Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 6
Synthesis of 1H-pyrazolo[1, 2-b]phthalazine-5,10-dione derivatives using NiFe2O4 Nanoparticle as a Heterogeneous Catalyst
Vijay V Dabholkar, Swapnil K Kurade* and Keshav S Badhe
Department of Chemistry, Organic Research Laboratory, Kishichand Chellaram College, Churchgate, Mumbai-400020, India
- *Corresponding Author:
- Swapnil K Kurade
Department of Chemistry
Organic Research Laboratory
Kishichand Chellaram College
Churchgate, Mumbai-400020, India
Abstract
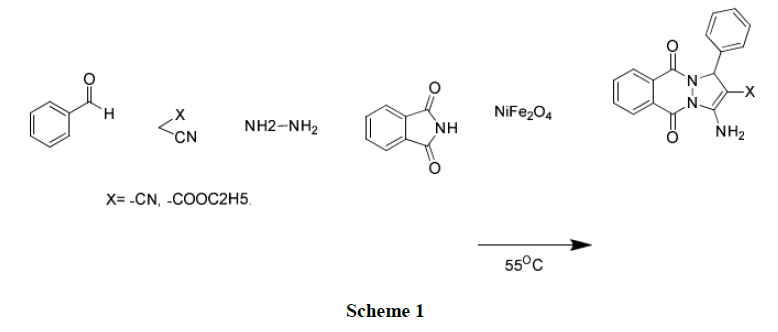
A direct and efficient approach for the preparation of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones has been developed through one pot four component reaction of easily available phthalic anhydride, hydrazine monohydrate, aromatic aldehyde and malononitrile or ethyl acetoacetate catalyzed by nano particulate NiFe2O4, a heterogeneous base catalyst at 55°C. This protocol offers excellent yield of products within short reaction time, easy work up, easy to separate and recyclable catalyst.
Keywords
One-pot synthesis, Hydrotalcite, 1H-pyrazolo [1,2-b]phthalazine-5,10-dione, Heterogeneous
Introduction
Nitrogen containing heterocyclic compounds are wide spread in nature and their application to pharmaceuticals, agrochemicals and functional materials are becoming more and more important [1-5]. 1H-pyrazolo [1,2-b]phthalazine-5,10-diones are important nitrogen containing heterocycles that are known to possess multiple pharmacological and biological activities such as anticancer [6], antimicrobial [7], antifungal [8], anti-inflammatory [9] and anticonvulsant [10]. Phthalazine derivatives are also found to possess cardiotonic [11] and vasorelaxant [12]. Therefore, development of simple method for 1H-pyrazolo [1,2-b]phthalazine-5,10-diones is very important. Multicomponent reaction are one of the most promising methods for the synthesis of heterocycles [13,14]. Multicomponent reactions are often accompanied by significant increase in molecular complexity and impressive selectivity within single step in order to avoid time consuming and costly processes for the purification of various precursors and isolation of intermediate [15,16]. Therefore the development of new methodology for multicomponent reactions is very important in the field of organic and medicinal chemistry. One-pot three component condensation of phthalhydrazide, malononitrile/ethyl cynoacetate and benzaldehyde was reported for synthesis of 1H-pyrazolo [1,2-b]phthalazine-5,10-diones using following conditions [Bmim] OH under microwave irradiation at 100 W power and 45°C [17], triethylamine in ethanol at 50°C for 1 h and ultrasonication with frequency of 50 KHz and 350 W an output power [18], p-TSA in ionic liquid,1-Butyl-3-Methyl Imidazolium Bromide([BMIM]Br) as solvent at 100°C [19]. Synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones were also reported by one-pot four component reaction of phthalimide, hydrazine hydrate, malononitrile/ethyl cynoacetate and benzaldehyde employing basic ionic liquids such as 1,8- diazabicyclo[5,4,0]-undec-7-en-8-ium acetate [20], pyrrolidinium acetate [21] and triethyl amine as a catalyst under ultrasonication condition [22]. But existing methodologies are suffered from several drawbacks such as longer reaction time, high temperature, harsch reaction condition, use of toxic and expensive catalyst, lack of recyclability of catalyst and so forth. Consequently, there is a scope to develop alternative methods for the synthesis of 1H-pyrazolo [1,2-b]phthalazine- 5,10-diones under environmentally benign conditions.
