Review Article - Der Pharma Chemica ( 2024) Volume 16, Issue 2
Protein Assembly: An Understanding and Clarification
Sivakumar B1*, Eldhose M J2, Asish S1, Vipuldas MK2, Anila Kumar2 and Vidhya PM32Department of Pharmacology, Sanjo College of Pharmaceutical Studies, Vellapara, Chithali Palakkad, India
3Department of Pharmaceutical Chemistry, Sanjo College of Pharmaceutical Studies, Vellapara, Chithali Palakkad, India
Sivakumar B, Department of Pharmacognosy, Sanjo College of Pharmaceutical Studies, Vellapara, Chithali Palakkad, India, Email: sivapharm003@yahoo.co.in
Received: 09-Feb-2024, Manuscript No. Dpc-24-127344; Editor assigned: 14-Feb-2024, Pre QC No. Dpc-24-127344 (PQ); Reviewed: 28-Feb-2024, QC No. Dpc-24-127344; Revised: 01-Mar-2024, Manuscript No. Dpc-24-127344 (R); Published: 28-Mar-2024, DOI: 10.4172/0975-413X.16.2.239-242
Abstract
Alzheimer's disease is characterized by the accumulation of mis folded proteins that form fibrillary amyloid deposits in specific regions of the central nervous system. This leads to memory loss, changes in personality, abnormal behavior and a decline in cognitive abilities. In the early stages of the disease, individuals may experience short-term memory loss, difficulty remembering names and addresses and an inability to learn new information. As the condition progresses, these changes become more pronounce and individuals may even forget the way to their own home. AD patients often exhibit emotional features such as frustration, irritability and hostility. Genetic factors account for seventy percent of Alzheimer's cases, while environmental factors contribute to twenty-one percent. Late-onset Alzheimer's, which occurs after the age of 60, is the most common form of the disease. Currently, the most widely accepted treatment strategy for Alzheimer's involves the use of cholinesterase inhibitors, which work by inhibiting the activity of the enzyme Acetylcholinesterase (AChE) and increasing levels of acetylcholine in the brain. Examples of these inhibitors include rivastigmine, donepezil, tacrine, galantamine and memantine. However, there is currently no cure for Alzheimer's disease and available medications only help to alleviate symptoms. Herbal medicine offers an alternative approach to managing and slowing down the progression of AD.
Keywords
Protein assembly; Alzheimer; Sinapic acid; Amyloid; Phytoconstituents
Introduction
Proteins within the cell have the ability to self-assemble into more complex structures or assemblies. Many of these proteins contain regions known as Intrinsically Disordered Regions (IDRs). Unlike other protein sequences, IDRs do not adopt a single three-dimensional structure. Instead, they provide proteins with the flexibility to exist in a range of states, from completely unstructured to partially structured [1]. This flexibility allows IDRs to interact with multiple partners and participate in various types of interactions that are crucial for the initiation of protein assembly. These interactions include specific interactions between folded domains and unfolded sequences [2-4], as well as non-specific weak interactions among IDRs [5,6].
A wide range of assembly phenomena can occur depending on the strength and avidity of interactions, as well as other factors such as the physicalchemical state of the cellular environment at one end of the spectrum, proteins can form highly dynamic and metastable assemblies with liquid-like properties [7-9]. On the other end, these initial interactions can lead to more ordered interactions, resulting in stable higher order aggregates like amyloid fibers. In the following sections, we will provide a concise overview of these distinct classes of supramolecular assemblies, highlighting their key properties and notable examples. Within the cell, protein components have the ability to self-assemble into higher-order complexes or assemblies. Many of these proteins share a common feature, which is the presence of Intrinsically Disordered Regions (IDRs). IDRs are protein sequences that lack a single three-dimensional structure, instead providing flexibility for proteins to adopt a range of states, from unstructured to partially structure.
IDRs offer a great deal of flexibility, allowing proteins to interact with multiple partners and engage in various types of interactions that facilitate the initiation of protein assembly. These interactions can include specific interactions between folded domains and unfolded sequences, as well as non-specific weak interactions among IDRs. The strength and avidity of these interactions, along with other factors such as the cellular environment, can lead to a wide range of assembly phenomena. At one end of the spectrum, proteins can form highly dynamic, metastable assemblies with liquid-like properties. At the other end, these initial interactions can result in more ordered interactions that give rise to stable higher-order aggregates, such as amyloid fibers. In the following sections, we will provide a brief overview of these different classes of supramolecular assemblies, highlighting their key properties and providing examples, including amyloid fibers. Amyloids have been associated with various human diseases, including diabetes and systemic amyloidosis [10,11]. Notably, amyloid deposits are characteristic features of neurodegenerative disorders and their formation has been linked to the development of diseases such as Alzheimer's disease (AD) and Parkinson's Disease (PD) [12].
