Research - Der Pharma Chemica ( 2022) Volume 14, Issue 1
Phytochemical Evaluation of Asparagus racemosus and Chlorophytum borivilianum Leaves
Sumitra Nain*, Ruchi Singh, Samriti Faujdar and Sarvesh PaliwalSumitra Nain, Department of Pharmacy, Banasthali Vidyapith, Banasthali, Rajasthan-304022, India, Email: nainsumitra@gmail.com
Received: 21-Dec-2021, Manuscript No. dpc-21-43612; Accepted Date: Dec 23, 2021 ; Editor assigned: 23-Dec-2021, Pre QC No. dpc-21-43612; Reviewed: 08-Jan-2022, QC No. dpc-21-43612; Revised: 14-Jan-2022, Manuscript No. dpc-21-43612; Published: 26-Jan-2022, DOI: 10.4172/0975-413X.14.1.10-21
Abstract
Background: This study had basically focused in revealing the existence of various active principles viz. steroids, triterpenoids, glycosides, alkaloids, etc. in the leaves of Asparagus racemosus by performing phytochemical evaluation of various types of extracts. Subsequently, explore the leaf extracts of Chlorophytum borivilianum for revealing the existence of several phytoconstituents. The extracts obtained by Soxhlet method were evaluated for pH, color, and percentage yield. Thus, in this research work, researchers evaluated the physicochemical, phytochemical evaluation and characterization by subjected to standard procedures.
Results: This study claimed that the ethanolic and Aqueous extract of Asparagus racemosus and Chlorophytum borivilianum displayed overwhelming concentration of phytoconstituents viz., carbohydrates, terpenoid (saponins), steroids, flavonoids, proteins, free amino acids, glycosides, alkaloids, tannins, mucilages, etc.
Conclusions: Thus, the ethanolic and aqueous extract of both plants has shown positive and effective reports. Therefore, these both extracts were subjected to standard procedure for the characterization by HPTLC and TLC. The present study includes the detailed exploration of phytochemical constituents found in the leaves of Asparagus racemosus and Chlorophytum borivilianum.
Keywords
Safed musli; Steroids; Triterpenoids; Saponins
Introduction
Currently in developing countries, medicinal plants are in high trend for different therapeutic application and for maintaining good health. Chlorophytum borivilianum is commonly known as safed musli or musli [1]. It is a herb belonging to a family “Asparagaceae” consist of lanceolate leaves. Its leaves are eaten as vegetable & roots are good health tonics. It is indigenous in thick forests of India. Since antiquity Chlorophytum borivilianum have a great economic potential due to high medicinal properties. It consists of saponins, alkaloids, terpenoids, carbohydrates, phenols, resins, mucilage sugars etc. Chlorophytum borivilianum have approx 55-60 cm length with short, white flowers, long slender leaves (20-70 cm long & 2-3 cm wide) and thick, fleshy rhizome [2, 3].
The Latin word “herba” and French word “herbe” gives an English word “herb” [1, 2]. In recent studies “herb” means any part of the plant like roots, stem, leaves, fruits, flowers etc. and also non-woody plants but in old age “herb” means only non- woody plants [2, 3]. The herbal plants have various medicinal properties and used as food, perfume, medicine, ornamental plants, spiritual faith, etc. Since old ages, when the mankind itself started, human beings started depending on nature for their basic needs like shelter, clothing, food, medicine, fragrance, transportation, etc [1-3]. In developing countries many researchers are working on herbal plants as these are showing dominant effect from continuous long use in the health care system. In developed as well as developing countries herbal plants have dynamic part in developing new drug discovery [3]. Herbal plants have sole role in treating deadly diseases like heart attacks, cancer, hepatitis, AIDS, etc [4]. Globally, India is the vast biodiversity- rich country having traditional and medicinal importance of herbal plants in the field of homeopathy, Ayurveda, unani and siddha since ancient times.
Various phytochemical constituents like steroidal saponins, beta sitosterol, stigmasterol are found in Chlorophytum borivilianum but the key pharmacologic phytoconstituent is saponin having various therapeutic applications. Saponins have unique physical property i.e., “soap like formation”. If it is dissolved in liquid, then it will give foam like formation while agitation. It is miscible in both aqueous as well as in oily solution [7, 8]. The chemical structure of saponin includes the attachment of glycosides and sugars to another organic moiety i.e., steroid or triterpenoid. Thus, saponin is also known as triterpenoid saponins or steroidal saponins. Many researchers have done pharmacological studies on tubers of Chlorophytum borivilianum showing many beneficial therapeutic effects viz., hypocholesterolemic, hypolipidemic, anti-oxidant and various other activities [9].
Asparagus racemosus is commonly known as Satavar, Shatavari, etc. It is indigenous to the Himalayan region of north India. Its height is 2-3m with short, spiky stems having white flowers and blackish-purple, globular berries. Its roots are adventitious and tuberous going deep in gravelly, rocky soil. It belongs to a family “Asparagaceae”. Carbohydrates, glycosides, steroidal saponins, flavonoids, alkaloids and mucilage are the main active principles of Asparagus racemosus [10].
Chlorophytum borivilianum
Traditionally, Chlorophytum borivilianum plant is a rare ayurvedic herb having lanceolate leaves found in thick forest regions of India [11]. Now it is being cultivated because of strong economic potential and several usage properties viz. adaptogenic compounds, aphrodisiac, anti-inflammatory and used in arthritis, cancer, and diabetes. Chlorophytum borivilianum is commonly known as safed musli but due to the coloration of its root, referred as White gold [12]. The roots are rich in several active constituents like saponins and polysaccharides. There is various pharmacological activity of Chlorophytum borivilianum viz. anxiolytic, aphrodisiac, immunomodulatory activity, anthelmintic, antiulcer, antistress, anti-tumor, antioxidant, antidiabetic, antimicrobial, etc. Chlorophytum borivilianum is a peculiar gift of nature to mankind. Traditionally the leaves of Chlorophytum borivilianum are used for different activities like – aphrodisiac activity, culinary as well as vegetables [10-13].
