Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 9
One Pot Synthesis of (4z)-4-(Benzylidene/Substituted Benzylidene)-1n-Methyl-2-(Styryl/Substituted Styryl)-1h-Imidazol-5(4h)-One Derivatives and their Anti-Bacterial Activity Evaluation
Anitha Rani V1* and Bharathi Kumari Y2
1Department of Chemistry, Institute of Aeronautical Engineering, Dundigal, Hyderabad
2Department of Chemistry, Jawaharlal Nehru Technological University Hyderabad, College of Engineering, Kukatpally, Hyderabad-500085, Andhra Pradesh, India
- *Corresponding Author:
- Anitha Rani V
Department of Chemistry
Institute of Aeronautical Engineering
Dundigal, Hyderabad
Abstract
One pot synthesis of (4z)-4-(benzylidene/substituted benzylidene)-1n-methyl-2-(styryl/substituted styryl)-1h-imidazol-5(4H)-one derivatives have been developed. Initially, (Z)-4-benzylidene-2-methyl-oxazol-5(4H)-one was treated with methylamine and refluxed for 5 h in ethanol to produce (Z)-2-acetamido-N-methyl-3-(phenyl/substituted phenyl) prop-2-enamides. which were made to react with the schiff bases benzylidine/substituted benzylidine anilines in the presence of Et3N as a catalyst in ethanol medium under reflux condition for 2 hr to produce in situ (4Z)-4-(benzylidene/ substituted benzylidene)-1N-methyl-2-(styryl/substituted styryl)-1H-imidazol-5(4H)-one derivatives in good yields and tested for their anti-bacterial activities against Escherichia coli, Providencia aeruginosa, Pseudomonas azotogensis and Baccilus Subtillis. Some of the synthesized compounds possess good activity against Escherichia coli and Baccilus Subtillis compared to standard drug streptomycin.
Keywords
One pot synthesis, Schiff bases, Anti-bacterial activity.
Introduction
Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a 1,3-diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents. This ring system is present in important biological building blocks, such as histidine, and the related hormone histamine. Imidazole can serve as a base and as a weak acid. Many drugs contain an imidazole ring, such as antifungal drugs and Nitroimidazole [1-5].
Imidazole was first synthesized by Heinrich Debus in 1858, but various imidazole derivatives had been discovered as early as the 1840s, as shown below, used glyoxal and formaldehyde in ammonia to form imidazole [6]. This synthesis, while producing relatively low yields, is still used for creating C-substituted imidazoles. Synthesis various types of 2-imidazolines are biologically and pharmaceutically very important, since many imidazoline derivatives possess antidiabetic, antihypertensive, and anti-inflammatory activity. Apart of its use for pharmaceutical purpose it also has variety of applications in industries. One of the applications of imidazole is in the purification of His tagged proteins in immobilized metal affinity chromatography (IMAC). Moreover 2-substituted imidazolines are synthetically important due to their use as a synthetic intermediates [7], catalysts [8], chiral auxiliaries [9], chiral catalysts [10,11] and ligands for asymmetric catalysis [12,13] in various synthetic reactions. To date, there are several synthetic methods for 2-imidazolines starting mainly from aldehydes and ethylenediamine with NBS [14]. Some methods includes synthesis from nitriles [15,16], carboxylic acids [17], esters [18], ortho-esters [19], hydroxy-amides [20] and mono or disubstituted chlorodicyanovinyl benzene [21]. It is also called an important synthon for the preparation of biologically active compounds [22]. This prompted us to synthesize (4Z)-4-benzylidene-1-methyl-2-styryl-1H-imidazol-5(4H)-one derivatives of derivatives and evaluate them for anti-bacterial activity.
Materials and Methods
This article deals with simple facile synthesis of (4Z)-4-(benzylidene/ substituted benzylidene-1N-methyl-2-(styryl/substituted styryl)-1H-imidazol-5(4H)-one derivatives possessing different functional groups attached to 1H-imidazol-5(4H)-one ring with a sole view to arrive a new heterocyclic system of high antibacterial activity.
