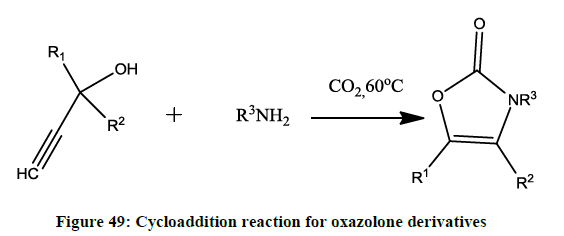Review Article - Der Pharma Chemica ( 2019) Volume 11, Issue 1
Review on Chemistry and Therapeutic Activity of the Derivatives of Furan and Oxazole: The Oxygen Containing Heterocycles
Emdor Mi Rymbai1, Anurag Chakraborty2, Ratna Choudhury3, Neeraj Verma4 and Biplab De1*2Department of Chemistry, Jadavpur University, Kolkata, WB, India
3Rajnagar H. S. School, Agartala, Tripura, India
4Hygia Institute of Pharmaceutical Education and Research, Lucknow, UP, India
Biplab De, Regional Institute of Pharmaceutical Science and Technology, Abhoynagar, Agartala, Tripura, India, Email: principalripsat@gmail.com
Abstract
Heterocyclic compounds occupy a position of importance in organic chemistry. The present compilation is designed for searching of some new chemical moiety of Furan and Oxazole nucleus-two important heterocycles, having potent therapeutic activity along with their chemistry.
Keywords
Heterocyclic, Furan, Oxazole, Synthesis, Chemistry, Therapeutic review
Introduction
The heterocyclic compounds are widely used in biological, industrial and chemical sectors. So heterocyclics occupy a position of importance in organic chemistry. The sulphur, nitrogen, oxygen, phosphorus and selenium containing heterocyclic compounds have great significance in historical development of organic synthesis. There is large number of heterocyclic bioactive compounds available in nature such as papaverine, theobromine, quinine, emetine, theophylline, atropine, procaine, codeine, reserpine and morphine. Synthetic heterocycles also have wide range of medicinal importance as broad spectrum antimicrobial, antileishmanial agents, genotoxic, CNS active agents both centrally and peripherally, fungicides, herbicides, antioxidant agent etc.
The fundamental process of life involving various biochemical reactions like energy generation and utilization, nerve impulses, sights, metabolism and hereditary information are also based on the participation of many heterocyclic compounds in the form of enzymes, coenzymes, nucleic acids, few vitamins, ATP, serotonin, histamine, few amino acids and so on. Heterocycles are also key components of nucleic acid molecules that control the protein synthesis and the sequence of amino acids [1].
Keeping all discussion in consideration, the present study is designed for searching of some new chemical moiety of Furan and Oxazole nucleus of potent therapeutic activity along with their chemistry.
Materials and Methods
Furan
Furfural as a derivative of furan (Figure 1) was isolated in 1832 by the German chemist Johann Wolfgang Dobereiner as a byproduct during formic acid synthesis [2]. In 1901 the German chemist Carl Harries deduced furfural’s structure. Furan can be prepared from tetra-phenol [3]. They are important structural fragment for many active pharmaceutical ingredient and pharmacologically active compounds [4].
Synthesis of furan and its derivatives
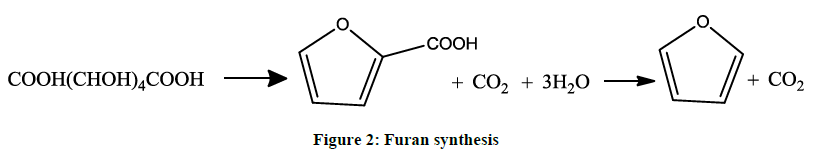
Furan can be obtained from pine-wood [5]. It may be prepared by distillation of mucic acid (Figure 2). Upon heating of mucic acid, the furoic acid is obtained. Furic acid then can be converted to furan by decarboxylation.
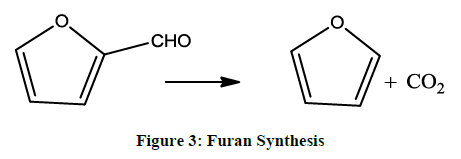
Furan can also be prepared from furfural by oxide catalyst (Figure 3).
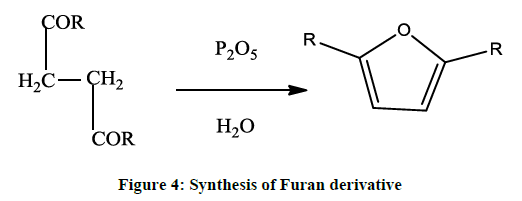
A general method of preparing furan derivative is by dehydrating 1, 4-diketones or dialdehydes with phosphorous pentoxide / sulphuric acid etc., (Figure 4).
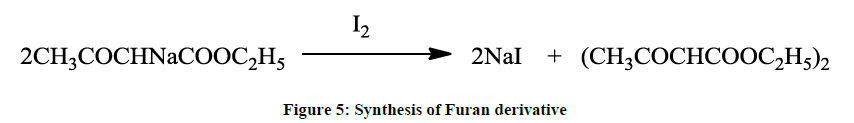
Alternatively, furan derivatives may also be prepared from ethylacetoacetate in presence of iodine (Figure 5).