Classification
Kingdom: Plantae
Order: Asparagales
Family: Asparagaceae
Sub family: Agavoideae
Genus: Chlorophytum
Species: borivilianum
Asparagus racemosus
Asparagus racemosus is a traditional plant found in India and regions of Himalayas used for treating dyspepsia, constipation, diarrhea, bronchitis, dementia, diabetes, etc. Asparagus racemosus is a species of Asparagus, commonly known as satavar, shatavari, etc and indigenous to tropical and subtropical region of India [14]. It is a woody climber grows 2-3m tall but roots go deep in soil. The demand of Asparagus racemosus is constantly high due to its various medicinal uses [15]. The whole plant of Asparagus racemosus including leaves and roots are especially useful in traditional Ayurvedic medicine [16]. Generally, Asparagus racemosus plants are found in India, some regions of Himalayas and all over the region of Sri Lanka, Asia, Australia, etc. It has many pharmacological effects namely: antitussive, antisecretory, gastrointestinal effects, antibacterial, antiprotozoal activity, apoptogenic activity, molluscicidal effect, antihepatotoxic activity, effect on uterus, etc. Generally, all the therapeutic uses of Asparagus racemosus are described in I.P. and B.P. and in Ayurveda, Siddha and Unani. Asparagus racemosus crop are generally resistant to pest and insects [17-21]. These are properly grown in hot climate and well- drained black soil [22].
Classification
Kingdom: Plantae
Order: Asparagales
Family: Asparagaceae
Subfamily: Asparagoideae
Genus: Asparagus
Species: racemosus
Materials and Methods
Selection of the plants on the basis of medicinal use and ethnobotanical survey
The leaves of Chlorophytum borivilianum and Asparagus racemosus are abundant in saponins, sterols, β-sitosterols, flavonoids, etc., according to different reports [23]. The test components would be the leaves of Chlorophytum borivilianum and Asparagus racemosus.
Collection, storage and authenticity of identification
Asparagus racemosus leaves have been collected from the campus of the University of Gopal Narayan Singh, Jamuhar, Sasaram (Bihar), and Chlorophytum borivilianum leaves was collected from the Indrapuri-Rohtas Sasaram district (Bihar). Plant authentication was performed by the Botanist, Department of Botany, National College of Bihar, University of Patna (Bihar). The leaves of these two plants were desiccated without drying.
Physicochemical Evaluation
The dried parts were subjected to the normal process of determining the different physicochemical parameters.
Successive Extraction of Plant material
Samples were broken and tested with 40 meshes. Dried shade into coarse powdered plant material (250 gms) i.e., dried leaves of Asparagus racemosus and Chlorophytum borivilianum were loaded into Soxhlet machines and extracted with petroleum ether (60-62ºC), Chloroform, ethanol and water until complete extraction. After completing the extraction, the solvent was extracted by distillation. Dried the extracts using a rotator evaporator. The rest was then stored in a dessicator and a percentage yield was determined [12-14].
Phytochemical investigation
According to the standard procedures, the various leaves extracts of Asparagus racemosus and Chlorophytum borivilianum i.e., Pet. Ether extract, Chloroform extract, Ethanolic extract and Aqueous extract were obtained and after extraction they were subjected for phytochemical screening to determine the presence of various phytochemicals present in the extracts [11-13].
Various extracts obtained after extraction were subjected to phytochemical experiments to determine the existence of several phytochemicals present in extracts. Standard procedures were adopted to conduct the research [14, 15].
Characterization of extracts by TLC and HPTLC
The HPTLC instrument was used for the identification of respective chemical constituents present in the leaves of Chlorophytum borivilianum and Asparagus racemosus.
Development of thin layer chromatography
The TLC profile was determined for ethanolic and aqueous extract of ARL: Asparagus racemosus (Leaves) and CBL: Chlorophytum borivilianum (Leaves) using following technique.
Experimental technique [12]
Preparation and activation of plate
Liquid mud (1 part silica G gel and 3 parts pure water) was filtered out of the pestle glass and spread over glass plates (10cm by 20cm) by pouring and allowed to dry in air. The plates work an hour at 110-120 degrees and are placed on desiccators to cool.
Preparation of sample solution
About 1% solution of methanolic extract was prepared and suspended impurities were filtered off.
Saturation of chamber
The solvent system was repaired and poured into the TLC chamber. A sheet of filter paper was inserted into it to provide quick filling and to prevent the bending effect. The room was closed by placing a glass plate at the mouth of the room with paraffin wax.
Application of spots
Spots of sample solutions are applied with the help of small capillaries on a plate, at a distance of about 1.5 cm from the ground and allowed to dry in the air. The distance between the two spots is kept at least 10 mm.
Development of chromatograms
After filling, the plates are placed in a chamber and the solvent is allowed to work until the solvent height of about 15 cm is reached at the point of view, removed and marked. It is then allowed to dry in air and sprayed with detecting reagent and stored in the oven for 5 minutes. Then the Rf values are calculated.
