mg of API per unit).
• Employment of non-moisture sensitive substances.
Preformulation studies mouth dissolving tablet [24, 25]
Preformulation study relates to pharmaceutical and analytical investigation carried out proceeding and supporting formulation development efforts of the dosage form of the drug substance. Preformulation yields basic knowledge necessary to develop suitable formulation for the toxicological use. It gives information needed to define the nature of the drug substance and provide frame work for the drug combination with pharmaceutical excipients in the dosage form. Hence, the following preformulation studies were performed on the obtained sample of drug.
Bulk Density (Db)
It is the ratio of total mass of powder to the bulk volume of powder. It was measured by pouring the weight powder (passed through standard sieve # 20) into a measuring cylinder and initial weight was noted. This initial volume is called the bulk volume. From this the bulk density is calculated according to the formula mentioned below. It is expressed in g/ml and is given by
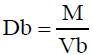
Where, M is the mass of powder
Vb is the bulk volume of the powder.
Tapped Density (Dt)
It is the ratio of total mass of the powder to the tapped volume of the powder. Volume was measured by tapping the powder for 750 times and the tapped volume was noted if the difference between these two volumes is less than 2%. If it is more than 2%, tapping is continued for 1250 times and tapped volume was noted. Tapping was continued until the difference between successive volumes is less than 2 % (in a bulk density apparatus). It is expressed in g/ml and is given by
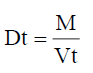
Where, M is the mass of powder
Vt is the tapped volume of the powder.
Angle of Repose (q)
The friction forces in a loose powder can be measured by the angle of repose (q). It is an indicative of the flow properties of the powder. It is defined as maximum angle possible between the surface of the pile of powder and the horizontal plane
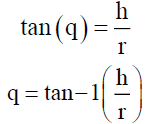
Where, q is the angle of repose.
h is the height in cms
r is the radius in cms.
The powder mixture was allowed to flow through the funnel fixed to a stand at definite height (h). The angle of repose was then calculated by measuring the height and radius of the heap of powder formed. Care was taken to see that the powder particles slip and roll over each other through the sides of the funnel. Relationship between angle of repose and powder flow property (Table 1).
| Sr. No. | Angle of Repose | Type of Flow |
|---|---|---|
| >1 | <20 | Excellent |
| 2 | 20-30 | Good |
| 3 | 30-34 | Passable |
| 4 | >34 | Very poor |
Carr’s index (or) % compressibility
It indicates powder flow properties. It is expressed in percentage and is give
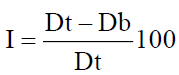
Where, Dt is the tapped density of the powder and Db is the bulk density of the powder (Talbe 2).
| % Compressibility | Flow ability |
|---|---|
| >12-May | Excellent |
| 16-Dec | Good |
| 18-21 | Fair Passable |
| 23-35 | Poor |
| 33-38 | Very Poor |
| <40 | Very Very Poor |
Evaluation of mouth dissolving tablets
Weight variation [26]
20 tablets were selected randomly from the lot and weighted individually to check for weight variation. Weight variation specification as per I.P. is shown in Table 3.
| Average Weight of Tablet | % Deviation |
|---|---|
| >80 mg or less | ±10 |
| More than 80 mg but less than | ±7.5 |
| 250 mg | |
| 250 mg or more | ±5 |
Hardness
Hardness or tablet crushing strength (fc), the force required to break a tablet in a diametric compression was measured using Monsanto tablet hardness tester. It is expressed in kg/cm2.
Friability (F) [27]
Friability of the tablet determined using Roche friabilator or Electro lab friabilator. This device subjects the tablet to the combined effect of abrasion and shock in a plastic chamber revolving at 25 rpm and dropping a tablet at I height of 6 inches in each revolution. Preweighted sample of tablets was placed in the friabilator and were subjected to the 100 revolutions. Tablets were dusted using a soft muslin cloth and reweighed. The friability (F) is given by the formula.
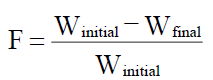
Mechanical Strength
Tablets should possess adequate strength to withstand mechanical shocks of handling in manufacturing, packaging and shipping. Crushing strength and friability are two important parameter to evaluate a tablet for its mechanical strength.
Crushing Strength
It is the force required to break a tablet by compression in the radial direction, it is an important parameter in formulation of mouth dissolve tablets because excessive crushing strength significantly reduces the disintegration time. In the present study the crushing strength of the tablet was measured using Pfizer hardness testers. An average of three observations is reported.
Wetting time [28]
Wetting time is closely related to the inner structure of the tablets and to the hydrophilicity of the excipient. According to the following equation proposed by Washburn E.W (1921), the water penetration rate into the powder bed is proportional to the pore radius and is affected by the hydrophilicity of the powders.
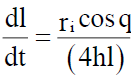
Where l is the length of penetration, r is the capillary radius, ¡ is the surface tension, h is the liquid viscosity, t is the time, and q is the contact angle. It is obvious that pores size becomes smaller and wetting time increases with an increase in compression force or a decrease in porosity. A linear relationship exists between wetting time and disintegration time. Thus wetting is the important step for disintegration process to take place. A piece of tissue paper folded double was placed in a Petri plate (internal diameter is 6.5 cm) containing 6ml of water. The tablet was placed on the paper and the time for complete wetting of the tablet was measured in seconds. The method was slightly modified by maintaining water at 37ºC. Wetting time corresponds to the time taken for the tablet to disintegrate when kept motionless on the tongue.