Literature Review
Amyloids
Amyloid fibrils possess remarkable strength and exhibit a high degree of organization, making them capable of forming from various proteins and peptides. Their stability and insolubility render them valuable in both natural occurrences and bio nanotechnology applications. However, these characteristics also contribute to their destructive nature and their tendency to accumulate in diseased tissues. While significant progress has been made in comprehending the structure of amyloid-like fibrils, a deeper understanding of the assembly mechanism is crucial in combating amyloid diseases and harnessing the potential advantages of protein/peptide design.
Previous biochemical analysis of amyloid deposits revealed that amyloid is not solely made up of fibrils, but also includes the presence of Serum Amyloid Protein (SAP) and proteoglycans. While SAP is thought to play a scavenging role in stabilizing amyloid formation, the exact contribution of these proteins in the process of amyloid formation and deposition is still not fully understood. In 1988, the Pepys laboratory utilized radiolabeled SAP as an in vivo tracer to track the development of amyloid disorders in patients.
Serpin A3, commonly known as alpha-1-antichymotrypsin, serves as a significant inhibitor of plasma serine proteinase. Its primary function is to inhibit neutrophil cathepsin G, an enzyme crucial for neutrophils in their bacterial killing process. Additionally, this protein acts as a molecular chaperone for Aβ peptides, which are derived from the amyloid precursor protein in the brain. Furthermore, it is a component of the senile plaques, also known as amyloid.
Alzheimer's disease is marked by the presence of amyloid plaques and neurofibrillary tangles, which are significant pathological characteristics. Additionally, neuro inflammation and neuronal degeneration are observed in the pathology of Alzheimer's disease, with proteins associated with inflammatory responses closely associated with the plaques. Among these proteins is the serine protease inhibitor Alpha-1-Antichymotrypsin (ACT). Studies have demonstrated that ACT promotes the polymerization of A beta both in vitro and in vivo. Furthermore, the levels of ACT protein in the plasma and cerebrospinal fluid of Alzheimer's patients have been found to correlate with the progression of dementia.
Causes of Alzheimer
Due to the process of urbanization, changing lifestyles and the aging of populations, stroke rates are increasing on a global scale. Individuals in their mid-forties who maintained healthy and low-risk lifestyles, such as quitting smoking, engaging in regular exercise, consuming alcohol in moderation and maintaining a moderate weight, experienced a reduced occurrence of neurodegenerative diseases. Consequently, the regular exposure to risk factors such as stress, sedentary behavior, poor nutrition and obesity, high levels of cholesterol in the blood, smoking, excessive alcohol consumption and arterial hypertension may be responsible for the relatively high incidence of neurodegenerative diseases [13-16]. A highfat, high-cholesterol diet has been identified as one of the main dietary factors associated with an increased risk of atherosclerosis and cerebrovascular disease [17]. Conversely, unhealthy eating habits can worsen metabolic diseases such as high blood pressure, metabolic syndrome, cardiovascular disease, stroke, insulin resistance and Type 2 Diabetes (T2DM). These conditions are all triggered by systemic and persistent inflammation, also referred to as metabolic arthritis or meta-inflammation [18].
Cytokines, adipokines, sphingolipids and eicosanoids are all believed to play a role in this process by triggering negative regulatory responses in target cells such as macrophages. The hypothalamus, which controls energy balance, is influenced by factors such as high income, food scarcity, dietary preferences and lifestyle choices, all of which can lead to weight gain. At a molecular level, O-GlcNAc-transferase regulates body weight by facilitating the transfer of N-acetyl glucosamine from uridine-diphosphate to serine or threonine residues in nucleocytoplasmic proteins. Inhibition of O-N-acetyl glucosamine transferase, along with a high-fat diet, can result in obesity and insulin resistance. Several dietary and lifestyle factors, including cigarette and coca/coffee consumption, as well as levels of 25(OH) vitamin D, have been strongly associated with Alzheimer's disease (AD). Higher levels of 25(OH) vitamin D, coffee consumption and cigarette smoking have been linked to a decreased risk of AD conversely; an increased risk of dementia and stroke is associated with the higher consumption of artificially sweetened beverages. Sugary beverages or soft drinks with added sugar have also been linked to an increased risk of dementia and stroke [19].