Development of HPTLC
Fingerprint of ethanolic and aqueous extracts of ARL: Asparagus racemosus (Leaves) and CBL: Chlorophytum borivilianum (Leaves) is made using the CAMAG HPTLC (Switzerland) system with the automatic Linomat IV additive. . The analysis was performed in an air-conditioned room maintained at 220ºC. HPTLC is made of pre-assembled silica gel HPTLC aluminum plates 60 F254 (20cm 10cm / 10cm 10cm, 0.2mm thick, 5-6μm particle size, E. Merck, Germany). 5-10μl sample solution (1μg / ml) was identified as a 4mm or 5mm diameter band using automatic samples fitted with a 100 μl Hamilton syringe. Plates are developed using a solvent system prepared i.e. EA: M: H2O; 7.5: 2: 4 for ethanolic and methanolic extraction respectively in the CAMAG double plate development room which was lined with filter paper and filled in front with a 30ml cell section. Enhanced plates are dried in the air and photographed. A spectrodensitometer (Scan 3, CAMAG) equipped with WINCATS software for planar chromatography controller was used for densitometry measurement and data processing. Absorbance / emission was a measurement mode with a scan speed of 20 mm / sec. Fragment dots are scanned from 200 to 800 nm to record their UV-VIS spectrum and detect high wavelength wavelengths. Densitogram was recorded at wavelength of high absorption of a different sample observed [13-14].
Result
In this research work different solvents were used to prepare various extracts and researchers evaluated the percentage yield of different extracts of both the plants i.e. Asparagus racemosus and Chlorophytum borivilianum, shown below in Table 1 and Table-2.
| S/No. | Extract | Parameters | |||
|---|---|---|---|---|---|
| Nature of Extract | Color | pH | % Yield | ||
| 1. | PEEARL | Semi Solid | Dark Brown | 5.89 | 0.67 |
| 2. | CEARL | Semi solid | Creamish Brown | 6.03 | 3.09 |
| 3. | EEARL | Semi Solid | Blackish Brown | 6.09 | 8.11 |
| 4. | AEARL | Solid Powder | Light brown | 6.05 | 15.09 |
| 5. | PEECBL | Semi Solid | Dark Green | 6.09 | 0.86 |
| 6. | CECBL | Semi Solid | Green | 6.09 | 2.98 |
| 7. | EECBL | Semi solid | Light Brown | 7.87 | 7.77 |
| 8. | AECBL | Semi Solid | Dark brown | 6.08 | 11.99 |
| Abbreviations PEEARL=Petroleum Ether extract of Asparagus racemosus (Leaves) CEARL=Chloroform extract of Asparagus racemosus (Leaves) EEARL=Ethanolic extract of Asparagus racemosus (Leaves) AEARL=Aqueous extract of Asparagus racemosus (Leaves) PEECBL=Petroleum Ether extract of Chlorophytum borivilianum(Leaves) CECBL=Chloroform extract of Chlorophytum borivilianum (Leaves) EECBL=Ethanolic extract of Chlorophytum borivilianum (Leaves) AECBL=Aqueous extract of Chlorophytum borivilianum (Leaves) |
|||||
| S/No. | Constituents | ARL | |||
|---|---|---|---|---|---|
| PEEARL | CEARL | EEARL | AEARL | ||
| 1 | Carbohydrates | √ | √ | X | √ |
| 2 | Glycosides | X | X | √ | √ |
| 3 | Alkaloids | X | X | √ | √ |
| 4 | Protein & Amino acid | X | X | √ | √ |
| 5 | Tannins & Phenolic compounds | X | X | √ | X |
| 6 | Flavonoids | X | √ | √ | X |
| 7 | Fixed oil and Fats | X | X | X | X |
| 8 | Steriods & Triterpenoids | √ | √ | √ | √ |
| 9 | Waxes | X | X | X | X |
| 10 | Mucilage & Gums | √ | X | X | X |
| √ = Present X =Absent |
|||||
Physicochemical Evaluation
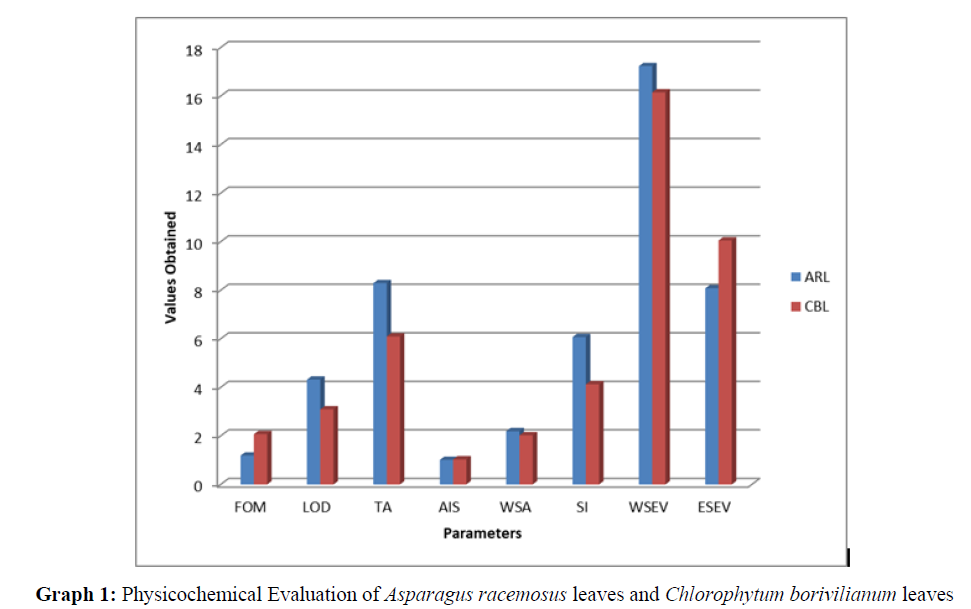
According to the standard procedures the dried plant parts of ARL: Asparagus racemosus (Leaves) and CBL: Chlorophytum borivilianum (Leaves) were used for the determination of various physicochemical parameters (Table 3-5). The results were presented in table 5.1 and Graph 1.
