Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 8
Reactions of Triacetonamine Part II, Synthesis of Novel Pyrazolo(4,3-c)pyridine Derivatives, and 2-(Piperidin-4-ylidene)hydrazinecarbothioamide Derivatives Derived From 2,2,6,6-Tetramethyl-piperidin-4-one for In vitro Anticancer Evaluation
Mahmoud NM Yousif1*, Ahmed A Fayed1,2 and Nabil M Yousif1
1Department of Photochemistry, Chemical Industries Research Division, National Research Centre, Dokki, 12622, Giza, Egypt
2Department of Respiratory Therapy, Faculty of Medical Rehabilitation Sciences, Taibah University, Madinah Munawara, 22624, Saudi Arabia
- *Corresponding Author:
- Mahmoud NM Yousif
Department of Photochemistry
Chemical Industries Research Division
National Research Centre
Dokki, 12622, Giza, Egypt
Abstract
2,2,6,6-Tetramethylpiperidin-4-one reacts with 2 moles of aromatic aldehydes and thiosemicarbazide or semicarbazide hydrochloride in different conditions to afford piperidine derivatives 1a-f and pyrazolo(4,3-c)pyridine derivatives 2a-f. Pyrazolo[4,3-c]pyridine-2-carbothioamide derivative 2b reacts with benzophenone, 4-cholorobenzophenone, and 4-bromobenzophenone to give 5-(pyrazolo[4,3-c]pyridine-2-yl) thiazole derivatives 3a-c. Piperidine derivative 1b reacts with acetic anhydride to afford compounds 4a-b. Pyrazolo(4,3-c)pyridine derivative 2c reacts with acetic anhydride to afford compounds 5a-b. Hydrazinecarbothioamide derivative 1a reacts with benzoin and benzoin oxime to give compounds 6, and 7 respectively. Anticancer evaluation of some of synthesized compounds is reported.
Keywords
2,2,6,6-Tetramethylpiperidin-4-one, Pyrazolo(4,3-c)pyridines, 5-pyrazolo[4,3-c]pyridin-2-yl)thiazoles, Anticancer evaluation.
Introduction
Sterically hindered amines such as 2,2,6,6-tetramethyl-piperidines are potent ganglionic blocking agent used as antihypertensive [1]. Also, these amines are used for spin labeling methods [2,3] and for industrial use in a variety of gas treating processes [4,5]. 3,5-Bis(benzylidene)-4-piperidone derivatives were reported to show cytotoxic activity against leukemia cell lines and colon cancer [6-8]. Our previous work on 2,2,6,6tetramethylpiperidine derivatives reveal that 3,5-bis(4-(dimethylamino)benzylidene)-2,2,6,6-tetramethylpiperidin-4-one, and 8-(4-chlorobenzylidene)-4-(4-chlorophenyl)-5,5,7,7-tetramethyl-3,4,5,6,7,8-hexahydropyrido[4,3-d]pyrimidine-2(1H)-thione show more anticancer effect against breast cancer cell lines than standard reference (part I) [9].. These results directed us to synthesize novel pyrazolopyridines for anticancer evaluation.
Materials and Methods
Experimental
All melting points are uncorrected and measured using Electro-thermal IA 9100 apparatus (Shimadzu, Tokyo, Japan). Infrared (IR) spectra were recorded as potassium bromide pellets on a Perkin-Elmer 1650 spectrophotometer (Perkin-Elmer, Norwalk, CT, USA). Proton Nuclear Magnetic Resonance (1H-NMR) was determined on a Jeol-Ex-400 NMR spectrometer (Jeol, Tokyo, Japan) and chemical shifts were expressed as part per million; ppm (δ values) against TMS as internal standard. Mass spectra were recorded on VG 2AM-3F mass spectrometer (Thermo electron corporation, USA). Microanalyses were operated using Mario El Mentar apparatus and satisfactory results for C, H, and N were within the accepted range (± 0.30) of the calculated values. Follow up the reactions and checking the purity of the compounds was made by Thin Layer Chromatography (TLC) on silica gel-protected aluminium sheets (Type 60 F254, Merck). All used chemicals were of reagent grade and were used as supplied directly unless otherwise stated. Anticancer activity was performed by Dr. Mamdouh M. Ali, Biochemistry Department, Genetic Engineering and biotechnology Division, National Research Centre, Dokki, Giza, Egypt.