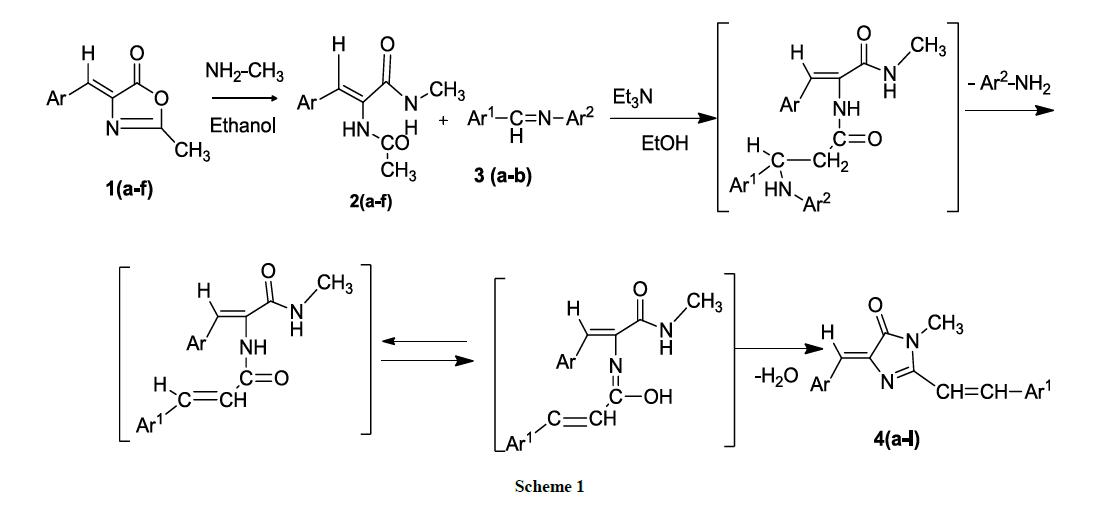
The title compounds (4Z)-4-(benzylidene/substitutedbenzylidene-1N-methyl-2-(styryl/substituted styryl)-1H-imidazol-5(4H)-one derivatives 4(a-l) have been synthesized by green approach through synthetic sequence shown in Scheme 1. Initially, (Z)-4-benzylidene-2-methyl-oxazol-5(4H)-one (1) was treated with methylamine and refluxed for 5 h in ethanol to produce (Z)-2-acetamido-N-methyl-3-(phenyl/substituted phenyl) prop-2-enamides 2 (a-f). which were made to react with the schiff bases benzylidine/substituted benzylidine anilines 3 (a,b) in the presence of L-tyrosine as a catalyst in ethanol medium under reflux condition for 2 h to produce in situ (4Z)-4-(benzylidene/ substituted benzylidene)-1N-methyl- 2-(styryl/substituted styryl)-1H-imidazol-5(4H)-one derivatives 4(a-l). A reasonable mechanism has been formulated for the formation of these imidazoline 5-one derivatives 4(a-l). The IR spectrum of the compounds 4(a-l) showed the absence of absorption for –NH group and the 1H-NMR (DMSO-d6) showed signals for methyl protons for N-methyl group and two trans olefinic protons along with additional signals for aromatic compounds. The mass spectrum of the compounds 4(a-l) showed the molecular ion peaks corresponding to their molecular weights. Thus the structure of compounds 4(a-l) were confirmed from their spectral analysis.