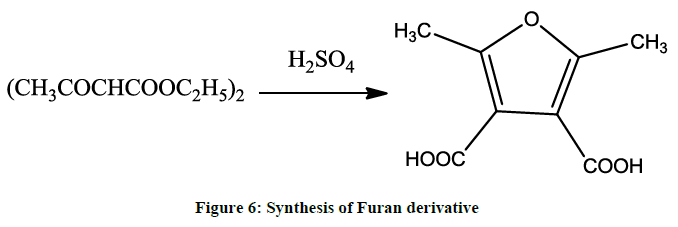
2, 5-Dimethylfuran-3, 4 -dicarboxylicacid is formed on heating of diacetosuccinic ester with dilute sulphuric acid (Figure 6).
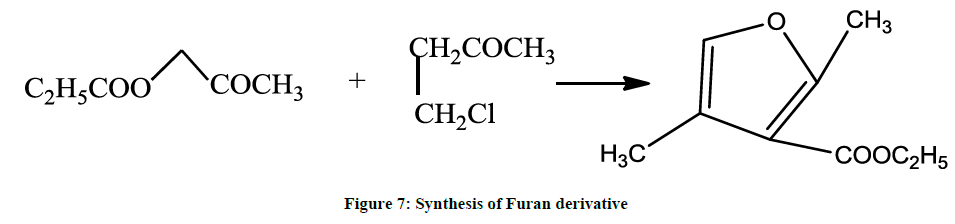
Furan derivatives may also be prepared by the Feist-Benary synthesis (1902, 1911) where α-chloro ketone is condensed with a β-keto ester in the presence of pyridine (Figure 7) [5].
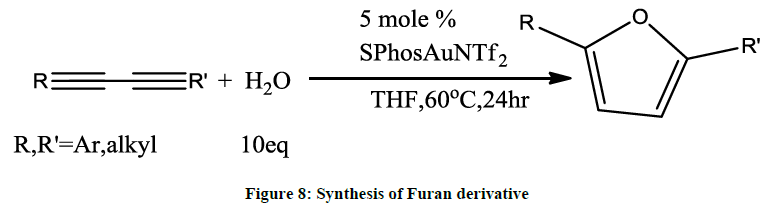
Au (I)- catalysed hydroamination [6] or hydration of 1, 3-diynes allows formation of 2, 5-diamidofurans (Figure 8). This method can also be expanded for 2, 5-disubstituted furans.
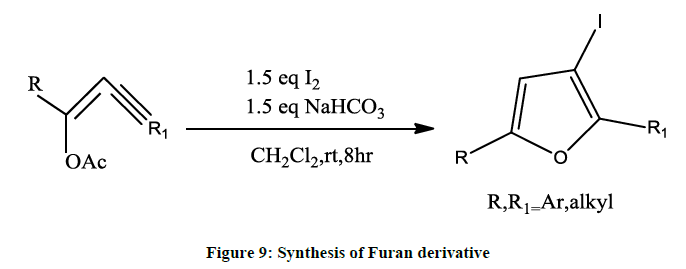
2, 5-disubstituted 3-iodofurans [7] can be prepared in presence of palladium or copper as catalyst. The cross coupling reaction is taken place between beta bromoenol acetate and terminal alkynes followed by iodocyclization (Figure 9).
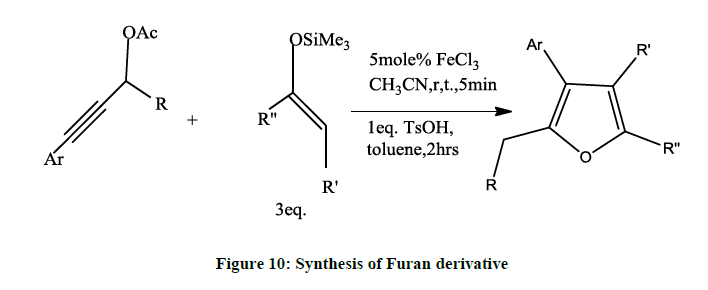
An efficient substitution reaction [8] of propargylic acetates in enoxy-silances under mild conditions in presence of FeCl3 as catalyst affords γ- alkynyl ketone (Figure 10). The intermediate ketone in presence of TsOH as catalyst forms tri or tetra substituted furan.
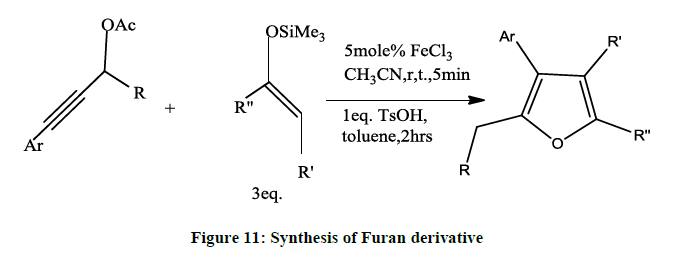
Synthesis of Furan [9] can also be obtained by reaction between propargyl alcohols and terminal alkynes, which yields 1, 4-diynes, poly substituted furan and water as a byproduct (Figure 11).
Chemistry of furan
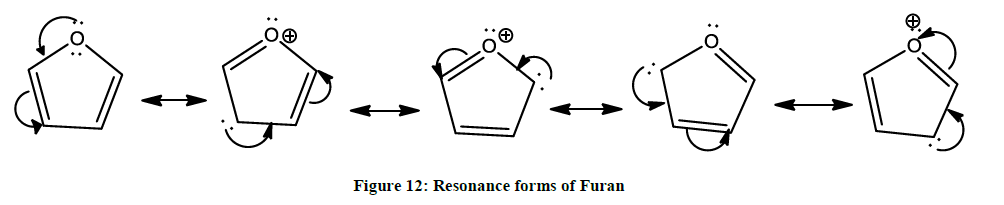
Being a five membered aromatic compound (Figure 12, resonance forms), furan’s behaviour is quite dissimilar to that of the more typical heterocyclic ethers such as tetrahydrofuran.