| S/No. | Constituents | CBL | |||
|---|---|---|---|---|---|
| PEECBL | CEPCBL | EECBL | AECBL | ||
| 1 | Carbohydrates | X | √ | √ | √ |
| 2 | Glycosides | X | √ | √ | √ |
| 3 | Alkaloids | √ | √ | √ | √ |
| 4 | Protein & Amino acid | X | X | √ | √ |
| 5 | Tannins & Phenolic compounds | √ | √ | √ | √ |
| 6 | Flavonoids | X | X | X | X |
| 7 | Fixed oil and Fats | X | X | X | X |
| 8 | Steriods & Triterpenoids | √ | √ | √ | √ |
| 9 | Waxes | X | X | X | X |
| 10 | Mucilage & Gums | X | X | X | X |
| √ = Present; X = Absent | |||||
| S. No. | Solvent System | Inference |
|---|---|---|
| 1. | Chloroform | Overlapping |
| 2. | Ethyl acetate | Overlapping |
| 3. | Methanol | Overlapping |
| 4. | Ethyl acetate: hexane: methanol (9:90:1) | Overlapping |
| 5. | Chloroform: benzene: methanol (20:2:1) | Tailing |
| 6. | Ethyl acetate: hexane: chloroform (50:50:5) | Tailing |
| 7. | Ethyl acetate: hexane: chloroform (50:60:3) | Poor |
| 8. | Chloroform: Ethyl acetate: formic acid (2.5: 2: 0.5v/v) | Satisfactory |
| 9. | Toluene: ethyl acetate (50:50) | Overlapping |
| 10. | Toluene: ethyl acetate: Glacial acetic acid: formic acid (20: 45: 20:5) | Overlapping |
| 11. | Toluene: ethyl acetate: formic acid (3: 2: 0.4v/v) | Satisfactory |
| 12. | Ethyl Acetate:methanol:water (7.5: 2: 4) | Best |
| Adsorbent: Silica gel, Detecting reagent: Ferric chloride solution and in UV at 254 nm | ||
| S./No. | Name | No. of spots (Visible) | No. of spots | Rf values |
|---|---|---|---|---|
| (At 254nm) | ( in visible) | |||
| 1 | EEAARL | 2 | 2 | 0.51, 0.68 |
| 2 | AEARL | 2 | 2 | 0.49, 0.70 |
| 3 | EECBL | 2 | 2 | 0.47, 0.72 |
| 4 | AECBL | 2 | 2 | 0.48. 0.71 |
| Adsorbent: Silica gel G, Detecting reagent: Ferric chloride solution and in UV at 254 nm, Solvent System: Ethyl Acetate: methanol: water (7.5: 2: 4 v/v) | ||||
| S/No. | Parameters | ARL | CBL |
|---|---|---|---|
| 1. | FOM | 1.19±0.02 | 2.07±0.28 |
| 2. | LOD | 4.32±0.15 | 3.09±0.12 |
| 3. | TA | 8.29±0.08 | 6.09±0.12 |
| 4. | AIS | 1.01±0.01 | 1.03±0.02 |
| 5. | WSA | 2.19±0.12 | 2.02±0.03 |
| 6. | SI | 6.07±0.13 | 4.12±0.11 |
| 7. | WSEV | 17.23±1.08 | 16.15±1.12 |
| 8 | ESEV | 8.08±1.11 | 10.05±1.03 |
Successive extraction of selected herbs
The shade dried coarsely powdered plant material of ARL: Asparagus racemosus leaves) and CBL: Chlorophytum borivilianum (Leaves) was extracted with petroleum ether, Chloroform, ethanol and water. The extraction was done by soxhlet method for dried plant material of ARL and CBL.
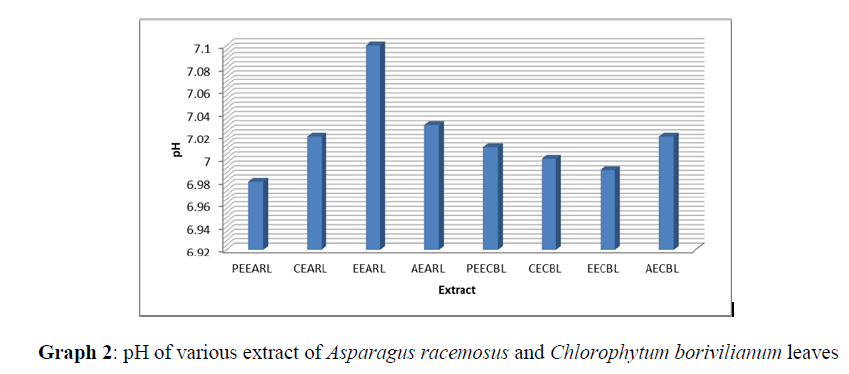
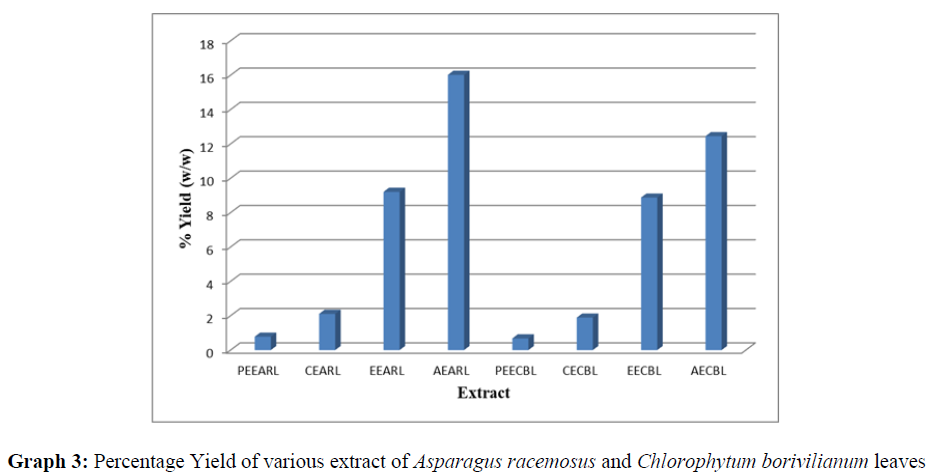
The extracts obtained were evaluated for pH, color and % yield. The results were presented in table 5.2, 5.3 and graph 2 & 3.