Mechanism
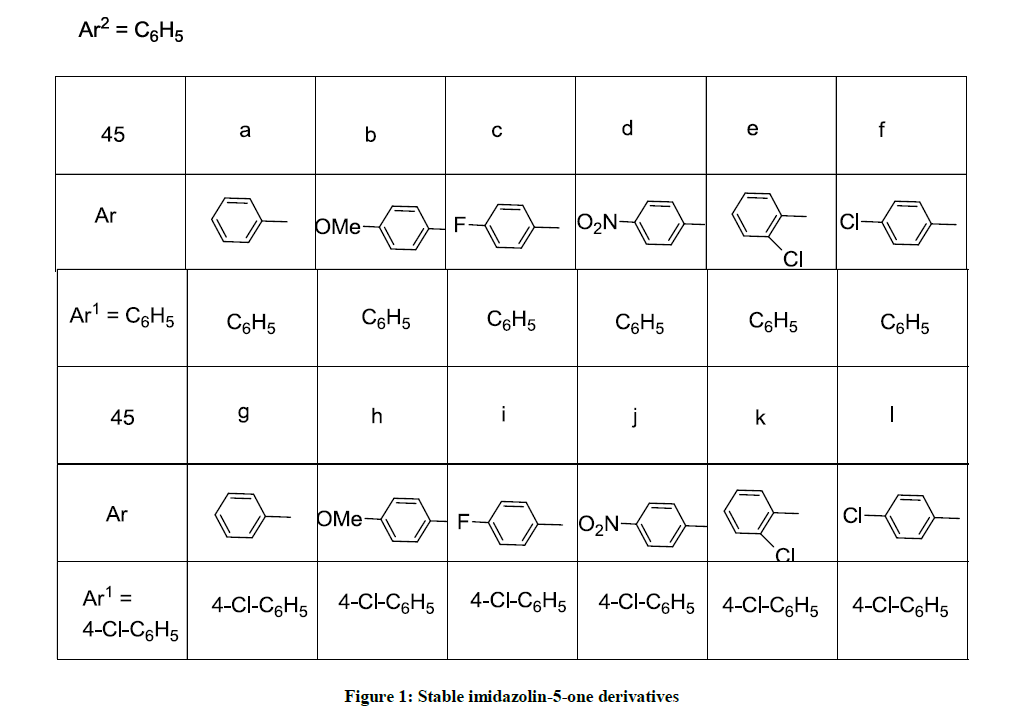
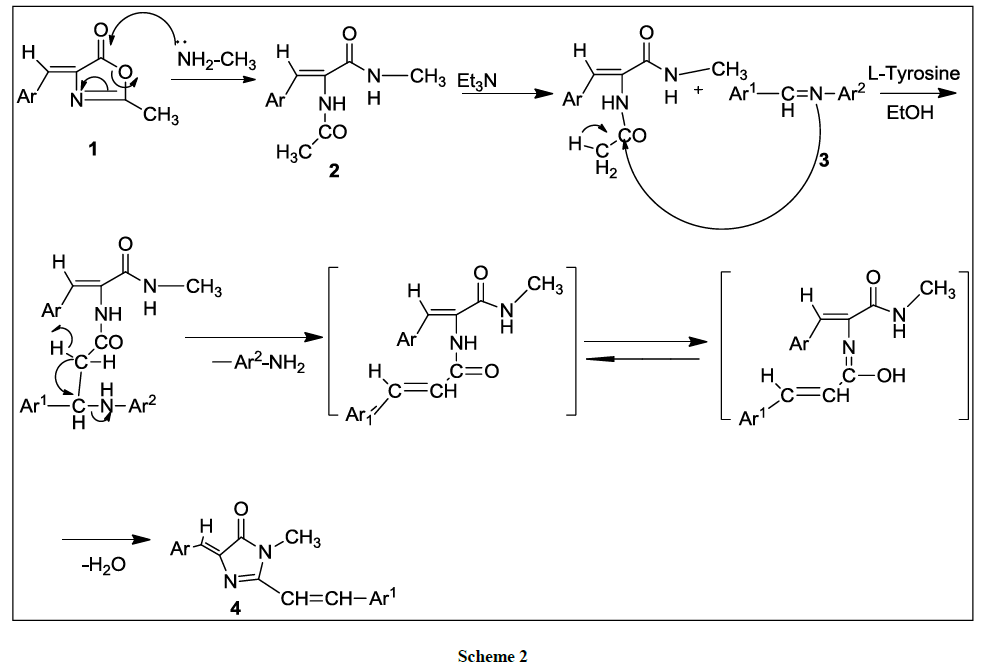
The mechanism of the formation of (4Z)-4-(benzylidene/ substituted benzylidene)-1N-methyl-2-(styryl/substituted styryl)-1H-imidazol-5(4H)- one derivatives 4(a-l) is given as follows. Initially nucleophilic addition of methylamine to 4-benzylidene-2-methyl oxazolin-5-ones 1(a-f) produces (Z)-2-acetamido-N-methyl-3-(phenyl/substituted phenyl) prop-2-enamides 2(a-f). The mechanism can be explained as Michael type addition of the active methyl group of 2(a-f) across the carbon-nitrogen double bond of the Shiff bases leading to the generation of styryl group by the loss of aniline. The resulting unstable intermediates readily undergo cyclocondensation to form stable imidazolin-5-one derivatives 4(a-l) (Figure 1).
The IR spectra of 2(a-f) showed the presence of NH-stretching absorptions for NH and absence of stretching absorptions of lactone ring. The 1H-NMR data showed signals for NH, which are D2O exchangeable and mass spectra confirmed the molecular weight of the compounds.