Heterogeneous catalyst has received tremendous amount of interest, both from a scientific and an industrial perspective. Heterogeneous catalysis is leading to a deeper understanding of how chemical reaction takes place at surface [22-24]. Nowdays, Nanoparticles have drawn major attention because they connect the gap between bulk materials and atomic or molecular structures. Research on nanocatalyst has focused on particle size in order to correlating the catalytic activity, many other factor such as geometry, composition and chemical/physical environment can play a role in determining the nanoparticle reactivity.
Nano particulate NiFe2O4 is one of the most versatile and technologically important soft ferrite materials because of its catalytic behaviour, low conductivity and thus lower eddy current losses, ferromagnetic properties [25]. On account of these properties, this spinel ferrite has many applications such as nanodevices [26], magnetic pigment [27] and catalysis [28]. In view of these observations, it was thought worthily to employed nano particulate NiFe2O4, a heterogeneous base catalyst for synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones by one-pot four component reaction of phthalic anhydride, hydrazine hydrate, aromatioc benzaldehyde and malononitrile/ethyl cynoacetate at 55°C.
Materials and Methods
Chemicals and analysis
All chemicals were purchased from S.D. Fine Ltd. Mumbai, India and used without further purification. Melting points of all synthesized compounds were determined in open capillary tubes on an electro thermal apparatus. The purity of the compounds was monitored by thin layer chromatography on silica gel coated aluminum plates (Merck) as adsorbent and UV light as visualizing agent. FT-IR Spectra were recorded on Bruker Spectrometer in the region of 400-4000 cm-1. Proton Nuclear Magnetic Resonance (1H-NMR) and Carbon-13 Nuclear Magnetic Resonance (13C-NMR) spectra were recorded on Varian 500 MHz Nuclear Magnetic Resonance (NMR) spectrophotometer using CDCl3/DMSO-d6 as solvent and TMS as an internal standard (chemical shifts in δ ppm). Powder X-ray diffraction pattern were collected with monochromatic Cu Kα radiation (λ=1.54059 Å) at 40 kV and 15 mA using Shimadzu 7000S diffractometer.
General procedure for synthesis of NiFe2O4
NiFe2O4 nanoparticles were synthesized by co-precipitation method [29]. Nickel nitrate, ferric nitrate and sodium hydroxide were used as starting materials. Aqueous solutions of ferric nitrate and nickel nitrate were prepared in de-ionized water; NaOH solution was then added to it slowly and stirred continuously using a magnetic stirrer until a pH reached to 10-11. This solution was then heated at 80°C for an hour. The obtained precipitate was thoroughly washed with distilled water till pH of filtrate become 7. It was kept overnight for drying. The obtained powder was grounded and calcinied at temperatures 500°C for 3 h.
General procedure for synthesis of 1H-pyrazolo [1,2-b] phthalazine-5, 10-dione
Phthalimide (3 mmol) and hydrazine hydrate (3 mmol) was heated at 50°C in water for 10 min to form phthalihydrazide as intermediate. Then to this mixture, malononitrile (3 mmol), benzaldehyde (3 mmol) and 0.03 g NiFe2O4 were added and the mixture stirred for at 50°C. The completion of reaction was checked by Thin Layer Chromatography (TLC). After that, reaction mixture diluted with ethanol and heated to dissolved solid formed. The solid catalyst was recovered by magnet. The removal of solvent resulted in recovery of solid product. The product was recrystallised using ethanol. Purified product characterized by M.P., NMR and Infrared Radiation (IR).
Results and Discussion
The reaction between phthalimide, hydrazine hydrate, aromatic aldehyde and malononitrile/ethyl aceto-acetate at room temperature in presence of nano particulate NiFe2O4 as a heterogeneous base catalyst was selected as the model reaction for optimization of various parameters.