| S/No. | Extract | Parameters | |||
|---|---|---|---|---|---|
| Nature of Extract | Color | pH | % Yield | ||
| 1 | PEEARL | Semi Solid | Creamish brown | 6.98 | 0.78 |
| 2 | CEARL | Semi solid | Brown | 7.02 | 2.11 |
| 3 | EEARL | Semi Solid | Light brown | 7.1 | 9.21 |
| 4 | AEARL | Solid Powder | Dark brown | 7.03 | 16.02 |
| S/No. | Extract | Parameters | |||
|---|---|---|---|---|---|
| Nature of Extract | Color | pH | % Yield | ||
| 1 | PEECBL | Semi Solid | Green | 7.01 | 0.68 |
| 2 | CECBL | Semi Solid | Light green | 7 | 1.89 |
| 3 | EECBL | Semi solid | Brown | 6.99 | 8.88 |
| 4 | AECBL | Semi Solid | Blackish brown | 7.02 | 12.45 |
Preliminary phytochemical investigation of extracts
The various extract of ARL: Asparagus racemosus (Leaves) and CBL: Chlorophytum borivilianum (Leaves) obtained post-extraction were subjected to phytochemical experiments to determine the presence of existing phytochemical extracts. A standard procedure was adopted to conduct research.
The preliminary phytochemical screening revealed the presence of Carbohydrates, Alkaloids, Glycosides, Flavonoids, Tannins, Phenolic compounds, and Amino acid, Saponins, Steroids, Gums and Proteins.
The results for ARL are presented in table 5.4 and for CBL are presented in table 5.5.
| S/No. | Constituents | ARL | |||
|---|---|---|---|---|---|
| PEEARL | CEARL | EEARL | AEARL | ||
| 1 | Carbohydrates | + | + | - | + |
| 2 | Glycosides | - | - | + | + |
| 3 | Alkaloids | - | - | + | + |
| 4 | Protein & Amino acid | - | - | + | + |
| 5 | Tannins & Phenolic compounds | - | - | + | - |
| 6 | Flavonoids | - | + | + | + |
| 7 | Fixed oil and Fats | - | - | - | - |
| 8 | Steriods & Triterpenoids | + | + | + | + |
| 9 | Waxes | - | - | - | - |
| 10 | Mucilage & Gums | - | - | - | - |
| + = Present; - = Absent | |||||
| S/No. | Constituents | CBL | |||
|---|---|---|---|---|---|
| PEECBL | CEPCBL | EECBL | AECBL | ||
| 1 | Carbohydrates | - | + | + | + |
| 2 | Glycosides | - | + | + | + |
| 3 | Alkaloids | + | + | + | + |
| 4 | Protein & Amino acid | - | - | + | + |
| 5 | Tannins & Phenolic compounds | + | + | + | + |
| 6 | Flavonoids | - | - | + | + |
| 7 | Fixed oil and Fats | - | - | - | - |
| 8 | Steriods & Triterpenoids | + | + | + | + |
| 9 | Waxes | - | - | - | - |
| 10 | Mucilage & Gums | - | - | - | - |
| + = Present; - = Absent | |||||
Characterization of extracts by TLC and HPTLC
The various extract of ARL: Asparagus racemosus (Leaves) and CBL: Chlorophytum borivilianum (Leaves) post-extraction were subjected to phytochemical experiments to determine the presence of various phytochemicals present in extracts. In ethanolic extract and aqueous extract the maximum phyto-constitunets were present in ARL and CBL. Therefore these two extracts of ARL: Asparagus racemosus (Leaves) and CBL: Chlorophytum borivilianum (Leaves) were subject to standard procedure for the characterization by TLC and HPTLC.