The cyclocondensation of 2(a-f) with schiff bases to produce (4Z)-4-(benzylidene/ substituted benzylidene-1N-methyl-2-(styryl/substituted styryl)-1H-imidazol-5(4H)-one derivatives 4(a-l) which is supported by IR spectra showing the absence of N-H stretching absorptions of the amide group. The 1HNMR spectra showed the disappearance of peaks for NH and appearance of peaks for 2-styryl protons. Compound 13C NMR spectra of the compound 4(a-l) shows signals for the presence of Ar(C=C), N(C=O), N-CH3, and C-N. Finally the mass spectrum of the compounds 4(a-l) confirmed the molecular weight of the compounds there by supporting the structure of the title compound (4Z)-4- (benzylidene/substituted benzylidene-1N-methyl-2-(styryl/substituted styryl)-1H-imidazol-5(4H)-one derivatives 4(a-l). The mass fragmentation pattern of 4(c) is shown in Scheme 2.
Experimental
Melting points are uncorrected and taken in open capillary tubes in sulphuric acid bath. TLC was run on silica gel-G and visualization was done using UV light. IR spectra were recorded using Perkin- Elmer 1000 instrument in KBr pellets. 1H-NMR spectra were recorded in CDCl3 using TMS as internal standard with 400 MHZ spectrometer. Mass spectra were recorded on Agilent-LCMS instrument under CI conditions and given by Q+1 value only.
Results and Discussion
Preparation of (Z)-2-acetamido-N-methyl-3-(phenyl/substituted phenyl)prop-2-enamide 2(a-f)
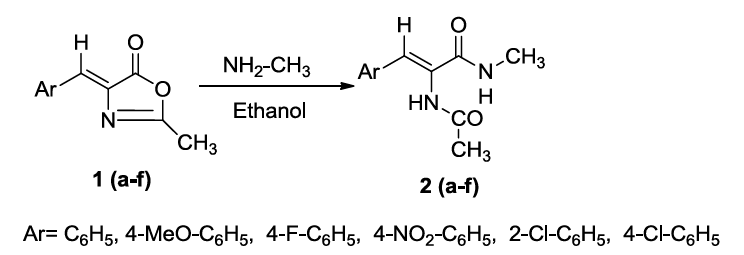
A mixture of (Z)-4-(benzylidene/substituted benzylidene)-2-methyl oxazolin -5-ones 1(a-f) (10 mm) and methylamine (10 mm) was dissolved in ethanol and refluxed for 5 h at 80oC. The completion of the reaction was monitored by TLC (1: 3 of EtOAc: Hexane) then this reaction mixture was cooled to room temperature and poured into ice-cold water (50 ml). Solid separated out was collected, washed with water (10 ml) and dried. The product was recrystallised from ethanol to obtain (Z)-2-acetamido-N-methyl-3-(phenyl/substituted phenyl)prop-2-enamide 2(a-f): The formation of 2(a-f) from oxazolin-5-one derivatives 36(a-f) has been confirmed from the spectral data.
The IR spectrum of the enamides [2(a-f)] showed peaks for NH group and C=O and absence of the peak for lactone ring. The 1H-NMR spectra of the enamides showed signals for NHCH3 and NHCO groups. The mass spectrum of the compounds have given molecular ion peacks (M+) corresponding to their molecular weights.
(Z)-2-acetamido-N-methyl-3-phenyl prop-2-enamide (2a)
Yield: 83%, Melting point: 142°C-144°C, IR (KBr) cm-1: 3464 (-NH), 2989 (Ar), 1720 (-C=O), 1694 (-C=O). 1H-NMR (DMSO-d6): δ (ppm)=1.8 (s, 3H, N-CH3), δ (ppm)=2.8 (s, 3H, CO-CH3), δ (ppm)=5.9 (s, 1H, -NH), δ (ppm)=7.4-7.8 (m, 5H, Ar-H), δ (ppm)=8.2 (s, 1H, NH), δ (ppm)=8.6 (s, 1H, C=CH). Mass (m/z): 219 (M+1) (100%).
(Z)-2-acetamido-N-methyl-3-(4-methoxyphenyl) prop-2-enamide (2b)
Yield: 81%, Melting point: 152°C-153oC; IR (KBr) cm-1: 3468 (-NH-), 2987 (Ar) 1710 (-C=O), 1674 (-C=O). 1H-NMR (DMSO-d6): δ (ppm)=1.7 (s, 3H, N-CH3), δ (ppm)=2.9 (s, 3H, CO-CH3), δ (ppm)=3.7 (s, 3H, -OCH3), δ (ppm)=5.8 (s, 1H, -NH), δ (ppm)=7.4-7.8 (m, 4H, Ar-H), δ (ppm)=8.4 (s, 1H, NH), δ (ppm)=8.7 (s, 1H, C=CH). Mass (m/z): 249 (M+1) (100%).