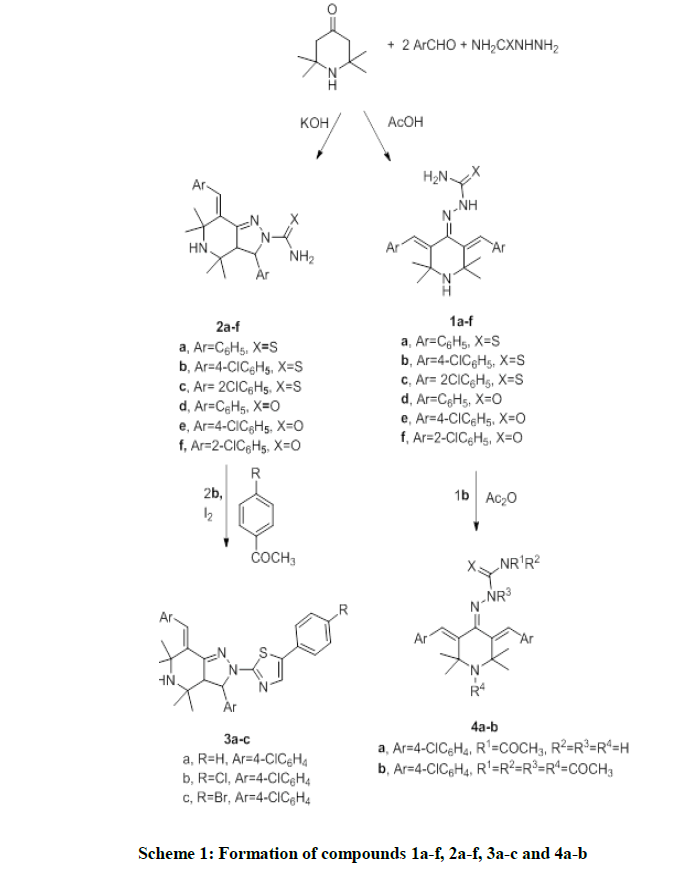
General method for preparation of compounds 1a-f
A mixture of 2,2,6,6-tetramethyl-4-piperidone (0.01 mol), aromatic aldehyde (0.02 mol), semicarbazide hydrochloride or thiosemicarbazide (0.01 mol), 20 ml acetic acid and 1 g. sodium acetate are refluxed. Then, the mixture is concentrated, poured into water, filtered, dried and crystallized from proper solvent.
2-(3,5-Dibenzylidene-2,2,6,6-tetramethyl-piperidin-4-ylidene)hydrazinecarbothioamide 1a: Yield 91%, M. P. 140°C-142°C, reaction time; 2 h, solvent of crystallization; ethanol/ water, yellow needles. IR for compound 1a (KBr) υ, cm-1: 3367 (br, NH), 3263 (br, NH2).
2-(3,5-Bis(4-chlorobenzylidene)-2,2,6,6-tetramethyl-piperidin-4-ylidene)hydrazine-carbothioamide 1b: Yield 94%, M. P. 220°C-222°C, reaction time; 2 h, solvent of crystallization; ethanol/ water, yellow needles; IR for compound 1b (KBr) υ, cm-1: 3458 (br, NH), 3168 (br, NH2).
2-(3,5-Bis(2-chlorobenzylidene)-2,2,6,6-tetramethylpiperidin-4-ylidene)hydrazinecarbothioamide 1c: Yield 81%, M. P. 138°C-140°C, reaction time; 3 h, solvent of crystallization; ethanol/ water, yellow powder; IR for compound 1c (KBr) υ, cm-1: 3481 (br, NH), 3203 (br, NH2); 1H-NMR for compound 1c (400 MHz, CDCl3): δ H 1.24 (s, 6H, 2CH3), 1.70 (s, 6H, 2CH3), 6.63 (s, 2H, C=CH), 7.20-7.38 (m, 4H, Ar), 7.85-7.96 (m, 4H, Ar), 8.46 (brs, 2H, 2NH), 9.50 (brs, 2H, NH2). MS, m/z (%) (473, 80%, M+), (475, 60%, M+2), (477, 20%, M+4).