The TLC was performed for EEARL, AEARL, EECBL and AECBL. The findings were recorded in table 5.6 and table 5.7
| S. No. | Solvent System | Inference |
|---|---|---|
| 1 | Chloroform | Overlapping |
| 2 | Ethyl acetate | Overlapping |
| 3 | Methanol | Overlapping |
| 4 | Ethyl acetate: hexane: methanol (9:90:1) | Overlapping |
| 5 | Chloroform: benzene: methanol (20:2:1) | Tailing |
| 6 | Ethyl acetate: hexane: chloroform (50:50:5) | Tailing |
| 7 | Ethyl acetate: hexane: chloroform (50:60:3) | Poor |
| 8 | Chloroform: Ethyl acetate: formic acid (2.5: 2: 0.5v/v) | Satisfactory |
| 9 | Toluene: ethyl acetate (50:50) | Overlapping |
| 10 | Toluene: ethyl acetate: Glacial acetic acid: formic acid (20: 45: 20:5) | Overlapping |
| 11 | Toluene: ethyl acetate: formic acid (3: 2: 0.4v/v) | Satisfactory |
| 12 | Ethyl Acetate:methanol:water (7.5: 2: 4) | Best |
| Adsorbent: Silica gel, Detecting reagent: Ferric chloride solution and in UV at 254 nm | ||
| S./No. | Name | No. of spots (Visible) | No. of spots(At 254nm) | Rf values ( in visible) |
|---|---|---|---|---|
| 1 | EEAARL | 2 | 2 | 0.51, 0.68 |
| 2 | AEARL | 2 | 2 | 0.49, 0.70 |
| 3 | EECBL | 2 | 2 | 0.47, 0.72 |
| 4 | AECBL | 2 | 2 | 0.48. 0.71 |
| Adsorbent: Silica gel G, Detecting reagent: Ferric chloride solution and in UV at 254 nm, Solvent System: Ethyl Acetate: methanol: water (7.5: 2: 4 v/v) | ||||
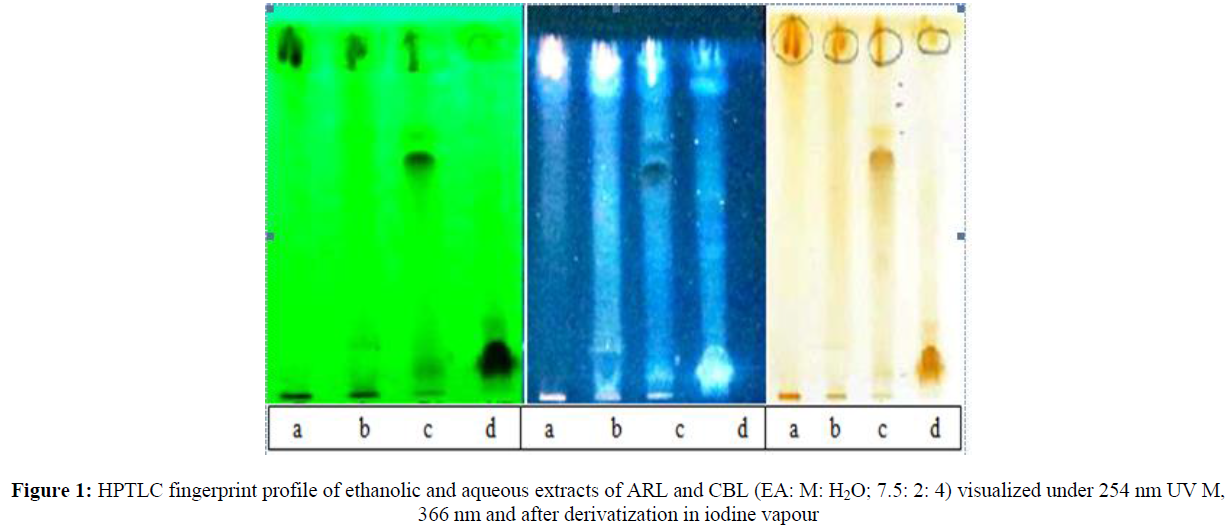
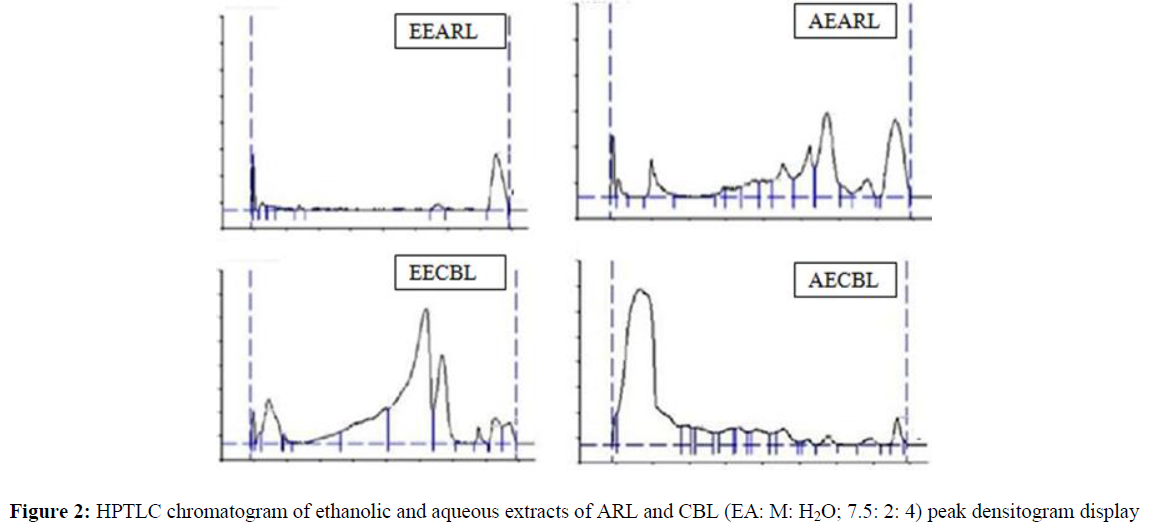
EEARL, AEARL, EECBL and AECBL were subjected to HPTLC analysis by specific solvent systems i.e., EA: M: H2O; 7.5: 2: 4 for respective extracts and detected under UV at 25 4nm to identify the active phyto-constituents present in these extract. The results were presented in table 5.8. Figure 1 shows the number of reported peaks. HPTLC chromatograms were presented in Figure 2.