(Z)-2-acetamido-N-methyl-3-(4-fluorophenyl) prop-2-enamide (2c)
Yield: 78%, Melting point: 148°C-150oC, IR (KBr) cm-1: 3465 (-NH-), 2986 (Ar) 1712 (-C=O), 1670 (-C=O); 1H-NMR (DMSO-d6): δ (ppm)=1.8 (s, 3H, N-CH3), δ (ppm)=2.9 (s, 3H, CO-CH3), δ (ppm)=5.8 (s, 1H, -NH), δ (ppm)=7.4-7.8 (m, 4H, -H), δ (ppm)=8.3 (s, 1H, NH), δ (ppm)=8.8 (s, 1H, C=CH). Mass (m/z): 237 (M+1) (100%).
(Z)-2-acetamido-N-methyl-3-(4-nitrophenyl) prop-2-enamide (2d)
Yield: 88%, Melting point: 158°C-160oC, IR (KBr) cm-1: 3460 (-NH-), 2996 (Ar), 1708 (-C=O), 1675 (-C=O); 1H-NMR (DMSO-d6): δ (ppm)=1.6 (s, 3H, N-CH3), δ (ppm)=2.7 (s, 3H, CO-CH3), δ (ppm)=5.8 (s, 1H, -NH), δ (ppm)=7.4-7.8 (m, 4H, -H), δ (ppm)=8.4 (s, 1H, NH), δ (ppm)=8.9 (s, 1H,C=CH). Mass (m/z): 264 (M+1) (100%).
(Z)-2-acetamido-N-methyl-3-(2-chlorophenyl) prop-2-enamide (2e)
Yield: 84%, Melting point: 159°C-161oC; IR (KBr) cm-1: 3462(-NH-), 2986 (Ar), 1705 (-C=O), 1670 (-C=O); 1H-NMR (DMSO-d6): δ (ppm)=1.7 (s, 3H, N-CH3), δ 2.8 (s, 3H, CO-CH3), δ (ppm)=5.9 (s, 1H, -NH), δ (ppm)=7.4-7.8 (m, 4H, -Ar), δ (ppm)=8.3 (s, 1H, NH), δ (ppm)=8.5 (s, 1H, C=CH). Mass (m/z): 253 (M+1) (100%).
(Z)-2-acetamido-N-methyl-3-(4-chlorophenyl) prop-2-enamide (2f)
Yield: 85%, Melting point:153°C-155oC; IR (KBr) cm-1: 3465 (-NH-), 2986 (Ar), 1704 (-C=O), 1679 (-C=O); 1H-NMR (DMSO-d6): δ (ppm)=1.8 (s, 3H, N-CH3), δ (ppm)=2.9 (s, 3H, CO-CH3), δ (ppm)=5.7 (s, 1H, -NH), δ (ppm)=7.4-7.8 (m, 4H, -H), δ 8.4 (s, 1H, NH), δ (ppm)=8.8 (s, 1H, C=CH). Mass (m/z): 253 (M+1) (100%).
Preparation of (4Z)-4-(benzylidene/substituted benzylidene)-1-methyl-(styryl/substituted styryl)-1H-imidazol-5(4H)-one derivatives 4 (a-l)
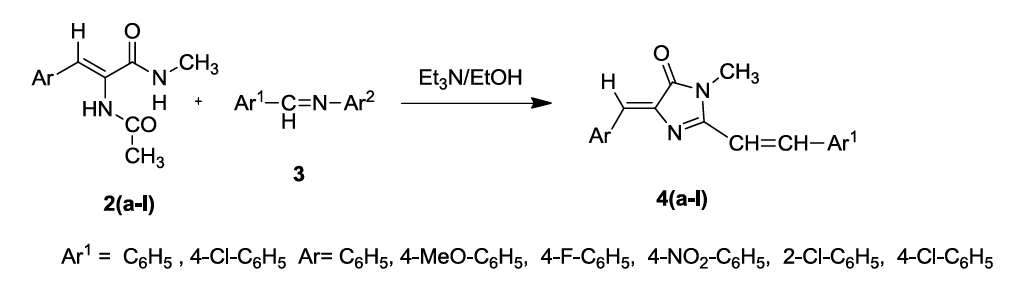
Equimolar quantities of (Z)-2-acetamido-N-methyl-3-(phenyl/substituted phenyl)prop-2-enamide 2(a-f) (10 mM) and schiff bases (10 mm) were mixed together in 20 ml of ethanol and L-Tyrosine (1 mm) as a catalyst. The mixture was refluxed for 2 h. The completion of the reaction was monitored by TLC (1: 3 of EtOAc: Hexane), then this reaction mixture was cooled to room temperature and poured into ice-cold water (50 ml). The solid separated out was collected, washed with water (10 ml) and dried. The product was recrystallized from ethanol to obtain (4Z)-4- (benzylidene/substituted benzylidene)-1-methyl-(styryl/substituted styryl)-1H-imidazol-5(4H)-one derivatives 4(a-l). The physical and spectral analysis of the compounds is given below (Table 1).