In vitro dispersion time [29]
Tablet was placed in 10 ml phosphate buffer solution, pH 6.8±0.5ºC. Time required for complete dispersion of a tablet was measured.
In-vitro disintegration time [29]
The process of breakdown of a tablet into smaller particles is called as disintegration. The in-vitro disintegration time of a tablet was determined using disintegration test apparatus as per I.P. specifications. One tablet was placed in each of the 6 tubes of the basket. Add a disc to each tube and run the apparatus using pH 6.8 (simulated saliva fluid) maintained at 37±20C as the immersion liquid. The assembly should be raised and lowered between 30 cycles per minute in the pH 6.8 maintained at 37±20C. The time in seconds taken for complete disintegration of the tablet with no palpable mass remaining in the apparatus was measured and recorded.
Thickness Variation [30]
Ten tablets from each formulation were taken randomly and their thickness was measured with a digital screw gauge micrometer. The mean SD values were calculated.
CONCLUSION
Mouth dissolving tablets can offer several biopharmaceutical advantages such as improved efficiency over conventional dosage forms. For example, they require smaller amounts of active ingredient to be effective, improve absorption profiles, and offer better drug bioavailability than regular tablets and capsules.
References
- Chein YW. Oral Drug Delivery and Delivery Systems. 2nd ed. New York: Marcel Dekker; 1992.
- Kuchekar BS, Bhise SB, Arungam V. Indian J Pharm Edu. 2005, 35: p. 150.
- Slowson M and Slowson S. Pharma Times. 1985, 51: p. 90‐96.
- Indurwade NH, Rajyaguru TH, Nakhat PD. Indian Drugs. 2002, 39: p. 405‐441.
- Seager HJ. Pharm Pharmcol. 1998, 50: p. 375‐382.
- Gohel M, Patel M, Amin A, et al., AAPS Pharm Sci Tech. 2004, 5: p. 36.
- Reddy LH, Ghosh B and Rajneesh. Indian J Pharm Sci. 2002, 64: p. 331‐336.
- Habib W, Khankari R, Hontz J. Drug Carrier Systems. 2002, 17: p. 61‐72.
- Biradar SS, Bhagavati ST, Kuppasad IJ. The Int J Pharmacol. 2006, 4.
- kaur T, Gill B, kumar S, et al., Int J Current Pharma res. 2011, 3: p. 1-7.
- Debjit B, Chiranjib B, Krishnakanth P, et al., J Chem Pharm Res. 2009, 1: p. 163-177.
- Rishi RK. The Pharma Review. 2004, 2: p. 32.
- Kuchekar SB, Badhan CA, Mahajan SH. Pharma Times, 2003, 35: p. 7-14.
- Kamal Saroha, Pooja Mathur, Surender Verma, et al., Der Pharmacia Sinica. 2010, 1: p. 179-187.
- Kuchekar BS, Badhan CA, Mahajan HS. Pharma Times. 2003, 35: p. 7‐10.
- Yarwood RJ, Kearny P, Thomson AR. US Patent. 1998, No. 5738875.
- Uddhav B, Kishore G, Nancy P, et al., Int J Pharm Sci. 2010, 2: p. 76-80.
- Anupama k, Shelly k, Neena b. Int J Pharm Pharm Sci. 2009, 1: p. 12-23.
- Panigrahi D, Baghel S, Mishra B. J Pharm Res. 2005, 4: p. 35‐38.
- Kamal Saroha, Pooja Mathur, Surender Verma, et al., Der Pharmacia Sinica. 2010, 1: p. 179-187.
- Rangasamy M. Int J Pharma Res Dev. 2009, 1: p. 1-10.
- Dinesh V, Sharma I, Sharma V. J Appl Pharm Sci. 2011, 1: p. 50-58.
- Sreenivas SA, Dandagi PM, Gadad AP, et al., Indian J Pharm Educ. 2005, 39: p. 177.
- Rasheed SH, Arief M, Gajavalli SR, et al., Res J Pharm Biol Chem Sci. 2011, 2: p.155-163.
- Mukesh PR, Mohant GP, Upadhyay L. RJPT. 2009, 2: p. 5-12.
- Basawaraj SP, Kulkarni U, Bhavik P, et al., Res J Pharm Biol Chem Sci. p. 587-592.
- Singh SK, Mishra DN, Jassal R, et al., J pharm clin res. 2009, 2: p. 1-9.
- Mohanachandran PR, Krishnamohan PR, Saju F, et al., Int J Appl Pharm. 2010, 2.
- Ahad HA, Kumar CS, Reddy KK, et al., JAPST. 2010, 1: p. 101-107.
- Bhupendra G Prajapati, Satish N Patel. J Sci & Tech. 2011, p. 9-21.
Indexed at, Google Scholar, Crossref