2-(3,5-Dibenzylidene-2,2,6,6-tetra-methyl-piperidin-4-ylidene)hydrazine-carboxamide 1d: Yield 95%, M. P. 235°C-237°C, reaction time; 3 h, solvent of crystallization; ethanol, yellow powder; IR for compound 1d (KBr) υ, cm-1: 3478 (br, NH), 3248 (br, NH2).
2-(3,5-Bis(4-chlorobenzylidene)-2,2,6,6-tetramethylpiperidin-4-ylidene)hydrazinecarboxamide 1e: Yield 81%, M. P. 138°C-140°C, reaction time; 3 h, solvent of crystallization; ethanol/ water, yellow powder; IR for compound 1e (KBr) υ, cm-1: 3781 (br, NH), 3103 (br, NH2).
2-(3,5-Bis(2-chlorobenzylidene)-2,2,6,6-tetramethylpiperidin-4-ylidene)hydrazinecarboxamide 1f: Yield 90%, M. P. 255°C-257°C, reaction time; 3.5 h, solvent of crystallization; ethanol, yellow powder; IR for compound 1f (KBr) υ, cm-1: 3477 (br, NH), 3140 (br, NH2).
General method for preparation of compounds 2a-f
A mixture of 2,2,6,6-tetramethyl-4-piperidone (0.01 mol), aromatic aldehyde ( 0.02 mol), semicarbazide hydrochloride or thiosemicarbazide (0.01 mol) in 2 g. potassium hydroxide in 50 ml ethanol is refluxed under TLC control. The reaction mixture poured into cold dilute HCL and the precipitate is filtered, dried, and crystallized from proper solvent.
7-Benzylidene-4,4,6,6-tetramethyl-3-phenyl-3,3a,4,5,6,7-hexahydro-2H-pyrazolo[4,3-c]pyridine-2-carbothioamide 2a: Yield 87%, M. P. 171°C-173°C, solvent of crystallization, ethanol, brown powder; IR for compound 2a (KBr) υ, cm-1: 3478 (br, NH), 3248 (br, NH2).
7-(4-Chlorobenzylidene)-3-(4-chlorophenyl)-4,4,6,6-tetramethyl-3,3a,4,5,6,7-hexahydro-2H-pyrazolo[4,3-c]pyridine-2-carbothioamide 2b: Yield 78 %, M. P. 180°C-182°C, solvent of crystallization, ethanol, yellow powder; IR for compound 2b (KBr) υ, cm-1: 3483 (br, NH), 3238 (br, NH2).
7-(2-Chlorobenzylidene)-3-(2-chlorophenyl)-4,4,6,6-tetramethyl-3,3a,4,5,6,7-hexahydro-2H-pyrazolo[4,3-c]pyridine-2-carbothioamide 2c: Yield 61%, M. P. 150°C-152°C, solvent of crystallization, ethanol/dioxane, yellow powder; IR for compound 2c (KBr) υ, cm-1: 3430 (br, NH), 3148 (br, NH2), 1650 (C=O); 1H-NMR for compound 2c (400 MHz, CDCl3): δ H 1.24 (s, 6H, 2CH3), 1.70 (s, 6H, 2CH3), 1.93 (d, 1H, CH), 5.34 (d, 1H, CHN), 6.63 (s, 1H, C=CH), 7.20-7.38 (m, 8H, Ar), 10.05 (brs, 1H, NH), 14.50 (brs, 2H, NH2).MS, m/z (%) (473, 80%, M+), (475, 60%, M+2), (477, 20%, M+4).
7-Benzylidene-4,4,6,6-tetramethyl-3-phenyl-3,3a,4,5,6,7-hexahydro-2H-pyrazolo[4,3-c]pyridine-2-carboxamide 2d: Yield 36%, M. P. 110°C-112°C, solvent of crystallization, dilute ethanol, dark green powder; IR for compound 2d (KBr) υ, cm-1: 3430 (br, NH), 3148 (br, NH2), 1650 (C=O).