| Entry | Starting material | Product | Time (h) | Yield* | M. P. (ºC) |
|---|---|---|---|---|---|
| 1 | 2a | 3a | 2 | 75 | 182-184 |
| 2 | 2a | 3b | 2.5 | 70 | 195-197 |
| 3 | 2a | 3c | 2.5 | 65 | 180-182 |
| 4 | 2a | 3d | 2 | 60 | 210-212 |
| 5 | 2a | 3e | 2.5 | 55 | 189-191 |
| 6 | 2a | 3f | 2.5 | 50 | 220-222 |
| 7 | 2b | 3a | 2 | 70 | 195-197 |
| 8 | 2b | 3b | 2.5 | 70 | 182-185 |
| 9 | 2b | 3c | 2.5 | 65 | 218-220 |
| 10 | 2b | 3d | 2 | 65 | >230 |
| 11 | 2b | 3e | 2.5 | 55 | 222-224 |
| 12 | 2b | 3f | 2.5 | 60 | >230 |
*Refers to yields of crude products only
Table 1: Synthesis of {4(a-l)} from {2(a-f)} and {3(a-b)}
(4Z)-4-benzylidene-1-methyl-2-styryl-1H-imidazol-5(4H)-one derivatives {4(a-l)}
(4Z)-4-benzylidene-1N-methyl-2-styryl-1H-imidazol-5(4H)-one (4a): IR (KBr) cm-1: 1661 (-C=O); 1H-NMR (400 MHz, DMSO-d6/TMS): δ (ppm)=3.3 (s, 3H, -CH3), 7.4-8.3 (m, 10H, Ar-H and s, 3H, =CH-Ar); 13C-NMR (100 MHz, DMSO-d6/TMS): 28.9, 112.2, 112.9, 118.0, 118.3, 118.6, 122.1, 123.5, 128.1, 128.6, 136.4, 137.1, 138.2, 138.9, 150.9, 162.8. M+H=291.
(4Z)-4-(4-methoxybenzylidene)-1N-methyl-2-styryl-1H-imidazol-5(4H)-one (4b): IR (KBr) cm-1: 1665 (-C=O); 1H-NMR (400MHz, DMSO-d6/TMS): δ (ppm)=3.3 (s, 3H, -CH3), 4.5 (s, 3H, -CH3), 7.4-8.3 (m, 9H, Ar-H and s, 3H, =CH-Ar); 13C-NMR (100 MHz, DMSO-d6/TMS): 28.9, 43.8, 111.1, 112.7, 117.5, 118.1, 118.8, 122.7, 122.9, 127.4, 128.4, 135.4, 136.4, 138.6, 138.5, 145.9, 164.3. M+H=321.
(4Z)-4-(4-fluorobenzylidene)-1N-methyl-2-styryl-1H-imidazol-5(4H)-one (4c): IR (KBr) cm-1: 1670 (-C=O); 1H-NMR (400 MHz, DMSO-d6/TMS): δ (ppm)=3.4 (s, 3H, -CH3), 7.4-8.4 (m, 9H, Ar-H and s, 3H, =CH-Ar); 13C-NMR (100 MHz, DMSO-d6/TMS): 30.8, 110.4, 111.5, 117.4, 118.2, 118.7, 122.3, 123.4, 125.4, 128.4, 133.2, 134.5, 137.3, 138.5, 152.8, 160.7. M+H=310.
(4Z)-4-(4-nitrobenzylidene)-1N-methyl-2-styryl-1H-imidazol-5(4H)-one (4d): IR (KBr) cm-1: 1661 (-C=O); 1H-NMR (400 MHz, DMSO-d6/TMS): δ (ppm)=3.3 (s, 3H, -CH3), 7.4-8.3 (m, 10H, Ar-H and s, 3H, =CH-Ar), 13C-NMR (100 MHz, DMSO-d6/TMS): 28.9, 112.2, 112.9, 118.0, 118.3, 118.6, 122.1, 123.5, 128.1, 128.6, 136.4, 137.1, 138.2, 138.9, 150.9, 162.8. M+H=336.