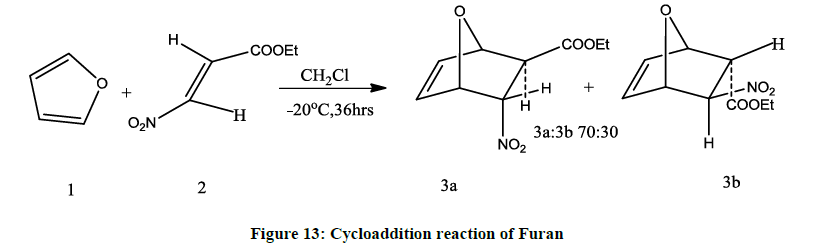
It is considerably more reactive towards electrophilic substitutions [5]. It also undergoes cycloaddition reaction preferably endo isomer [10] (Figure 13).
Therapeutic review of furan derivatives
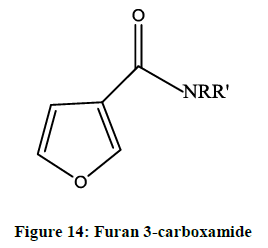
Furan derivatives as antimicrobial agent: NiloZanatta et al. reported [11] the antimicrobial activityof furan 3-carboxamide derivatives (Figure 14) against yeast, filamentous fungi, bacteria and algae. The derivatives were prepared by aromatization and nucleophilic displacement in 4- trichloracetyl-2, 3-dihydrofuran.
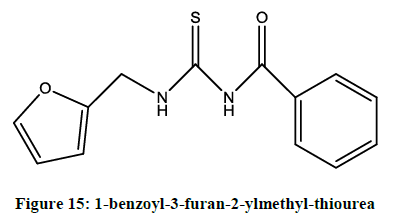
Karipcin et al. reported [12] antimicrobial activity of 1-benzoyl-3-furan-2-ylmethyl-thiourea (Figure 15).
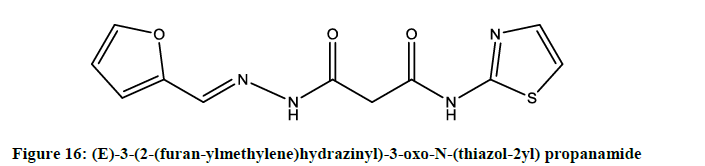
Zaky et al. also reported [13] antimicrobial effect for the (E)-3-(2-(furan-ylmethylene)hydrazinyl)-3-oxo-N-(thiazol-2yl) propanamide (Figure 16).
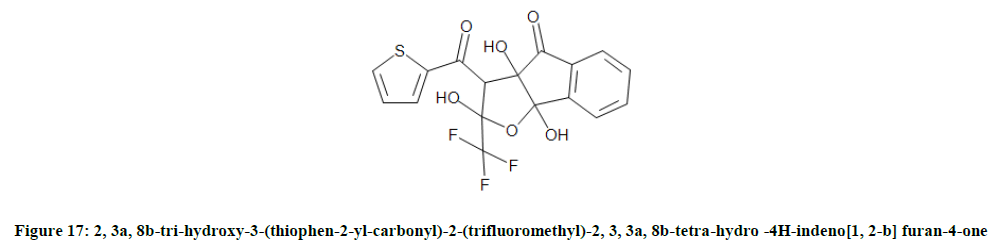
The synthesis of 2, 3a, 8b-tri-hydroxy-3-(thiophen-2-yl-carbonyl)-2-(trifluoromethyl)-2, 3, 3a, 8b-tetra-hydro-4H-indeno[1, 2-b] furan-4-one (Figure 17) [14] was reported by Obafemiet et al. which showed broad spectrum activity against different strains of bacteria of gram positive and negative.
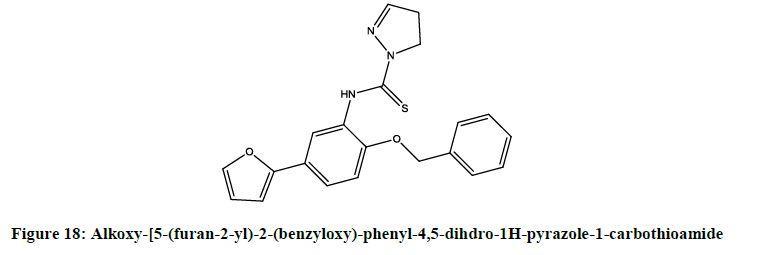
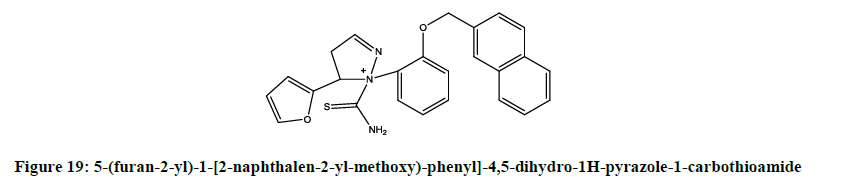
A series of novel [15] analogues of [5-(furan-2-yl)-3-[2-(alkoxy)-phenyl]-4,5-dihydro-1H-pyrazole-1-carbothioamide were synthesized by Rani et al. Alkoxy-[5-(furan-2-yl)-2-(benzyloxy)-phenyl-4,5-dihdro-1H-pyrazole-1-carbothioamide (Figure 18) and 5-(furan-2-yl)-1-[2-naphthalen-2- yl-methoxy)-phenyl]-4,5-dihydro-1H-pyrazole-1-carbothioamide (Figure 19) shown better activity compared to standard against A. hydrophila, Y. enterocolitica, L. monocytogenes and S. aureus.