7-(4-Chlorobenzylidene)-3-(4-chlorophenyl)-4,4,6,6-tetramethyl-3,3a,4,5,6,7-hexahydro-2H-pyrazolo[4,3-c]pyridine-2-carboxamide 2e: Yield 39%, M. P. 126-128°C, solvent of crystallization, dilute ethanol, dark green powder; IR for compound 2e (KBr) υ, cm-1: 3430 (br, NH), 3148 (br, NH2), 1650 (C=O).
7-(4-Chlorobenzylidene)-3-(4-chlorophenyl)-4,4,6,6-tetramethyl-3,3a,4,5,6,7-hexahydro-2H-pyrazolo[4,3-c]pyridine-2-carboxamide 2f: Yield 36%, M. P. 240°C-242°C, solvent of crystallization, ethanol, pale yellow powder; IR for compound 2f (KBr) υ, cm-1: 3520 (br, NH), 3170 (br, NH2), 1650 (C=O).
General procedure for preparation of compounds 3a-c
A mixture of compound 2b (0.05 mol) is heated under reflux with acetophenone derivatives (0.05), and 1 g iodine in 10 ml absolute ethanol for 7 h. The reaction mixture is concentrated, poured into water and crystallized from proper solvent.
2-(7-(4-Chlorobenzylidene)-3-(4-chlorophenyl)-4,4,6,6-tetramethyl-3,3a,4,5,6,7-hexahydro-2H-pyrazolo[4,3-c]pyridin-2-yl)-5-phenylthiazole 3a: Yield 94%, M. P. 190°C-192°C, solvent of crystallization, ethanol, brown powder; IR for compound 3a (KBr) υ, cm-1: 1710 (C=O).
2-(7-(4-Chlorobenzylidene)-3-(4-chlorophenyl)-4,4,6,6-tetramethyl-3,3a,4,5,6,7-hexahydro-2H-pyrazolo[4,3-c]pyridin-2-yl)-5(4-chlorophenyl)-thiazole 3b: Yield 61%, M. P. 150-152°C, solvent of crystallization, ethanol/dioxane, yellow powder; IR for compound 3b (KBr) υ, cm-1: 1725 (C=O); 1H-NMR for compound 3b (400 MHz, CDCl3): δ H 1.84 (s, 6H, 2CH3), 2.09 (s, 6H, 2CH3), 2.46 (d, 1H, CHCHN), 3.48 (d, 1H, CHN), 6.84 (s, 1H, =CH), 7.03-7.28 (m, 6H, Ar), 7.31-7.33 (m, 6H, Ar), 8.23 (brs, 1H, NH). MS, m/z (%) (608, 61%, M+), (610, 27 %, M+2), (612, 1.5 %, M+4).
5-(4-Bromophenyl)-2-(7-(4-chlorobenzylidene)-3-(4-chlorophenyl)-4,4,6,6-tetramethyl-3,3a,4,5,6,7-hexahydro-2H-pyrazolo[4,3-c]pyridin-2-yl)thiazole 3c: Yield 83%, M. P. 230°C-232°C, solvent of crystallization, ethanol, brown powder; IR for compound 3c (KBr) υ, cm-1: 1715 (C=O).
General procedure for the preparation of compounds 4a-b
Compound 1b (0.1 mol) is refluxed with 20 ml acetic anhydride for 5 h. Then, the reaction mixture is concentrated, poured into water. The filtrate was concentrated, filtered, and crystallized to give compounds 4a-b.
N-(2-(3,5-Bis(4-chlorobenzylidene)-2,2,6,6-tetramethyl-piperidin-4-ylidene)hydrazine-carbonothioyl)acetamide 4a: Yield 13%, M. P. 200°C-202°C, solvent of crystallization, ethanol, pale brown powder; IR for compound 4a (KBr) υ, cm-1: 3478 (br, NH), 1648 (C=O); 1H-NMR for compound 4a (400 MHz, CDCl3): δH 1.84 (s, 6H, 2CH3), 2.09 (s, 6H, 2CH3), 2.19 (s, 3H, CH3CO), 4.21 (brs, 1H, NH), 6.84 (s, 2H, =CH), 7.24 (d, 4H, Ar), 7.31 (d, 4H, Ar), 7.89 (brs, 2H, 2NH). MS, m/z (%) (515, 45 %, M+), (517, 14 %, M+2), (519, 0.5%, M+4).