Treatment and cause
These are the five medications that have been approved by the FDA for treating the cognitive symptoms of Alzheimer's disease: Donepezil (Aricept), galantamine (Razadyne), rivastigmine (Exelon), memantine (Namenda) and the combination of donepezil and memantine (Namzaric). Donepezil, galantamine and rivastigmine are classified as cholinesterase inhibitors and are typically prescribed for individuals with mild to moderate Alzheimer's dementia. These drugs are believed to help with cognitive symptoms by preventing the breakdown of acetylcholine, a neurotransmitter in the brain that is important for memory and thinking. However, as the disease progresses and the brain produces less acetylcholine, these drugs may become less effective. Additionally, they can cause side effects such as nausea, vomiting, loss of appetite, weight loss and diarrhea. Memantine, on the other hand, is prescribed for severe Alzheimer's and works by regulating another neurotransmitter called glutamate, which is important for learning and memory. Excessive amounts of glutamate in Alzheimer's brains can lead to cell damage and death. Side effects of memantine include headache, constipation, diarrhea, confusion and dizziness. The combination of memantine and donepezil is used to treat moderate-to-severe Alzheimer's [20].
Most drug treatments currently under development are focused on addressing the pathology of Alzheimer's disease, which is the most prevalent form of dementia, accounting for approximately 60% to 70% of all cases. However, there are two significant challenges in finding an effective treatment for Alzheimer's. The first challenge is our limited understanding of the underlying biology of the disease. For instance, we are still uncertain about the precise mechanisms that regulate the accumulation of amyloid-β plaques and tau tangles in the brain, which are known to be toxic and are found in Alzheimer's patients. Furthermore, we are unsure about the specific types of these plaques and tangles that are toxic, as well as the reasons behind the varying progression rates of the disease among individuals. Complicating matters further, the symptoms of Alzheimer's develop gradually and slowly, often leading to a delayed diagnosis that occurs years after the brain has already undergone neurodegenerative changes. Additionally, it is not uncommon for Alzheimer's to coexist with other forms of dementia. Recognizing the signs of lewy body dementia may aid in expediting the diagnosis process.
The second major hurdle in finding a treatment lies in the fact that drugs must be able to penetrate the blood-brain barrier. This barrier serves as a defense mechanism against disease-causing pathogens and toxins that may be present in the bloodstream. Its purpose is to prevent foreign substances from entering the brain. However, this also means that the majority of potential drug treatments are unable to reach the brain due to the barrier's restrictive nature [21].
Antioxidants and oxidative stress in AD
It is widely acknowledged that oxidative stress plays a role in neurological disorders and the aging process. Ascorbic acid, by suppressing free radicals and regulating the expression of inflammation-causing genes, possesses neuroprotective properties that can reduce neuroinflammation and the build-up of amyloid-beta peptides. Additionally, the administration of ascorbic acid has been linked to a decrease in low-density lipoprotein composition and an increase in high-density lipoprotein levels in the bloodstream. This suggests that ascorbic acid treatment may also reduce atherosclerosis and the associated systemic inflammation by inhibiting the transformation of macrophages into foam cells. Furthermore, the consumption of ascorbic acid has been shown to decrease miR155 levels by 90%, indicating its ability to control inflammation by regulating miRNA levels. In an animal model of Alzheimer's disease (APPswe/PS1dE9 double transgenic mice), curcumin's antioxidant effects on synapseassociated proteins were observed. The transgenic mice exhibited reduced activity of PSD95 and Shank1 in the CA1 region of the hippocampus. By modulating PSD95 and Shank1 proteins, the consumption of curcumin may enhance synaptic structure and function. It is widely acknowledged that oxidative stress plays a role in neurological disorders and the aging process. Ascorbic acid, by suppressing free radicals and regulating the expression of inflammation causing genes, possesses neuroprotective properties that can reduce neuroinflammation and the buildup of amyloid-beta peptides. Additionally, the administration of ascorbic acid has been linked to a decrease in low-density lipoprotein composition and an increase in high-density lipoprotein levels in the bloodstream. This suggests that ascorbic acid treatment may also reduce atherosclerosis and the associated systemic inflammation by inhibiting the transformation of macrophages into foam cells. Furthermore, the consumption of ascorbic acid has been shown to decrease miR155 levels by 90%, indicating its ability to control inflammation by regulating miRNA levels. In an animal model of Alzheimer's disease (APPswe/PS1dE9 double transgenic mice), curcumin's antioxidant effects on synapse associated proteins were observed. The transgenic mice exhibited reduced activity of PSD95 and Shank1 in the CA1 region of the hippocampus. By modulating PSD95 and Shank1 proteins, the consumption of curcumin may enhance synaptic structure and function.