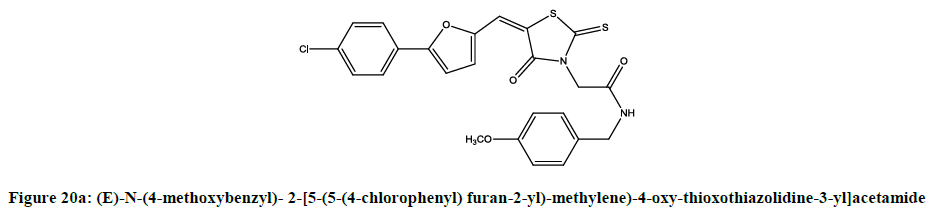
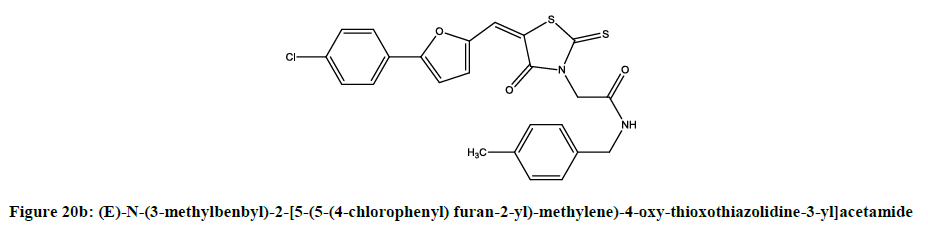
Furan derivatives as anticancer agent: Rangappa reported [16] anticancer activity of 2-[5-(5-(4-chlorophenyl) furan-2-yl)-methylene)-4-oxythioxothiazolidine- 3-yl] acetic acid derivatives (Figures 20a and 20b).
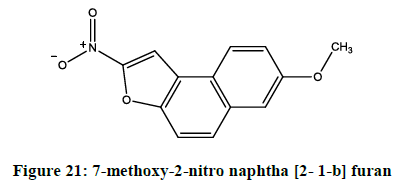
Hufnung et al. reported [17] in vivo mutagenic properties of a 5-nitrofuran. The 7-methoxy-2-nitro naphtha [2- 1-b] furan (Figure 21) was evaluated in transgenic mice (Big Blue).
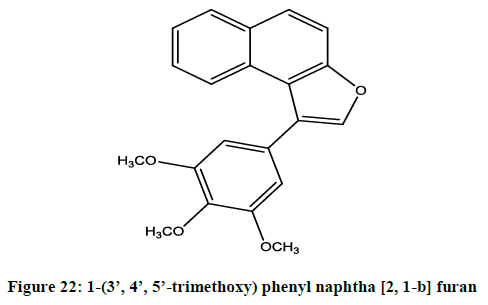
Negi et al. reported [18] anticancer activity of 1-(3’, 4’, 5’-trimethoxy) phenyl naphtha [2, 1-b] furan (Figure 22) by in vitro MTT assay.
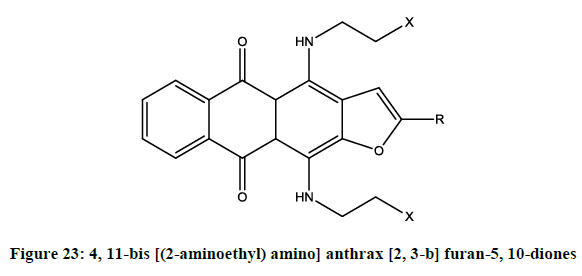
Shchekotikhin, et al. [19] reported cytotoxic activity properties of novel 4, 11-bis [(2-aminoethyl) amino] anthrax [2, 3-b] furan-5, 10-diones (Figure 23). The selected compound potently killed mammalian tumor cell lines, including-resistant-variants.
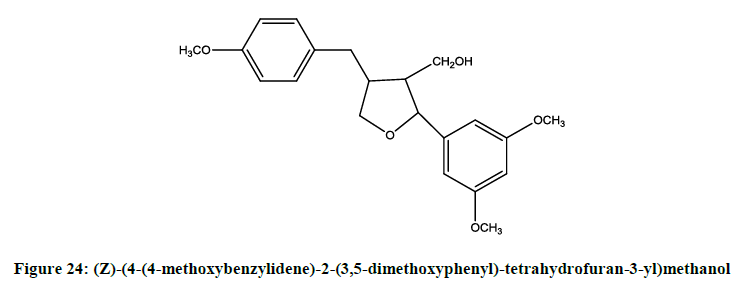
Li et al. [20] reported racemic mixture (Figure 24) of furan lignans. The optical isomers were obtained through a selective hydrolization. The isomers and the racemates were able to shown anticancer activity against QGY-7701 and HeLa cell lines.
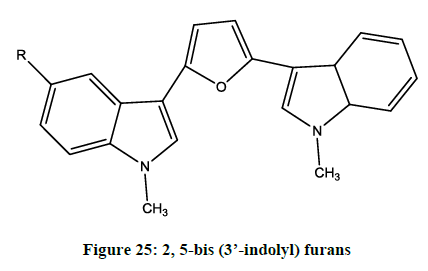
Cirrincione et al. reported [21] 2, 5-bis (3’-indolyl) furans (Figure 25) and 3,5-bis (3’-indolyl) isoxazoles as antitumor agents. The antiproliferative activity was also evaluated in vitro toward diverse human tumor cell lines.