(4Z)-4-(2-chloro benzylidene)-1N-methyl-2-styryl-1H-imidazol-5(4H)-one (45e): IR (KBr) cm-1: 1680 (-C=O); 1H-NMR (400 MHz, DMSO-d6/TMS): δ (ppm)=3.0 (s, 3H, -CH3), 7.4-8.5 (m, 9H, Ar-H and s, 3H, =CH-Ar); 13C- NMR (100 MHz, DMSO-d6/TMS): 29.3, 112.2, 113.5, 118.2, 119.3, 119.6, 122.3, 123.4, 126.4, 127.6, 134.3, 137.2, 138.5, 138.9, 143.5, 160.3. M+H=326.
(4Z)-4-(4-chlorobenzylidene)-1N-methyl-2-styryl-1H-imidazol-5(4H)-one (45f): IR (KBr) cm-1: 1690 (-C=O); 1H-NMR (400 MHz, DMSO-d6/TMS): δ (ppm)=3.2 (s, 3H, -CH3), 7.4-8.3 (m, 9H, Ar-H and s, 3H, =CH-Ar); 13C-NMR (100 MHz, DMSO-d6/TMS): 28.4, 110.3, 110.9, 115.0, 117.3, 118.6, 120.1, 123.4, 128.4, 128.8, 135.2, 137.2, 138.3, 138.9, 145.6, 160.6. M+H=326.
(4Z)-4-benzylidene-1N-methyl-2-(4-chlorostyryl)-1H-imidazol-5(4H)-one (45g): IR (KBr) cm-1: 1694 (-C=O); 1H-NMR (400 MHz, DMSO-d6/TMS): δ (ppm)=3.3 (s, 3H, -CH3), 7.4-8.3 (m, 9H, Ar-H and s, 3H, =CH-Ar); 13C-NMR (100 MHz, DMSO-d6/TMS): 28.5, 112.5, 112.6, 118.4, 118.8, 118.9, 122.3, 123.3, 128.4, 128.7, 133.2, 136.2, 138.4, 138.3, 150.4, 161.4. M+H=327.
(4Z)-4-(4-methoxy benzylidene)-1N-methyl-2- (4-chlorostyryl)-1H-imidazol-5(4H)-one (45h): IR (KBr) cm-1: 1660 (-C=O); 1H-NMR (400 MHz, DMSO-d6/TMS): δ (ppm)=3.2 (s, 3H, -CH3), 4.4 (s, 3H, -CH3), 7.4-8.3 (m, 8H, Ar-H and s, 3H, =CH-Ar); 13C-NMR (100 MHz, DMSO-d6/TMS): 28.7, 42.4, 110.2, 111.3, 115.6, 117.5, 118.5, 122.2, 122.3, 127.0, 128.3, 132.3, 134.4, 138.3, 138.7, 146.3, 164.2. M+H=357.
(4Z)-4-(4-fluoro benzylidene)-1N-methyl-2-(4-chlorostyryl)-1H-imidazol-5(4H)-one (4i): IR (KBr) cm-1: 1673 (-C=O); 1H-NMR (400 MHz, DMSO-d6/TMS): δ (ppm)=3.3 (s, 3H, -CH3), 7.4-8.4 (m, 8H, Ar-H and s, 3H, =CH-Ar); 13C-NMR (100 MHz, DMSO-d6/TMS): 30.2, 111.3, 111.5, 116.4, 118.4, 118.8, 122.2, 123.4, 125.5, 127.4, 130.2, 134.3, 137.2, 138.6, 150.7, 162.8. M+H=345.
(4Z)-4-(4-nitrobenzylidene)-1N-methyl-2-(4-chlorostyryl)-1H-imidazol-5(4H)-one (4j): IR (KBr) cm-1: 1661 (-C=O); 1H-NMR (400 MHz, DMSO-d6/TMS): δ (ppm)=3.3 (s, 3H, -CH3), 7.4-8.3 (m, 10H, Ar-H and s, 3H, =CH-Ar); 13C-NMR (100 MHz, DMSO-d6/TMS): 28.9, 112.2, 112.9, 118.0, 118.3, 118.6, 122.1, 123.5, 128.1, 128.6, 136.4, 137.1, 138.2, 138.9, 150.9, 162.8. M+H=372.