N-Acetyl-N-(1-acetyl-2-(1-acetyl-3,5-bis(4-chlorobenzylidene)-2,2,6,6-tetramethyl-piperidin-4-ylidene)-hydrazine-carbonothioyl)-acetamide 4b: Yield 72%, M. P. 250°C-252°C, solvent of crystallization, ethanol, brown powder; IR for compound 4b (KBr) υ, cm-1: 1641 (C=O); 1H-NMR for compound 4b (400 MHz, CDCl3): δ H 1.84 (s, 6H, 2CH3), 2.09 (s, 6H, 2CH3), 2.39 (s, 12H, 4CH3CO), 6.84 (s, 2H, =CH), 7.03 (d, 4H, Ar), 7.26 (d, 4H, Ar). MS, m/z (%) (641, 56%, M+), (643, 21 %, M+2), (645, 0.5%, M+4).
Preperation of compounds 5a-b
Compound 2c (0.01 mol) is refluxed with 20 ml acetic anhydride for 5 h. Then, the reaction mixture is concentrated, poured into water. The filtrate was concentrated, filtered, and crystallized to give compound 5a,b.
N-(7-(2-Chlorobenzylidene)-3-(2-chlorophenyl)-4,4,6,6-tetramethyl-3,3a,4,5,6,7-hexahydro-2H-pyrazolo[4,3-c]pyridine-2-carbonothioyl)acetamide 5a: Yield 53%, M. P. 161°C-163°C, solvent of crystallization, ethanol/dioxane, yellow powder; IR for compound 5a (KBr) υ, cm-1: 3444 (br, NH), 1705 (C=O); 1H-NMR for compound 5a (400 MHz, CDCl3): δH 1.98 (s, 6H, 2CH3), 2.24 (s, 6H, 2CH3), 2.46 (d, 2H, CHCHN), 2.46 (s, 3H, CH3CO), 6.84 (s, 1H, =CH), 7.04 (d, 1H, Ar), 7.33-7.49 (m, 6H, Ar), 7.73 (d, 1H, Ar), 11.82 (brs, 2H, NH, D2O exchangeable). MS, m/z (%) (515, 3.13%, M+), (517, 1.5 %, M+2), (519, 0.5%, M+4).
N-Acetyl-N-(5-acetyl-7-(2-chloro-benzylidene)-3-(2-chlorophenyl)-4,4,6,6-tetramethyl-3,3a,4,5,6,7-hexahydro-2H-pyrazolo[4,3-c]pyridine-2-carbonothioyl)acetamide 5b: Yield 61%, M. P. 150°C-152°C, solvent of crystallization, ethanol/dioxane, and yellow powder; IR for compound 5b (KBr) υ, cm-1: 1705 (C=O); 1H-NMR for compound 5b (400 MHz, CDCl3): δH 1.84 (s, 6H, 2CH3), 2.09 (s, 6H, 2CH3), 2.46 (d, 2H, CHCHN), 3.19 (s, 9H, 3CH3CO), 6.84 (s, 1H, =CH), 7.03 (d, 1H, Ar), 7.31-7.33 (m, 6H, Ar), 7.49 (d, 1H, Ar). MS, m/z (%) (599, 61%, M+), (601, 28 %, M+2), (603, 0.5%, M+4).