Initially, the reaction between phthalimide and hydrazine hydrate, when heated at 55°C in ethanol for 10 min led to formation of phthalhydrazide as intermediate. In resulting intermediate, malononitrile and NiFe2O4 catalyst was added followed by addition of benzaldehyde and whole reaction mixture run at 55°C.
The solvent affect the efficiency of the reaction (Table 1). Initially, model reaction (Scheme 1) was carried out under solvent free condition at 55°C; the product was obtained in very poor yield. Then, this reaction screens for various protic and aprotic solvents such as water, ethanol, methanol, Dimethylformamide (DMF) and Dimethyl sulfoxide (DMSO). We were pleased to see that, the reaction proceed smoothly in ethanol with excellent yield of product.
| Entry | Solvent | Yield of product (%) |
|---|---|---|
| 1 | H2O | 45 |
| 2 | EtOH | 88 |
| 3 | MeOH | 76 |
| 4 | DMF | 57 |
| 5 | DMSO | 48 |
| 6 | Without solvent | 35 |
Table 1: Effect of Solvent Selection
The reaction of benzaldehyde, malononitrile, Phthalimide and Hydrazine hydrate in the presence of NiFe2O4 in different solvents at 55°C.
Reaction conditions
Benzaldehyde (3 mmol), malononitrile (3 mmol), Phthalimide (3 mmol), Hydrazinehydrate (3 mmol), solvent (5 ml), NiFe2O4 (0.03 g), reaction temperature (55°C).
As the reaction required catalyst, we also evaluated the amount of catalyst required for the reaction and we observed that yield are affected by the amount of catalyst loading. The results revealed that as tabulated in (Table 2) best yield was obtained by using 0.03 g catalyst. With increasing the quantity of catalyst concentration beyond 0.03 g the yield of desire product was found to be constant. We also carried out the reaction in absence of catalyst, reaction did not proceed further. Therefore, the catalyst plays a crucial role in the success of reaction in terms of yields of the product.
| Entry | Catalyst quantity (g) | Yield of product (%) |
|---|---|---|
| 1 | 0.01 | 66 |
| 2 | 0.02 | 78 |
| 3 | 0.03 | 88 |
| 4 | 0.04 | 88 |
| 5 | 0.05 | 88 |
Table 2: Effect of Catalyst quantity
The reaction of Benz aldehyde, malononitrile, Phthalimide and Hydrazine hydrate using different quantity of NiFe2O4 in C2H5OH at 55°C.
Reaction conditions
Benzaldehyde (3 mmol), malononitrile (3 mmol), Phthalimide (3 mmol), Hydrazine hydrate (3 mmol), Ethanol (5 ml), NiFe2O4 (0.03 g), reaction temperature (55°C).
Nano particulate NiFe2O4, as a heterogeneous catalyst, recovery and reusability of the catalyst are very important factors. NiFe2O4 nanoparticle could be easily separated from the reaction mixture by using magnet. The recovered catalyst was used for number of times without loss of its catalytic activity. It was observed that there was no significant decrease in the yield of product (Table 3).
| Run | Yield of product (%) |
|---|---|
| 1 | 88 |
| 2 | 88 |
| 3 | 88 |
| 4 | 87 |
| 5 | 87 |
Table 3: Reusability of NiFe2O4 catalyst
Reaction conditions
Benzaldehyde (3 mmol), malononitrile (3 mmol), Phthalimide (3 mmol), Hydrazine hydrate (3 mmol), C2H5OH (5 ml), NiFe2O4 (0.03 g), reaction temperature (55°C).
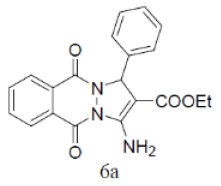
Under the above optimized condition, various aromatic aldehyde containing electron withdrawing and electron donating groups were reacted with malononitrile, phthalimide and hydrazine hydrate to generate derivatives of 1H-pyrazolo[1,2-b]phthalazine-5,10-diones in good to high yields (Table 4). It is observed that electron withdrawing group on aromatic aldehyde reacts rapidly as compared to aromatic aldehyde bearing electron donating group. To further extend the scope of the reaction, the reaction was attempted with ethyl acetoacetate in place of malononitrile under same conditions; it is observed that the corresponding products were obtained with high yields.