| Samples | Rf | Max Height | Max % | Area | Area % |
|---|---|---|---|---|---|
| EEARL | 0.43 | 50.4 | 5.23 | 592.7 | 1.99 |
| 0.11 | 102.6 | 10.65 | 1994.4 | 6.71 | |
| 0.68 | 28.8 | 2.99 | 389.4 | 1.31 | |
| AEARL | 0.49 | 47.2 | 4.90 | 1625.4 | 5.47 |
| 0.56 | 93.2 | 9.68 | 3170.0 | 10.66 | |
| 0.70 | 140.8 | 14.61 | 4151.7 | 13.97 | |
| 0.78 | 235.6 | 24.45 | 8067.7 | 27.1 | |
| 0.81 | 48.7 | 5.06 | 1454.7 | 4.89 | |
| 0.95 | 216.1 | 22.43 | 8278.3 | 27.85 | |
| EECBL | 0.13 | 135.0 | 7.94 | 890.7 | 1.26 |
| 0.21 | 183.9 | 10.82 | 6129.5 | 8.69 | |
| 0.29 | 37.6 | 2.21 | 382.6 | 0.54 | |
| 0.30 | 147.6 | 8.70 | 11804.5 | 16.73 | |
| 0.47 | 564.6 | 33.23 | 34856.8 | 49.41 | |
| 0.58 | 373.2 | 20.97 | 10930.1 | 15.40 | |
| 0.72 | 67.1 | 4.31 | 680.4 | 0.96 | |
| 0.84 | 104.17 | 6.20 | 2593.7 | 3.67 | |
| 0.91 | 83.7 | 4.93 | 2279.9 | 3.23 | |
| AECBL | 0.19 | 618.4 | 50.21 | 48110.3 | 74.67 |
| 0.41 | 78.8 | 6.53 | 1809.5 | 2.82 | |
| 0.48 | 68.9 | 5.71 | 2579.9 | 4.01 | |
| 0.69 | 66.1 | 5.48 | 2045.4 | 3.18 | |
| 0.71 | 67.8 | 5.61 | 1754.6 | 2.73 | |
| 0.75 | 61.1 | 5.06 | 2296.7 | 3.57 | |
| 0.84 | 62.9 | 5.21 | 2059.9 | 3.21 |
Discussion
There are various chemical constituents in medicinal plants which enhances the properties of prevention and treatment from various diseases [76, 77]. These chemical constituents are secondary metabolites, present in one or many parts of the plants. These chemical constituents are secondary metabolites, present in one or many parts of the plants. These secondary metabolites may be categorized as glycosides, alkaloids, flavonoids, saponins, steroids etc. The knowledge is still incomplete and it should go more and more especially regarding biosynthetic pathway due to which these medicinal plants are highly valuable in today’s life [78, 79]. The developing countries, forest areas, rural places have traditional uses of these medicinal plants. Since, last few decades, there is a drastic elevation in exportation of medicinal plants, due to which all over the world, traditional health system is in high demand [80, 81, 82].
There are various medicinal plants are now in extinction due to over-exploitation. Since last few decades, medicinal plants are in high demand for its properties. The tribal communities use different types of plants for their treatments [82, 83, 84].
Conclusion
This study revealed the presence of various phytochemical constituents, recorded above in which Steriods & Triterpenoids were in excessive amount in both Asparagaus racemosus and Chlorophytum borivilianum leaves and Tannins & Phenolic compounds in Chlorophytum borivilianum leaves and this study also proved that the percentage (%) yield of Aqueous extract of both the plants i.e. Asparagus racemosus and Chlorophytum borivilanum leaves was greater in comparison to other prepared extracts.
Acknowledgments
The authors are thankful to the Honorable Vice-Chancellor Banasthali Vidyapith, Rajasthan, for providing essential facilities.
References
- Cheung MC. Thromb and Vasc Biol. 2001. 21: p. 1320-1326.
- Doughari JH. J Med Plants Res. 2009, 3: p. 839-848.
- Arnoldus M Mangao. J Sci Food Agric. 2020, 100(3): p. 1185-1194.
- Salhi S. Lazaroa. 2010, 31: p. 133.
- Benkhnigue O. et.al. Acta Botanica Barc. 2019, 53: p. 191-216.
- Majambu Mbiky. Front Pharmacol. 2012, 20: p. 969-973.
- Lin X. et.al. Eur J Clin Nutr. 2010, 64: p. 1481-1487.
- Kamada C. Free Radi Res. 2005, 39: p. 185-194.
- Carroll KK. J Nutr. 1995, 125: p. 598S-605S.
- Radi R. Arch Biochem Biophys. 1991, 286: p. 117-125.
- Gylling H. Atheroscler. 2014, 232(2): p. 346-360.
- Parraga I. BMC Complement Altern Med. 2011, 11: p. 73.
- Carlos Eduardo Cabral. Arq Bras Cardiol. Arq Bras Cardiol. 2017, 109(5): p. 475-482.
- Mensink RP. Atheroscler. 2002, 160(1): p. 205-213.
- Plat J. et.al. J Nutr. 2009, 139(6): p. 1143-1149.
- Shattat F. ghassan. Biomed Pharmacol J. 2014, 7(2).
- Rita Kiss. Int J Mol Sci. 2018, 19(3): p. 771.
- Mang B. Eur J Clin Invest. 2006, 36(5): p. 340-344.
- Neelakantan Nithya. Nutr J. 2014, 13: p. 7.
- Subbarao D. J Nutr. 1997, 100: p. 1307-1309.
- Kitchen BJ. Biochem J. 1973, 135(1): p. 93-99.
- Pawar H. Med & Aromat Plants. 2018, 7: p. 1-7.