Phytochemical as alternative approach
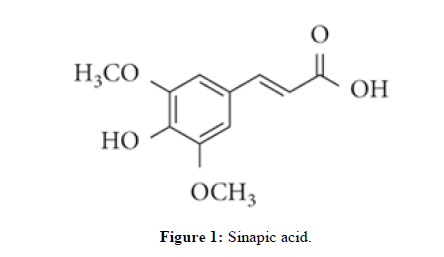
Sinapic acid, a phytochemical present in a variety of edible plants including spices, citrus and berry fruits, vegetables, cereals and oilseed crops, has been extensively studied for its potential benefits against various pathological conditions. These conditions include infections, oxidative stress inflammation, cancer, diabetes, neurodegeneration and anxiety. Additionally, some derivatives of sinapic acid, such as sinapine, 4-vinylsyringol and syringaldehyde, have been investigated for their acetylcholinesterase inhibition, antimutagenicity and antioxidant activity, respectively. 4- Vinylsyringol, also known as canolol, is a decarboxylated form of sinapic acid and was named as such due to its source, canola oil. The structural formulas of sinapic acid and its derivatives can be seen in Figure 1. Despite the lack of extensive research on the biological features of sinapic acid and its derivatives, this brief review article aims to highlight the existing studies and encourage the scientific community to further explore the biological aspects of these compounds.
Neuroprotective property
There is a limited amount of research available in the literature regarding the neuroprotective role of sinapic acid and its derivatives. One of these derivatives, sinapine, has been studied in vitro and has shown dose-dependent inhibition of Acetylcholine Esterase (AChE) activity. Both sinapine and ACh contain quaternary nitrogen, allowing them to bind competitively and reversibly to a specific region on AChE. Interestingly, sinapine exhibits greater effectiveness in the cerebral homogenate compared to the blood serum of rats, with IC50 values of 3.66 μM and 22.1 μM, respectively.
Chemistry of sinapic acid
Sinapic acid (Figure1) is a derivative of cinnamic acid that possesses two methoxy groups at the 3rd and 5th positions (ortho and para) of the phenyl ring, as well as a hydroxyl group at the 4th position (meta) of the phenyl ring.
The mechanism by which sinapic acid acts against OOH radicals involves the transfer of a hydrogen atom from its phenolic moiety. On the other hand, the mechanism against OH, OCCl3 and OOCCl3 radicals occurs through a single electron transfer. The reported melting point of sinapic acid is 192°C. Various derivatives of sinapic acid have been isolated and identified, including sinapine iodide, sinapine and sinapoyl glucose.
Discussion
There is the lack of extensive research on the biological features of sinapic acid and its derivatives .Since its neuroprotective property was scientifically proved which has dose dependent inhibition of of Acetylcholine Esterase (AChE) activity. Both sinapine and ACh contain quaternary nitrogen, allowing them to bind competitively and reversibly to a specific region on AChE. Since sinapic acid is the predominant phenolic compound present in rice as phenolic acids. There is no medicine has been formulated from sinapic acid which has a good neuroprotective property. The proportion of the sinapic acid and other phenoic compounds is higher in raw rice when compared to boiled rice. The purpose of this paper is to make awareness on the scientific community to bring more biological research particularly in targeting to distruct the beta amyloid plaques and bring more brighter life to the next generation by lifestyle modification like to have exercise, good food habit those contain phenolic compounds particularly to have raw rice. Since rice is the predominant food right from centuries and available abundantly in this planet. It has contain phenolic compounds abundantly particularly sinapic acid and its derivatives which has the inhibition and destructive action against the formation of the beta amyloid plaques and neurofibrillary tangles by inhibiting the self-assembling of proteins.