Preparation of 2-(3,5-dibenzylidene-2,2,6,6-tetramethyl-piperidin-4-ylidene)-N-(2-hydroxy-1,2-diphenyl-ethylidene)-hydrazine-carbothioamide 6: A mixture of compound 1a (0.05 mol), benzoin (0.05 mol), and glacial acetic acid (20 ml) are heated under reflux for 6 h. The reaction mixture is concentrated and poured into water. The precipitate formed is filtered and crystallized from dilute ethanol to give white crystals
Preparation of 2-(3,5-dibenzylidene-2,2,6,6-tetramethyl-piperidin-4-ylidene)-N-(2-(hydroxyl-imino)-1,2-diphenyl-ethyl)hydrazinecarbothioamide 7: A mixture of compound 1a (0.05 mol), benzoin oxime (0.05 mol), and glacial acetic acid (2 ml) are heated under reflux in absolute ethanol for 7 hours. The reaction mixture is concentrated and poured into water. The precipitate formed is filtered and crystallized from mixture of methanol/water to give round yellow powder (M. P. 80°C-82°C); IR for compound 7 (KBr) υ, cm-1: 3390 (NH); 1H-NMR for compound 7 (400 MHz, CDCl3): δ H 1.84 (s, 6H, 2CH3), 2.09 (s, 6H, 2CH3), 4.50 (s, 1H, CHN), 6.54 (s, 2H, =CH), 7.36-7.49 (m, 12 H, Ar), 7.67-7.89 (m, 8H, Ar), 8.05 (brs, 3H, 3NH), 9.50 (brs, 1H, OH). MS, m/z (%) (613.8, 21.72 %, M+).
Results and Discussion
Chemistry
2,2,6,6-Tetramethyl-piperidin-4-one reacts with 2 moles of different aromatic aldehydes, and thiosemicarbazide or semicarbazide hydrochloride in acetic acid under analogous reaction conditions [10] to afford compounds 1a-f. The IR spectra of compounds 1a-f show disappearance of absorption band for carbonyl group.
2,2,6,6-Tetramethyl-piperidin-4-one reacts with 2 moles of different aromatic aldehydes and thiosemicarbazide or semicarbazide hydrochloride to afford pyrazolo[4,3-c]pyridine derivatives 2a-f under analogous reaction conditions [11]. The structures of compounds 2a-f are elucidated from 1H-NMR, IR, and mass spectral data. The products 2a-f gives absorption bands for NH, and NH2 groups and disappearance of CO group in the IR spectrum. 1H-NMR spectrum for compound 2c shows two doublet signals at δ 1.93 and 5.34 characteristic to two CH groups in the nucleus of pyrazolo[4,3-c]pyridine derivative 2c. Compound 2b reacts with acetophenone, p-chloroacetophenone, and p-bromoacetophenone in iodine and absolute ethanol to afford compounds 3a-c.
The structures of compounds 3a-c are in agreement with mass spectrum, Infrared spectrum and 1H-NMR spectrum of compounds 3a-c. 2-(3,5Bis(4-chlorobenzylidene)-2,2,6,6-tetramethylpiperidin-4-ylidene)hydrazine-carbo-thioamide 1b reacts with acetic anhydride to give N-(2-(3,5bis(4-chlorobenzylidene)-2,2,6,6-tetramethylpiperidin-4-ylidene)hydrazine-carbonothioyl)-acetamide 4a and N-acetyl-N-(1-acetyl-2-(1-acetyl-3,5-bis(4-chlorobenzylidene)-2,2,6,6-tetramethylpiperidin-4-ylidene)-hydrazine-carbonothioyl)acetamide 4b.
The formation of tetraacetylated derivative 4b is a chemical proof for formation of open thiosemicarbazide structure 1b, as the maximum acetylation for the closed thiosemicarbazide products 2a-f are triacetylation (Scheme 1). The IR spectrum of compound 4b shows disappearance of absorption band for NH and NH2.
Compound 2c reacts with acetic anhydride to afford compounds 5a,b. Compound 1a reacts with benzoin in glacial acetic acid to afford compound 6 under analogous reaction condition [12]. Also, compound 1a reacts with benzoin oxime to afford compound 7. The nucleophilic NH2 in compound 1a can attack on two positions in benzoin oxime the electrophilic carbon linked to nitrogen, or electrophilic carbon linked to OH.
It is reported in analogous reaction that the attack of nucleophile will be to carbon linked to OH [13] (Scheme 2). The structures of compounds 6 and 7 are in agreement with mass, IR, 1H-NMR spectra. For example, the mass spectrum of compound 6 gives M+ at m/z 598.7.