(4Z)-4-(2-chloro benzylidene)-1N-methyl-2-(4-chloro styryl)-1H-imidazol-5(4H)-one (4k): IR (KBr) cm-1: 1690 (-C=O); 1H-NMR (400 MHz, DMSO-d6/TMS): δ (ppm)=3.3 (s, 3H, -CH3), 7.4-8.5 (m, 8H, Ar-H and s, 3H, =CH-Ar); 13C-NMR (100 MHz, DMSO-d6/TMS): 29.4, 111.2, 111.5, 116.2, 117.3, 118.6, 123.3, 123.4, 126.4, 127.6, 134.3, 135.2, 138.5, 138.9, 144.6, 162.5. M+H=362.
(4Z)-4-(4-chlorobenzylidene)-1N-methyl-2-(4-chlorostyryl)-1H-imidazol-5(4H)-one (45l): IR (KBr) cm-1: 1685 (-C=O); 1H-NMR (400 MHz, DMSO-d6/TMS): δ (ppm)=3.2 (s, 3H, -CH3), 7.4-8.5 (m, 8H, Ar-H and s, 3H, =CH-Ar); 13C-NMR (100 MHz, DMSO-d6/TMS): 28.4, 110.2, 112.5, 115.2, 117.3, 118.6, 120.3, 125.7, 126.8, 127.9, 130.7, 138.9, 139.0, 139.9, 143.6, 165.7. M+H=362.
Conclusion
One pot process for the preparation of compounds 4(a-l) has been developed with excellent yields.
Acknowledgement
Authors are very thankful to the authorities of Department of Chemistry, Institute of Aeronautical Engineering, Dundigal, Hyderabad for providing laboratory.
References
- A.R. Katritzky, Rees. Comprehensive Heterocyclic Chemistry., 1984, 5, 469-498.
- M.R. Grimmett, Imidazole and Benzimidazole Synthesis., 1997, Academic Press.
- E.G. Brown, Ring Nitrogen and Key Biomolecules., 1998, Kluwer Academic Press,.
- A.F. Pozharskii, Heterocycles in Life and Society., 1997, John Wiley & Sons,
- T.L. Gilchrist, Heterocyclic Chemistry The Bath press 1985.
- H. Debus, Annalen der Chemie und Pharmacie., 1858, 107(2), 199-208.
- F. Rondu, G. Le Bihan, X. Wang, A. Lamouri, E. Touboul, G. Dive, T. Bellahsene, B. Pfeiffer, P. Renard, B. Guardiola-Lemaitre, D. Manechez, L. Penicaud, A. Ktorza, J.J. Godfroid, J. Med. Chem., 1997, 40, 3793.
- P. Bousquet, Feldman, J. Drugs., 1999, 58, 799.
- M. Ueno, K. Imaizumi, T. Sugita, I. Takata, M. Takeshita, Inter. J. Immunopharmac., 1995, 17, 597.
- T. Hayashi, E. Kishi, V.A. Soloshonok, Y. Uozumi, Tetrahedron Lett., 1996, 37, 4969.
- M.E. Jung, A. Huang, Org. Lett., 2000, 2, 2659.
- E.J. Corey, M.J. Grogan, Org. Lett., 1999, 1, 157.
- T. Isobe, K. Fukuda, Y. Araki, T. Ishikawa, Chem. Commun., 2001, 243.
- H. Fujioka, K. Murai, Y. Ohba, A. Hiramastu, Y. Kita, Tetrahedron Lett., 2005, 46, 2197.
- E.E. Korshin, L.I. Sabirova, A.G. Akhadullin, Y.A. Levin, Russian Chem. Bull., 1994, 43, 431.
- G. Levesque, J.C. Gressier, M. Proust, Synthesis., 1981, 963.
- H. Vorbriiggen, K. Krolikiewicz, Tetrahedron. Lett., 1981, 22, 4471.
- G. Neef, U. Eder, G.J. Sauer, Org. Chem., 1981, 46, 2824.
- A.J. Hill, J.V. Johnston, J. Am. Chem. Soc., 1954, 76, 922.
- N.A. Boland, M. Casey, S.J. Hynes, J.W. Matthews, M.P. Smyth, J. Org. Chem., 2002, 67, 3919.
- G.I. Shin, J.I. Lee, J.H. Kim, Bull. Korean Chem. Soc., 1996, 17, 29.
- A. Davood, E. Alipour, A. Shafiee,Turk J Chem., 2008, 32, 389-395.