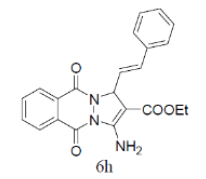
| S. No. | Substituted benzaldehyde | Product | Yield (%) | Time (min) | M.P. (°C) Found | M.P. (°C) Reported |
|---|---|---|---|---|---|---|
| 1 | 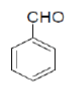 |
 |
88 | 50 | 275-276 | 276-278 [30] |
| 2 | 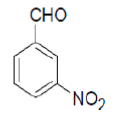 |
 |
84 | 45 | 268-270 | 269-271 [30] |
| 3 | 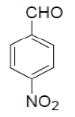 |
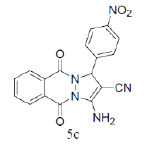 |
93 | 40 | 230-231 | 230-232 [30] |
| 4 | 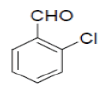 |
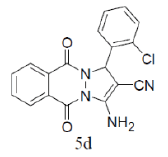 |
85 | 55 | 259-261 | 251-261 [30] |
| 5 |  |
 |
83 | 58 | 265-266 | 266-267 [30] |
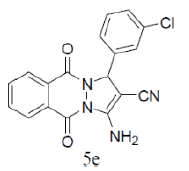
| 6 | 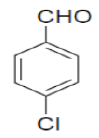 |
 |
85 | 53 | 269-270 | 270-272 [30] |
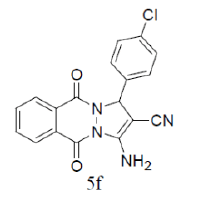
| 7 |  |
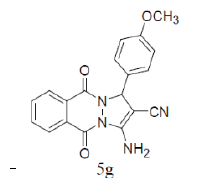 |
87 | 65 | 275-276 | --- |
| 8 | 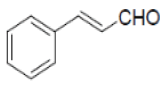 |
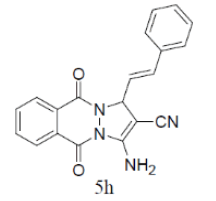 |
82 | 60 | 195-197 | --- |
| 9 | 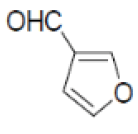 |
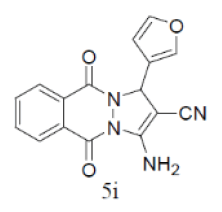 |
84 | 58 | 290-292 | --- |
| 10 | 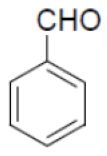 |
 |
90 | 55 | 345-347 | --- |
| 11 | 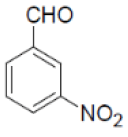 |
 |
86 | 50 | 332-334 | --- |
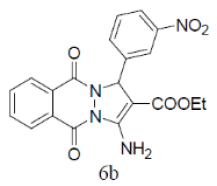
| 12 |  |
 |
87 | 45 | 336-338 | --- |
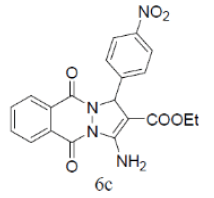
| 13 |  |
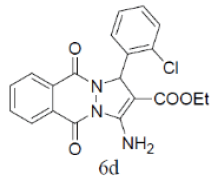 |
84 | 50 | 328-330 | --- |
| 14 | 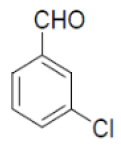 |
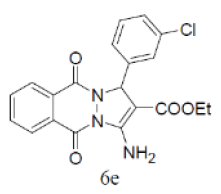 |
83 | 53 | 318-320 | --- |
| 15 | 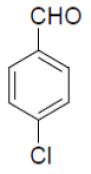 |
 |
86 | 48 | 326-328 | |
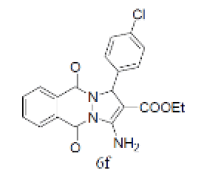
| 16 | 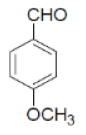 |
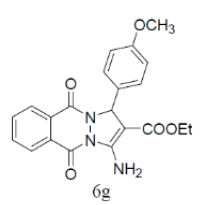 |
89 | 70 | 308-310 | --- |
| 17 | 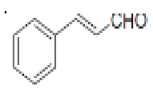 |
 |
86 | 60 | 325-327 | --- |
Table 4: Synthesis of substituted 1H-pyrazolo [1,2-b] phthalazine-5, 10-dione using NiFe2O4 at room temperature (55oC)
Reaction conditions
Substituted Aldehyde (3 mmol), Phthalimide (3 mmol), Hydrazine hydrate (3 mmol), Malononitrile (3 mmol), EtOH (5 ml), NiFe2O4 (0.03 g), reaction temperature (55°C).