The presence of phenolic compounds in rice grains has been highlighted in a thorough examination. Phenolic acids and their aldehydes are the most abundant phenolic compounds found in rice grains. Among the predominant phenolic acids in rice are p-coumaric and ferulic acids, which are derived from hydroxycinnamic acid. Additionally, chromogenic, caffeic and sinapic acids are also present as significant phenolic acids in rice.
Conclusion
Since sinapic acid is highly inhibiting the protein assembly and protect the brain from the formation of neurofibrillary tangles. It proved scientifically for its neuroprotective property. But these research has not implemented to target Alzheimer in the form of medicine. To avoid the multiple adverse reaction from the currently prescribed medicine and to protect the society it is the time to bring the more extensive research in sinapic acid an wonderful phytochemical which is easily available. The advance extraction and separation method has to be used to get these sinapic acid and derivatives without changing its originality. Finally it is concluded that in order to avoid the risk factors and associated diseases from the currently prescribed medicine it is better to develop the new formulation from sinapic acid and its derivatives to treat Alzheimer and other neuro generative disorders.
Acknowledgment
My sincere thanks to Dr.Vinod K R, Principal, Sanjo college of pharmaceutical study, Palakad for his support and encouragement to made the study possible.
References
- Tompa P, Schad E, Tantos A, et al. Curr Opin Struct Biol. 2015; 35: p. 49-59.
[Crossref] [Google Scholar] [PubMed]
- Banjade S, Wu Q, Mittal A, et al. Proc Natl Acad Sci. 2015; 112(47): p. 6426-6435.
[Crossref] [Google Scholar] [PubMed]
- Li P, Banjade S, Cheng HC, et al. Nature. 2012; 483(7389): p. 336-340.
[Crossref] [Google Scholar] [PubMed]
- Banjade S, Rosen MK. Elife. 2014; 3: p. 4123-4126.
[Crossref] [Google Scholar] [PubMed]
- Molliex A, Temirov J, Lee J, et al. Cell. 2015; 163(1): p. 123-133.
[Crossref] [Google Scholar] [PubMed]
- Martin EW, Mittag T. Biochemistry. 2018; 57(17): p. 2478-2487.
[Crossref] [Google Scholar] [PubMed]
- Brangwynne CP, Tompa P, Pappu RV. Nat Phy. 2015; 11(11): p. 899-904.
- Lin YH, Forman-Kay JD, Chan HS. Biochemistry. 2018; 57(17): p. 2499-2508.
[Crossref] [Google Scholar] [PubMed]
- Chiesa G, Kiriakov S, Khalil AS. BMC Biol. 2020; 18(1): p.1-8.
[Crossref] [Google Scholar] [PubMed]
- Mukherjee A, Morales-Scheihing D, Butler PC, et al. Trends Neuro Med. 2015; 21(7): p. 439-449.
[Crossref] [Google Scholar] [PubMed]
- Lachmann HJ, Hawkins PN. Curr Opin Pharmacol. 2006; 6(2): p. 214-220.
[Crossref] [Google Scholar] [PubMed]
- Buxbaum JN, Linke RP. J Mol Biol. 2012; 421(2-3): p. 142-159.
[Crossref] [Google Scholar] [PubMed]
- Rambaran RN, Serpell LC. Prion. 2008; 2(3): p. 112-117.
[Crossref] [Google Scholar] [PubMed]
- Padmanabhan J, Levy M, Dickson DW, et al. Brain. 2006; 129(11): p. 3020-3034.
[Crossref] [Google Scholar] [PubMed]
- Donnan GA, Baron JC, Ma H, et al. Lancet Neurol. 2009; 8(3): p. 261-269.
- Hu FB, Willett WC. Jama. 2002; 288(20): p. 2569-2578.
[Crossref] [Google Scholar] [PubMed]
- Olefsky JM, Glass CK. Annu Rev Physiol. 2010; 72: p. 219-246.
[Crossref] [Google Scholar] [PubMed]
- Khairnar SJ, Jadhav GB. Biosci Biotechnol Res Asia. 2023; 20(3): p. 793-816.
- Chen C. Oxidative Med Cell Longev. 2016; 20(6): P.235-240.
[Crossref] [Google Scholar] [PubMed]
- He L, Li HT, Guo SW, et al. Chin Med J. 2008; 33(7): p. 813-815.
[Google Scholar] [PubMed]
- Ferreres F, Fernandes F, Sousa C, et al. J Agric Food Chem. 2009; 57(19): p. 8884-8892.
[Crossref] [Google Scholar] [PubMed]