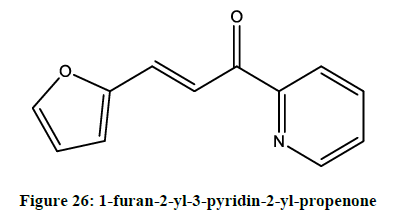
Furan derivatives as analgesic and anti-inflammatory agent: Lee reported [22] 1-furan-2-yl-3-pyridin-2-yl-propenone (Figure 26), as dual inhibitor of COX/5-LOX. The results suggest that FPP-3 may have a benefit in combating inflammation and pain by dual inhibition of COX and LOX.
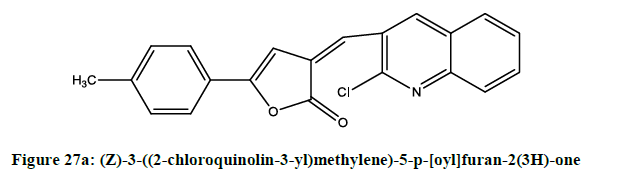
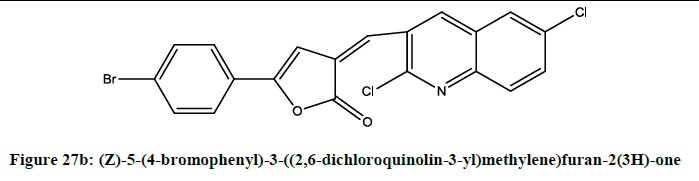
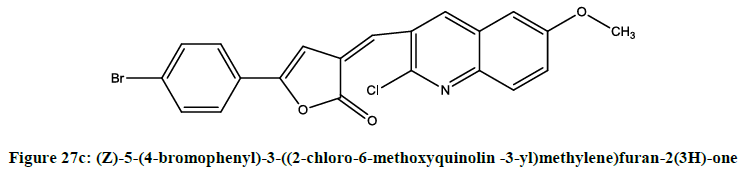
5-aryl-3-[(2-chloroquinolin-3-yl) methylene] furan-2(3H)-ones (Figures 27a-27c) were [23] synthesized by Alam. Compounds (i), (ii) and (iii) were showing potent anti-inflammatory activity.
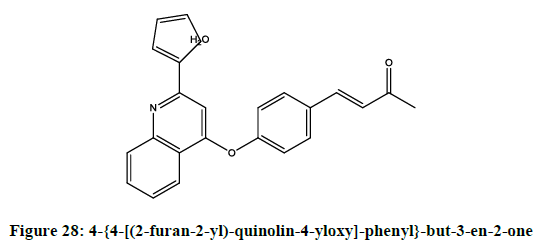
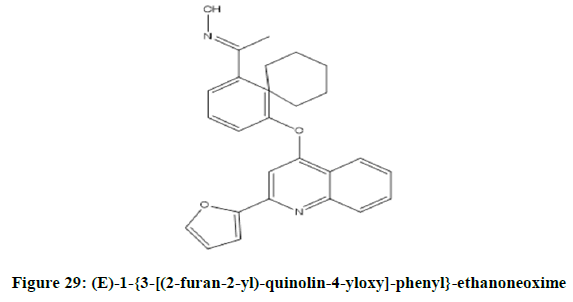
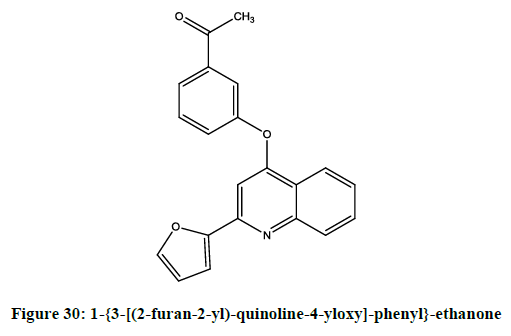
Cherng-ChyiTzeng, et al. reported [24] anti-inflammatory activity of 2-(furan-2-yl)-4-phenoxyquinolines. Among the reported compounds, 4-{4- [(2-furan-2-yl)-quinolin-4-yloxy]-phenyl}-but-3-en-2-one (Figure 28) was most potent compound. While (E)-1-{3-[(2-furan-2-yl)-quinolin-4- yloxy]-phenyl}-ethanoneoxime (Figure 29) and 1-{3-[(2-furan-2-yl)-quinoline-4-yloxy]-phenyl}-ethanone (Figure 30) was moderately potent than genistein.
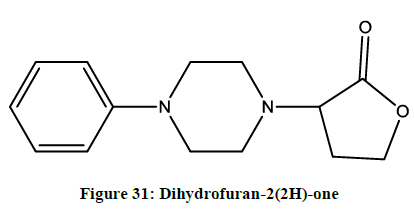
Anticonvulsant, neurotoxic and antinociceptive property for dihydrofuran-2(2H)-one (Figure 31) was reported [25] by Malawska, et al.
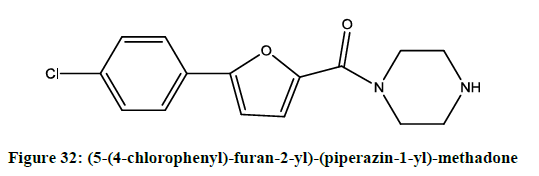
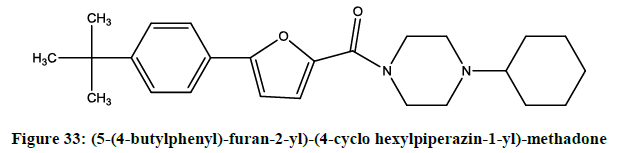
The synthesis and pharmacological evaluation [26] of novel furan derivative acted as voltage-gated sodium channel blockers were reported by Drizin et al. The compounds (5-(4-chlorophenyl)-furan-2-yl)-(piperazin-1-yl)-methadone (Figure 32), (5-(4-butylphenyl)-furan-2-yl)-(4-cyclo hexylpiperazin-1-yl)-methadone (Figure 33) showed more potent activity.