- Nitesh Visavadiya P. Evid Based Complement Alternat Med. 2009, 6(2): p. 219-26.
- Abu Saleh M. Bangladesh J Pharmacol. 2006, 1: p. 64-67.
- Gylling Heleng. J ISCP. 2010, 6(3): p. 18-21.
- Das Gitishree. Front Pharmacol. 2020, 11: p. 561248.
- Zahmatkesh M. Chron Dis J. 2013, 1(2): p. 74-82.
- Das Sayan. IJPCB. 2019, 8(6).
- Samani KG. Int J Health Sci. 2014, 8(1): p. 39-43.
- Gupta R. Int J of Biomed Adv Res. 2015, 6(01): p. 57-59.
- Willirson james T. Circulation. 2002, 106: p. 3143.
- Sambaiah K. Nahrung. 1991, 35(1): p. 47-51.
- Karen E. Friday. Exp Biol Med (Maywood). 2003, 228(7): p.769-778.
- Karkhaneh Azam. Lipids Health Dis. 2019.
- Muruganandan S. J Biol Chem. 2011, 286: p. 23982–23995.
- Islam D. J Diabet Mellit. 2017, 7: p. 55-70.
- Thamolwan, Suanarunsawat. Oxid Med Cell Longev. 2011, p. 962025.
- Vadiya DB Ashok. J Clin Biochem Nutr. 2007, 41(1): p. 1-11.
- Jihua zhang. AIJBM. 2019, 9(2):
p. 306-314. - Lemon Mark. J biomed life science. 2017, 4(3): 1-9.
- Ram Lokhande. Natural Science. 2010, 2: p. 26-32.
- MI Alam. J Pharmacology & Pharmacy. 2014, 5: p. 828-837.
- Hipólito Aguiar Hernández. J biomed & life science. 2016, 3: p. 1-15.
- Jamuna Senguttuvan. Asian Pac J Trop Biomed. 2014, 4(Suppl 1): p. S359-S367.
- Sunil Singh KR. Pharm Biol. 2009, 48(2): p.134-141.
- Upton Roy. Phytochemistry Reviews. 2020, 19: p. 1157–1177.
- Katiyar Chandrakant. NCBI. 2012, 33(1): p. 10-19.
- Lidia Szwajkowska-Michałek. MDPI. 2020, 10(19): p. 6907.
- Millett MA. ACS publications. 1964.
- Negi JS. Pharmacogn Rev. 2011, 5(10): p. 155-158.
- Kopila A. Pharmacogn J. 2018, 10(5): p. 963-968.
- Botham PA. Toxicol In Vitro. 2004, 18(2): p. 227-230.
- Gautam vinod Kr. J Adv Pharm Technol Res. 2013, 4(2): p. 108-117.
- Alam Sajid. CELLMED. 2019, 9(3): p. 5.1-5.2.
- Narayana DBA. Pharmacogn Mag. 2010, 6(23): p. 145-146.
- Hyder S. J Ethnopharmacol. 2011, 138(3): p. 741-747.
- Venu Pamidiboina. Int J Pharm and Pharm Sci. 2010, 2: p. 86 -91.
- Babu S. RJPP. 2018, 10(3): 241-245.
- Venu Pamidiboina. Int J Pharm and Pharm Sci. 2010, 2: p. 86 -91.
- Carroll KK. J Nutr. 1995, 125: p. 598S-605S.
- Bruno Chukwuemeka Chinko. JAMPS. 2020.
- Zulet M. J Am Coll Nutr. 1999, 18(1): p. 36-42.
- Scot M. AHA journal. 2018, 139: p. e1082–e1143.
- Alan R Needle. Sports Med. 2017, 47(7): p. 1271-1288.
- Asita Wongprikorn. PLoS One. 2016, 11(6): p. e0157531.
- Chem Clinton D. NIH. 2011, 21(5): p. 365-371.
- Jacek Rysz. Int J Mol Sci. 2020, 21(2): p. 601.
- Bergheanu SC. Neth Heart J. 2017, 25(4): p. 231-242.
- Harada-Shiba. J Atheroscler Thromb. 2018, 25(9): p. 846-984.
- Barbara Fletcher. AHA journal. 2015, 112: p. 3184-3209.
- Engelmann B. Biochim Biophys Acta. 1992, 1165: p. 32-37.
- Fenton WS. Biol Psychiatry. 2000, 47(1): p. 8-21.
- Michos ED. Prog Cardiovasc Dis. 2019, 16: p. 1557-1567.
- Condell RA. Arch Biochem Biophys. 1983, 223: p. 407-416.
- Shin-ichiro Kagami. Int Arch Allergy Immunol. 2008, 1: p. 61-66.
- Tamas Csont . Biochem Biophys Res Commun. 2002, 290: p. 1535-1538.
- Engelmann B. Biochim Biophys Acta. 1992, 1165: p. 32-37. \
- Verma Nihirika. Int J Curr Pharm Res. 2017, 9(1).
- Ghassan F Shattat. Biomed Pharmacol J. 2014, 7(2).
- Eric D Shirley. Sports Health. 2012, 4(5): p. 394-403.
- Sudipta Kumar Rath. Ayu. 2013, 34(3): p. 273-275.
- Thakur M. Evid Based Complement Alternat Med. 2007, 4 (4): p. 419-423.
- Shashi Alok. Asian Pac J Trop Dis. 2013, 3(3): p. 242-251.
- Fabio Firenzuoli. Evid Based Complement Alternat Med. 2007, 4: p. 37- 40.
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Cross ref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref
Indexed at Google Scholar Crossref