Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 10
Parthenin Isolation and Structural Elucidation from Parthenium hysterophorus Plant
Siva kumar Ramamurthy1* and Chenchugari Sridhar2
1Rayalaseema University Kurnool, Andhra Pradesh-518007, India
2Sri Padmavathi School of Pharmacy, Tiruchanoor, Andhra Pradesh-517503, India
- *Corresponding Author:
- Siva kumar Ramamurthy
Rayalaseema University Kurnool
Andhra Pradesh-518007, India
Abstract
Parthenium hysterophorus plant of Asteraceae family known as Gajarghas in south India and has medicinal potential. This plant is well known in Homeopathy and there is no commercial medicine in allopathy. Parthenin is the major chemical constituent of the plant which has various medicinal properties. The objective of this study is to extract the Parthenin from the plant and to derive the Parthenin structure by 1D and 2D Nuclear Magnetic Resonance (NMR) studies.
Keywords
Parthenium, Sesquiterpenoid, Parthenin asteraceae.
Introduction
Parthenium hysterophorus plant is native of tropical America and entered India in mid-fifties. It is widely known as Fever few, Congress weed, Carrot weed, Star weed, Bitter weed, Ramphol and Gajarghas. The word parthenium is derived from Latin parthenice suggesting medicinal uses [1].
John Lindley [2] in Flora Medica described the plant as follows:
The whole plant is bitter and strong-scented, reckoned tonic, stimulating and anti-hysteric. Root decoction is useful in dysentery. Parthenium is used as folk remedy in the Caribbean and Central America. It is applied externally on skin disorders and decoction of the plant is often taken internally as a remedy for a wide variety of ailments [3].
Chemical investigation of Parthenium hysterophorus plant revealed the presence of chemical constituents such as alkaloids, proteins, saponins, tannins, carbohydrate, glycosides, terpenoids, steroids, volatile oils, amino acids, amino sugars, lignans, phenolic compounds, flavonoids, metallic elements, organic acids, terpenoids and others [4-9]. Major component of the plant is Parthenin.
Materials and Methods
General experimental procedure
Melting point was recorded on fisher–john apparatus. The specific rotation was recorded on a Perkin Elmer precision model -343. The IR spectrum was recorded on an IFS 120H spectrometer. The 1H 13C-NMR were obtained on a Bruker 500 MHz spectrometer using TMS as an internal standard.
ESIMS was recorded on a ZAB-HS mass spectrometer. Column chromatography was performed on a silica gel 100-200 mesh. Thin Layer Chromatography (TLC) was carried out on coated silica gel G glass plate with thickness 1 mm (PF 254, art 7747, Merck). Solvents and reagents were purified according to standard procedures.
Plant material: Leaves of Parthenium hysterophorus 10 kg were collected from the forest of Tirumala, Andhra Pradesh, India in June 2016. It was identified by Dr. K. Madhava Chetty, Department of botany, Sri Venkateswara University, Tirupati. A voucher specimen of the plant was deposited in Herbarium, Department of Botany, with the accession number 1216.
Extraction and isolation: Air dried (in shade) leaves of Parthenium hysterophorus was powdered. 50 g of powdered and extracted with the petroleum ether, chloroform, methanol successively. The methanol extract was evaporated under reduced pressure to obtain a residue of 7 g. The residue was adsorbed on silica gel and subjected to column chromatography eluted with hexane and a mixture containing increasing amounts of ethyl acetate. The fraction eluted at 2% of ethyl acetate in hexane was collected separately concentrated and rechromatographed using silica gel column to obtain compound 1 (25 mg in pure form).
Results and Discussion
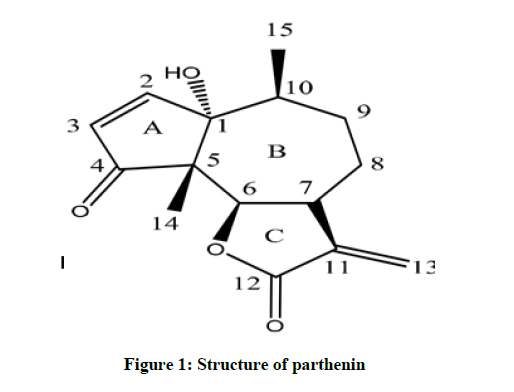
Compound 1 was isolated as a white powder. The molecular formula was deduced from the HRIEMS analysis at m/z 262.30 [M + Na]+, 13C-NMR and DEPT experiment’s as C15H18O4. IR spectrum indicates a broad spectrum at 1707 cm-1 (carbonyl group) [12].
The proton 1H-NMR spectra (MeOD, 500 MHz) of compound 1 showed a methyl singlet at 1.25 and a doublet for methyl at 1.12 (7.6 Hz), which were assigned to C-14, and C-15. Methyl protons, respectively. Four down field protons each showed doublets at 7.64 (5.9 Hz), 6.22 (2.5 Hz), 6.13 (5.9 Hz), and 5.73 (2.1 Hz) were attributed to a proton H-3, H-6a, H-5 and H-6b respectively. An upfield proton doublet at 4.97 (7.9Hz) was assigned to oxygenated proton H-8 [13-15].