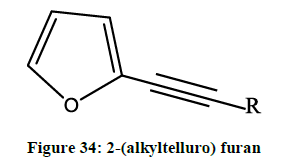
Zeni et al. reported [27] anti-inflammatory activity of acetylenic furan derivatives; which were synthesized via Pd-catalyzed coupling reactions of 2-(alkyltelluro) furan (Figure 34) with several terminal alkynes.
Furan derivatives as CNS active agent
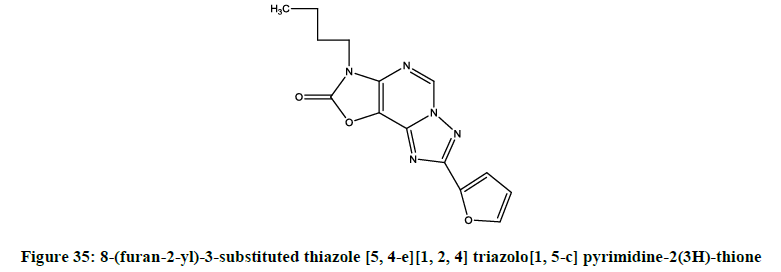
Luthra et al. reported [28] 8-(furan-2-yl)-3-substituted thiazole [5, 4-e][1, 2, 4] triazolo[1, 5-c] pyrimidine-2(3H)-thione (Figure 35) derivatives as potential adenosine A2A receptor antogonists.
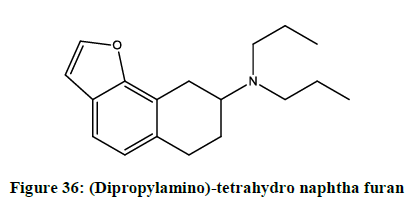
Stjernlof, et al. reported [29] (Dipropylamino)-tetrahydro naphtha furans (Figure 36) as centrally acting serotonin agonists and dopaminergic receptor blocker.
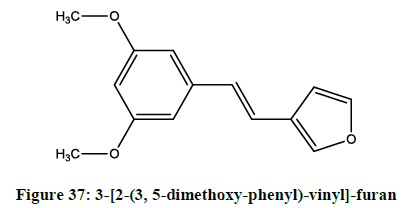
Choi et al. synthesized [30] 3-[2-(3, 5-Dimethoxy-phenyl)-vinyl]-furan (Figure 37) as synthetic resveratrol derivative. In addition, DPVF also inhibited ATP depletion following oxygen and glucose deprivation in the adult hippocampal slice.
Furan derivatives as antioxidant
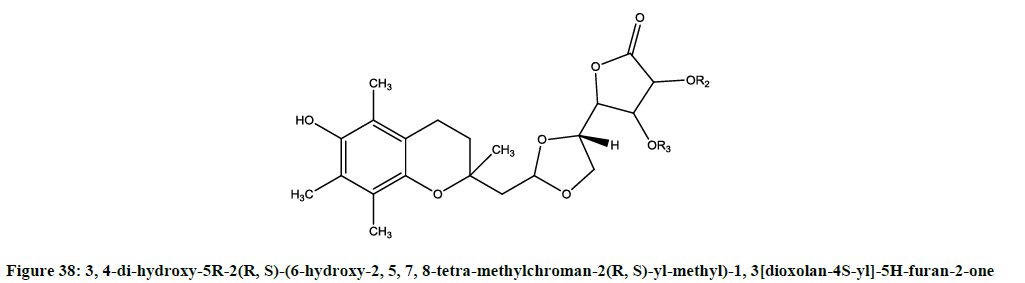
Mafredini et al. tested radical scavenging activities [31] of 2, 3-dihydroxy-2, 3-enono-1, 4-lactones. The 3, 4-di-hydroxy-5R-2(R, S)-(6-hydroxy- 2,5,7,8-tetra-methylchroman-2(R, S)-yl-methyl)-1, 3[dioxolan-4S-yl]-5H-furan-2-one (Figure 38) was able to show potent activity.
Furan dervatives as cytoprotective agent
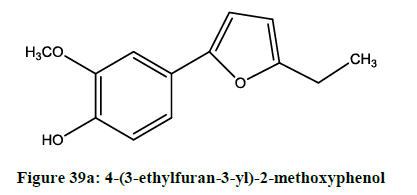
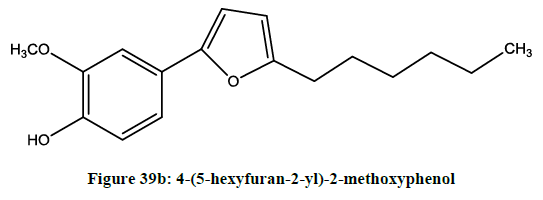
Saito et al. exmined [32] radical scavenging activity and cyto-protective effects of novel furan compounds Figures 39a and 39b, which have potent inhibitory activity against oxygenases such as COX-1, COX-2 and 5-LOX.