Characterisation of catalyst
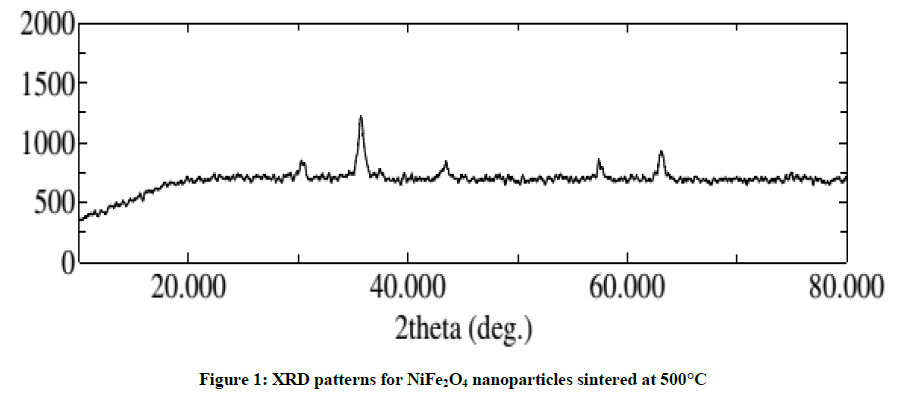
The structural characterization of NiFe2O4 nanoparticles were done by X-ray Diffraction using CuKα radiation (λ = 1.54059 Å) at 40 kV and 15 mA shown in Figure 1. The XRD patterns show the formation of single phase inverse cubic spinal nickel ferrite (the XRD peaks were compared to the standard PDF card number 742081 for inverse cubic nickel ferrite).
Morphological analysis
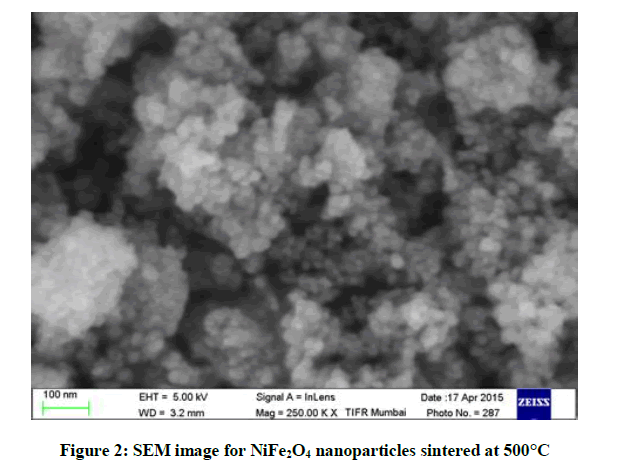
The morphology of NiFe2O4 nanoparticles were studied using SEM shown in Figure 2. Most of the particles are found to be spherical in shape.