Biological evaluation
The anticancer activities of the newly synthesized compounds against 3 different human cancer cell lines including liver cancer HepG2, breast cancer MCF-7 and lung carcinoma A549 will be evaluated (Table 1). The antiproliferative activity was measured in vitro using the Sulfo-Rhodamine-B stain (SRB) assay according to the previous reported standard procedure [14]. The antiproliferative activities were expressed by median growth inhibitory concentration (IC50).
| Compound | IC50 (μg/ml) | ||
|---|---|---|---|
| HepG2 | MCF-7 | A549 | |
| 1a | 22.43 | 37.95 | - |
| 1b | 35.72 | 38.64 | - |
| 1c | - | - | - |
| 2a | 29.39 | 35.75 | - |
| 2b | 44.3 | 15.3 | - |
| 2c | 21.6 | 23 | - |
| 3a | 30.2 | 10.3 | - |
| 3b | 9.7 | 6.1 | - |
| 3c | 14.8 | 19.87 | - |
| 4c | 15.74 | 18.3 | - |
| 5a | 45 | 25 | - |
| 6 | 39.1 | 19.6 | - |
| DMSO | - | - | - |
| Doxorubicin | 3.5 | 2.85 | 5.3 |
Table 1: Anticancer activities of the newly synthesized compounds against 3 different human cancer cell lines
All tested compounds show no activity against lung carcinoma A549 cell lines. Compounds 5a and 2b show highest anticancer activity against hepatocellular carcinoma HepG2 cell lines; Compounds 1b, 1a, and 2a exhibit highest anticancer activity against breast cancer MCF-7 cell lines.
Conclusion
New piperidine derivatives have been synthesized and structurally elucidated. Some of the prepared compounds were screened against reference drug doxorubicin.
References
- F.H. Smirk, I.V. Hodge, J. Chin. Pharm. Therap., 1960, I, 610.
- J.F.W. Keana, Chem. Rev., 1978, 78, 37-64.
- O. Brede, D. Beckert, C. Wmdolph, H.A. Gottinger, Phys. Chem. A., 1998, 102, 1457-1464.
- R. Hook, Ind. Eng. Chem. Res., 36, 1997, 1779-1790.
- A.K. Chakraboty, G. Astarita, K.B. Bischoff, Chem. Eng. Sci., 1986, 41, 997-1003.
- H.N. Pati, U. Das, S. Das, B. Bandy, E. De Clercq, J. Balzarini, M. Kawase, H. Sakagami, J.W. Quail, J.P. Stables, J.R. Dimmock, Eur. J. Med. Chem., 2009, 44(1), 54-62.
- U. Das, J. Alcorn, A. Shrivastav, R.K. Sharma, E. De Clercq, J. Balzarini, J.R. Dimmock, Eur. J. Med. Chem., 2007, 42(1), 71-80.
- U. Das, H.N. Pati, H. Sakagami, K. Hashimoto, M. Kawase, J. Balzarini, E. De Clercq, J.R. Dimmock, J. Med. Chem., 2011, 54 (9), 3445-3449.
- H.A. Soliman, M.N.M. Yousif, M.M. Said, N.A. Hassan, M.M. Ali, H.M. Awad, F.M.E. Abdel-Megeid, Der Pharma Chemica, 2014, 6(3), 394-410.
- H. Zhang, Y. Qian, D. Zhu, X. Yang, Eur. J. Med. Chem., 2011, 46(9), 4702-4708.
- M.N.A. Nasr, S.A. Said, Arch. Pharm., 2003, 336(12), 551-559.
- S.K. Bharti, S.K. Patel, G. Nath, R. Tilak, S.K. Singh, Trans. Met. Chem., 2010, 35(8), 917-925.
- A.R. Sudzhaev, I.A. Rzaeva, R.A. Nadzhafova, Y.S. Safarov, M.A. Allakhverdiev, Russian J. Appl. Chem., 2011, 84(8), 1394–1397.
- P. Skehan, R. Storeng, D. Scudiero, A. Monks, J. Mcmahon, D. Vistica, J.T. Warren, H. Bokesch, S. Kenney, M.R. Boyd, J. Nat. Canc. Inst., 1990, 82(13), 1107-1112.