Furan derivatives as miscellaneous therapeutic agents
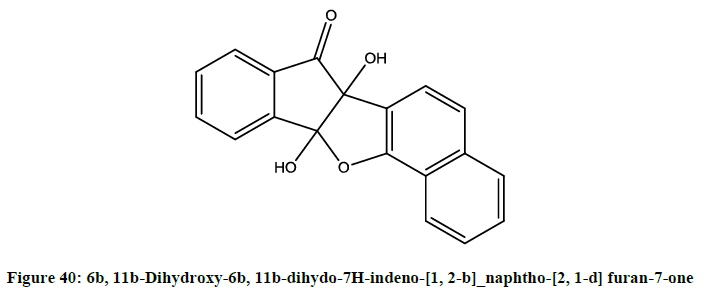
Unnikrishnan et al. reported [33] antioxidant, 5-lipogenase inhibitory,anti-inflammatory and peripheral analgesic activities of 6b, 11b- Dihydroxy-6b, 11b-dihydo-7H-indeno-[1, 2-b]_naphtho-[2, 1-d] furan-7-one (Figure 40). It also had promising non-ulcerogenic effect for immune pathogenic chronic inflammatory conditions.
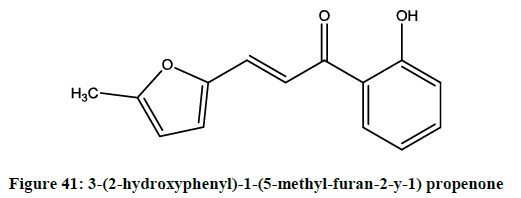
Israf et al. observed [34] that 3-(2-hydroxyphenyl)-1-(5-methyl-furan-2-y-1) propenone (Figure 41), was suppressing various proinflammatory mediators. HMP also selectively inhibited the p38/AFT-2 and AP-1 signaling pathways in the NO synthesis by the macrophage RAW 264.7.
Oxazole
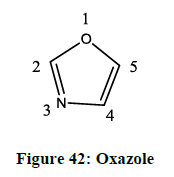
Oxazoles (Figure 42) are heterocyclic organic molecules, with an oxygen atom in the 1st position and a nitrogen atom in the 3rd position. Like pyridine they are weakly basic compounds, oxazoles might be considered as furan derivatives, in a sense that they can be derived from furan by a replacement of methane group by an azomethine nitrogen group in the 3rd position. Just like other heterocyclic organic compounds, they have certain pharmacological activities, which are as follows:- analgesics, antimicrobial, anticonvulsants, antiimflamatory, antideprssants, antidiabetic, antiobesity, diabetes. Depending on the different substituent, the oxazole moieties have different activities. In this section we have discussed about properties of oxazoles, different ways of synthesis, about naturally identified oxazoles and also about its several pharmacological activities.
Chemistry of oxazole
Though, oxazole entity was synthesised only in 1962, the chemistry of oxazole began as early as 1876 with synthesis of 2 methyl- oxazole, Oxazole chemistry came into limelight only after the first world war, when the antibiotic, penicillin was discovered. Further advancement of oxazole chemistry occurred with the invention of the dienes in the Diels Alder reaction and also in the reaction of mesoionicheterocycles involving 1,3 dipolar cycloaddition reactions [35].
Having several photophysical and photochemical activities, oxazoles are used in semiconductor devices as the electrophotographic photoreceptors and in the non-linear optical materials. Oxazoles show a cyclooxygenase-2 inhibitory action and also tyrosinase inhibitory property. They also show certain resembles with penicillin structure as well as its chemistry.
Oxazoles are used in certain polymerisation process and in the condensation of primary to many intermediates like homopolymers, telomers, peptides, pesticides, herbicides and also for agricultural intermediates [36].
Oxazoles play a vital role in several biologically active drug synthesis, which includes analgesics, anti-inflammatory, antimicrobial, anticancer, antidepressants, antidiabetics and anti-obesity too. Oxazole is an important component of spirocyclopropyloxazolones which is an inhibitor of herpes protease. Oxzole derivatives like phenacyclooxazolone are involved in the intermolecular Diels Alder reaction, ensuring the synthesis of the anticancer drug, pancratistamin and phenanthrene alkaloid [37].
Any functional group substitution at the C-4 and C-2 of the oxazole ring plays a vital role in the activity. For example immunosuppressive activities are greatly influenced by the C4 in the oxazole moiety. A substitution of the cinnamoyol residue combined with a functional group substitution at the C-4 and C-2 of the oxazole moiety are very fundamental for tyrosinase inhibitory activity. Also an addition of the double bond at the C-4 position with a phenyl ring being present at the C-2 position plays an imperative role in oxazolone ring. With an increase of the electron donating properties of the phenyl ring, substituted at the C-2 position, the oxazolone ring operating reaction decreases [38]. An exocyclic double bond generally shall act as a dienophile and N- substituted oxazole will participate in intermolecular Diels alder reaction. An electrophilic character is generated in the β carbon (5), if the carbonyl group of the unsaturated oxazolone gets activated by the Lewis acid. Oxazole [39] has a very distinct structure and a variety of application in the pharmaceutical field, natural product and certain bioactive product. For example, anticancer activity is shown by naturally occurring bioactive natural products like the diazonamide and phorboxazole family. I. J. Turchiin, during 1986, first covered the synthesis, chemistry and application of oxazoles with the many synthetic strategies heading directed for oxazole assembly as well as the use of the versatile heterocycles as the intermediates and other pharmaceutical building blocks vastly increased; i.e. several biological activities such as antitubercular, antibacterial, antihyperglycemic, antifungal, antiinflammatory and antiproliferation [40,41].