Spectral data
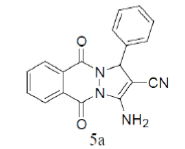
3-Amino-5,10-dioxo-1-phenyl-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile (Entry No. 1): Yellow powder, Yield = 90%, M.P.=275-276°C; IR (KBr) [cm-1]:3362 cm-1 & 3261 (NH2), 2198 (C≡N), 1661 (amido C=O); 1H-NMR (500 MHz, DMSO-d6) δ (ppm)=6.219 (s, 1H, CH), 8.036 (s, 2H.NH2),8.170-8.206 (t, 3H, Ar-H), 8.345- 8.376 (d, 2H, Ar-H) 7.401-7.539 (m, 4H, Ar-H); 13C-NMR (500 MHz, DMSO-d6) δ (ppm)=61.87(C-NH2), 63.51 (C-Ar), 100.02 (C-CN), 116.31 (CN), 127.18-154.09 (C=C, ArC),157.04 (C=O).
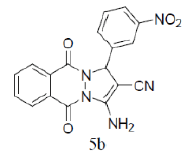
3-amino-1-(3-nitrophenyl)-5,10-dioxo-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine-2-carbonitrile (Entry No. 2): Yellow powder, Yield = 91%, M.P.=268-270°C; IR (KBr)[cm-1]:3433 & 3323 (NH2), 2198 (C≡N), 1658 (amido C=O) 1493 & 1330 (NO2); 1H NMR (500 MHz, DMSO-d6) δ (ppm)=6.330 (s, 1H, CH), 7.941 (s, 2H.NH2), 8.081-8.387 (m, 8H, Ar-H); 13C-NMR (500 MHz, DMSO-d6) δ (ppm)=60.67(C-NH2), 62.73 (C-Ar), 100.04 (C-CN), 116.10 (CN), 122.50-154.37 (C=C, ArC), 157.15 (C=O).
3-amino-1-(2-chlorophenyl)-5,10-dioxo-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine-2- carbonitrile(Entry No. 4): Yellow powder, Yield = 91%, M. P.=259-261°C; IR (KBr) [cm-1]: 3373 cm-1 & 3176 (NH2), 2210 (C≡N), 1659 (amido C=O); 1H-NMR (500 MHz, DMSO- d6) δ (ppm)=6.447 (s, 1H, CH), 7.287 (s, 2H.NH2),7.359-7.388 (t, 2H, Ar-H), 7.892-7.906 (d, 2H, Ar-H) 8.057-8.288 (m, 4H, Ar-H); 13C-NMR (500 MHz, DMSO-d6) δ (ppm)= 60.27 (C-NH2), 61.41 (C-Ar), 115.73 (CN), 127.25-153.84 (C=C, ArC), 156.94 (C=O).
3-amino-1-(4-chlorophenyl)-5,10-dioxo-5,10-dihydro-1H-pyrazolo[1,2-b]phthalazine-2-carbo-nitrile(Entry No. 6): Yellow powder, Yield = 92%, M. P.=269-270°C; IR (KBr) [cm-1]: 3363 cm-1 & 3261 (NH2), 2196(C≡N), 1660 (amido C=O); 1H-NMR (500 MHz, DMSO-d6) δ (ppm)= 6.164 (s, 1H, CH), 7.972 (s, 2H.NH2), 7.428-7.554 (q, 4H, Ar-H), 8.090- 8.294(m, 4H, Ar-H); 13C-NMR (500 MHz, DMSO-d6) δ (ppm)=61.61(C-NH2), 62.87 (C-Ar), 100.08 (C-CN),116.93 (CN), 126.99-154.40 (C=C, ArC), 157.42 (C=O).
Conclusion
We prepared NiFe2O4 nanoparticle by simple co-precipitation method and explored their potential use in the synthesis of 1H-pyrazolo [1, 2-b]-phthalazine-5,10-diones as a solid base heterogeneous catalyst. Catalytic activity results revealed that the NiFe2O4 catalyst showed excellent catalytic performance. Recyclability of the catalyst, non-toxic, operational simplicity and environmentally benign nature make it an attractive process. This method is best alternative to the existing methods and meets the requirements of clean organic reaction and increasing application of nanocatalyst in synthetic organic chemistry.
Acknowledgement
The Authors are thankful to the principal and Management of Guru Nanak college of Arts, Science & Commerce, Sion (E) for constant encouragement and providing necessary facilities. Authors are also thankful to TIFR, Mumbai for providing spectral data.