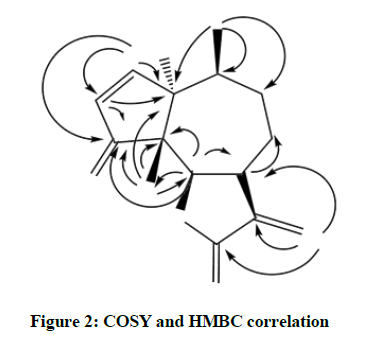
The 13C-NMR spectrum of compound 1 (CdCl3, 125 MHz) showed resonance for 15 carbon atoms including two methyl, three methylene, five methine, three quaternary carbons and two carbonyl carbons. The downfield resonances at 213.43 and 172.84 were assigned to carbonyl carbon and ketone and an ester, respectively. The carbons at two carbons at 86.29 and 75.71 were assigned to the oxygenated carbons C-8 and C-3 respectively. The resonance at 18.93 and 17.67 were ascribed to the methyl carbons C-14 and C-15 respectively. The HMBC spectrum of compound 1 showed the following correlation (Figures 1 and 2). Methyl protons at 1.25 showed correlation with carbons at 84.85, 30.91 and 41.60. The methyl protons at 1.12 showed correlation with 84.85, 213.43, 60.28 and 80.80. The exomethylene protons (Table 1) at 6.22 and 5.73 showed correlations with 45.54, 172.84 and 122.52. The oxymethine protons at 4.97 showed correlation at 213.43, 60.28, 29.42 and 17.67. The major COSY relations were observed in between H-2 and H-3, H-6 and H-7, H-10 and H-15. The overall NMR data were in good agreement with a sesquiterpene and is parthenin compound [16-18].
| S. No. | 1H NMR (500 MHz, MeOD) | 13C NMR (125 MHz, MeOD) |
|---|---|---|
| 1 | - | 84.85 |
| 2 | 7.64 (d, 5.5) | 166 |
| 3 | 6.13 (d, 5.5) | 131.63 |
| 4 | - | 213.43 |
| 5 | - | 60.28 |
| 6 | 4.97 (d, 8.0) | 80.8 |
| 7 | 3.53 (tt, 2.9, 10.4) | 45.54 |
| 8 | 2.25 1.9 |
29.42 |
| 9 | 2.29 1.67 |
30.91 |
| 10 | 2.33 | 41.6 |
| 11 | - | 172.84 |
| 12 | - | 142.15 |
| 13 | 6.22 (d, 2.5) 5.73 (d, 2.5) |
122.52 |
| 14 | 1.12 (d, 7.5) | 17.67 |
| 15 | 1.25 (s) | 18.93 |
Table 1: 1H and 13C-NMR spectral data
Conclusion
Parthenium hysterophorus plant known as allergic plant has medicinal potential and is a known remedy in Homeopathy. As there is no established medicine in market, Parthenin a major chemical constituent was extracted from the plant and Sesquiterpenoid lactone structure was determined in this study by 1D and 2D NMR studies. This will help for further exploration of the moiety for synthesis of Nano particles and to find pharmaceutical applications.
Acknowledgement
Author is very much thankful to Mysore University, Mysore for facilitating the analytical services.
References
- L.H. Bailey, Manual of cultivated plants, Macmillan, New York, 1960.
- John Lindley, Flora Medica; A Botanical Account Of All The More Important Plants Used In Medicine, In Different Parts Of The World, 1838.
- X.A. Dominguez, A. Sierra, Plant Medica., 1970, 18, 275-277.
- K. Lakshmanan, M. Arumugam, R. Mani, Asian J. Pharmaceut. Clin. Res., 2013, 5, 41-44.
- N.S. Sankar, P. Dipak, Scientific Res. J. India., 2014, 3(2), 80-86.
- K. Shashank, M. Amita, K.P. Abhay, Complemen. Alter. Med., 2013, 13, 120, 1-9.
- B. Das, R. Das, Allelopathy Journal., 1995, 2(1), 99-104.
- M. Bakhtiar, K. Rashid, A. Yasir, Afr. J. Biotechnol., 2012, 11(55), 11857-11860.
- S.K. Ramamurthy, P. Vishnu priya, R. Kotturi, P. Devi, S. Kumar, J. Pharmaceut. Sci. Rev. Res., 2011, 10, 95-99.
- Y. Prameela Devi, N.V. Nanda Kumar, The Baroda Journal of Nutrition., 1981, 8, 104-106.
- M. Mahadevappa, Parthenium, Prasaranga, University of Mysore, Mysore, Pp-66.
- D.C. Roy, Md.M. Shaik, J. Med. Plants Stud., 2013, 1(3), 126-141.
- S. Ranade, UAS Tech. Series., 1976, 16, 13-15.
- R.S. Rao, J. Bombay Nat. Hist. Soc., 1956, 54, 218-220.
- R.P. Rastogi, B.N. Mehrotra, Compendium of Indian Medicinal Plants, 1991, 2, 1970-1979.
- C. Reinhardt, S. Kraus, F. Walker, L. Foxcroft, P. Robbertse, K. Hurle, J. Plant Dis. Protection., 2004, 19(7), 253-261.
- E. Rodriguez, M.O. Dilton, T.J. Mabry, J.C. Mitchel, G.H.N. Towers, Experientia., 1975, 32(5), 236-238.
- G.L. Sharma, M.M. Bhutani, Planta Med., 1988,54, 120.