References
- A. Domling, Chem. Rev., 2006, 106, 17-89.
- M.D. Burke, S.L. Schreiber, Angew. Chem., Int. Ed., 2004, 43, 46-58.
- D.R. Spring, Org. Biomol. Chem., 2003, 1, 3867-3870.
- R.L. Strausberg, S.L. Schreiber, Science., 2003, 300, 294-295.
- D.A. Horton, G.T. Bourne, M.L. Smythe, Chem. Rev., 2003, 103, 893-930.
- J. Li, Y.F. Zhao, X.Y. Yuan, J.X. Xu, P. Gong, Molecules., 2006, 11, 574.
- S. S. El-Sakka, A. H. Soliman,A. M. Imam, Afinidad., 2009, 66, 167.
- C.K. Ryu, R.E. Park, M.Y, J.H. Nho, Bioorg. Med. Chem. Lett., 2007, 17, 2577.
- J. Sinkkonen, V. Ovcharenko, K.N. Zelenin, I.P. Bezhan, B.A. Chakchir, F. Al-Assar, K. Pihlaja, Eur. J. Org. Chem., 2002, 2046.
- L. Zhang, L.P. Guan, X.Y. Sun, C.X. Wei, K.Y. Chai, Z.S. Quan, Chem. Bio. Drug. Design., 2009, 73, 313.
- Y. Nomoto, H. Obase, H. Takai, M. Teranishi, J. Nakamura, K. Kubo, Chem. Pharm. Bull., 1990, 38, 2179-2183.
- N. Watanabe, Y. Kabasawa, Y. Takase, J. Med. Chem., 1998, 41, 3367-3372.
- A.F. Pozharskii, A.T. Soldatenkov, A.R. Katritzky, Heterocycles in Life and Society, 2nd (Edn.), Wiley & Sons: New York, NY, USA, 2011.
- K. Hemming, Annu. Rep. Prog. Chem. B, 2011, 107, 118-137.
- A. Dömling, I. Ugi, Angew. Chem. Int. Ed. Engl., 2000, 39, 3168.
- T.J. Müller, Beilstein J. Org. Chem., 2011, 7, 960-961.
- D.S. Raghuvanshi, K.N. Singh, Tetrahedron Letters., 2011, 52, 5702.
- M.R. Nabid, S.J. Tabatabaie, R. Gahremanzadeh and A. Bazgir, Ultrasonics. Sonochemistry., 2010,17,159.
- R. Ghahremanzadeh, G.I. Shakibaei, A. Bazgir, Synlett., 2008, 8, 1129.
- H.R. Shaterian, M. Mohammadnia, J. Mole. Liquids., 2012, 173, 55.
- L.P. Liu, J.M. Lu, M. Shi, M. Org. Lett., 2007, 9, 1303.
- H.J. Freund, Surf. Sci. Rep., 2008, 3, 63.
- G. Ertl, N. Thiele, Appl. Surf. Sci., 1979, 3, 99.
- R. Imbihl, M. P. Cox, G. Ertl, J. Chem. Phys., 1985, 83, 1578.
- J.L. Gunjakar, A.M. More, K.V. Gurav, C.D. Lokhande, Appl. Surf. Sci., 2008, 254(18), 5844-5848.
- P.M. Ajayan, P. Redlich, P.M. Ru"hle, J. Micro., 1997, 185(2), 275-282.
- X. Wang, G. Yang, Z. Zhang, L. Yan, J. Meng, Dyes. Pigm., 2007, 74(2), 269-272.
- J. Sloczynski, J. Janas, T. Machej, J. Rynkowski, J. Stoch, Appl. Catal. B., 2000, 24(1), 45-60.
- S. Joshi, M. Kumar, S. Chhoker, G. Srivastava, M. Jewariya, V.N. Singh, J. Mole. Str., 2014, 1076, 55-62.
- A. Mulik, M. Deshmukh, D. Chandam, P. Patil, S. Jagdale, D. Patil, Der Pharma Chemica., 2013, 5(2), 19-23.