In the modern day of organic chemistry, the heterocyclic chemistry is of great significance, and a foremost line of investigation. In drug discovery process, nitrogen, sulphur, and oxygen containing 5 membered ring and those heterocyclic compounds play a great role. The numbering of oxazole ring starts with oxygen being the 1st position, the acidity of the hydrogen atoms decreases as follows C2>C5>C4. When the acidity of the hydrogen at C2 position was calculated, the pka came around 20, while for oxazole itself the pKb is found to be 1.17. Oxazole exhibiting definite resonance can be seen in both 1H-NMR and 13C-NMR. The presence of any substituent can change the chemical shift up to 1 ppm. The parent compound shows a resonance between 7.00 and 8.00 in the 1H-NMR spectrum. A characteristic aromatic resonance is displayed by the 13C-NMR. The shielding or the deshielding effect of the C2 substitution on the C4 and C5 resonance is usually <2 ppm. Under IR spectra, oxazole shows absorbance around 1537, 1498, 1326 (stretch length), 1257 (C-H in plane deformation), 1143, 1080 (ring breathing). In the UV spectra the lamda max of the oxazoles depends highly on the substitution pattern. In methanol, the parent ring system indicates the lambda max at 205 nm [40,41].
Methods of oxazole synthesis
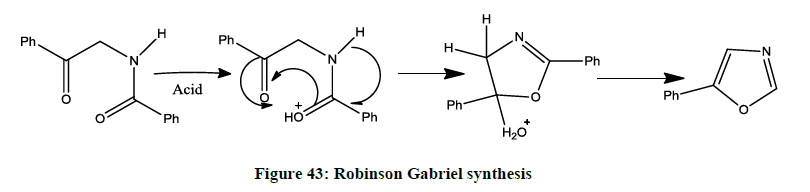
Robinson-Gabriel synthesis [42] (Figure 43).
Oxazole synthesis by dehydration of 2-acylaminoketones.
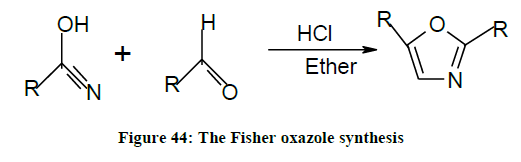
The Fisher Oxazole synthesis (Figure 44)
oxazole synthesis by condensation - cyanohydrin and carbonyl group.
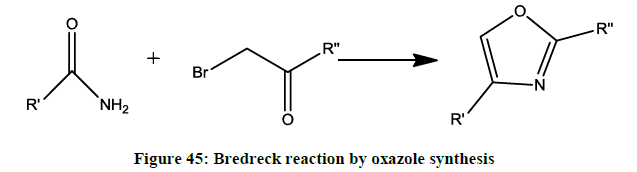
The Bredereck reaction (Figure 45)
Reaction between α-haloketones and formamide [43].
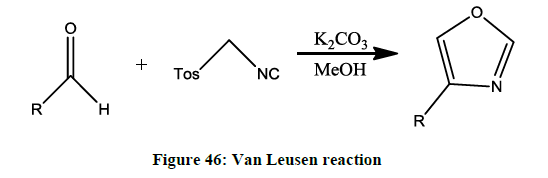
The Van Leusen reaction (Figure 46)
Synthesis of oxazole- reaction between an aldehyde and TosMIC [44].
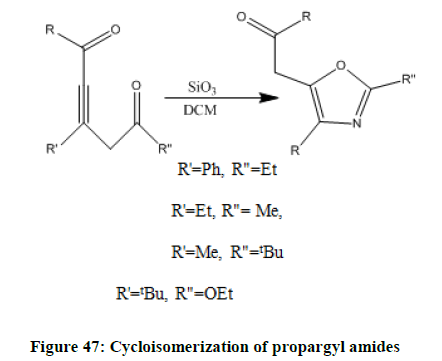
Cyclo-isomerization (Figure 47) of certain propargyl amides resulting oxazol.
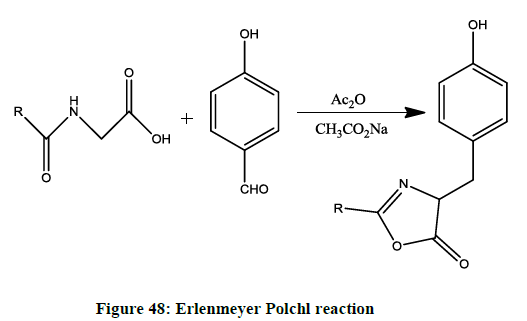
Erlenmeyer- Polchl reaction (Figure 48) [45]
Acetate anion catalysed oxazole synthesis in condensation of aldehyde and hippuric acid in presence of dry acetic anhydride.
Synthesis of N substituted oxazole taken place by cycloaddition reaction of propargylic alcohols and amines in the presence of carbon dioxide (Figure 49).
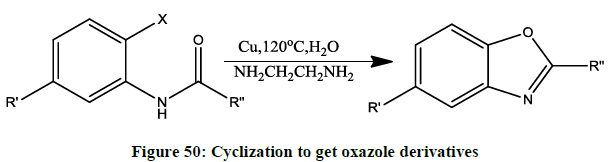
Intermolecular cyclization of oxazole-benoxazole from haloanilides by intermolecular O-arylation (Figure 50).
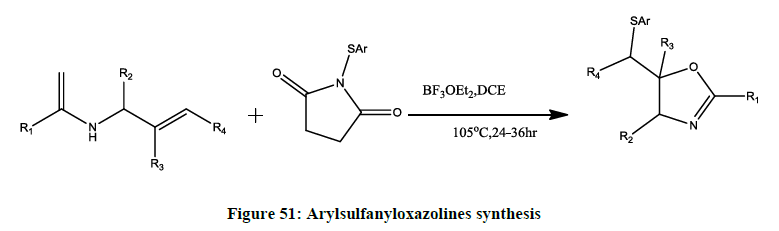
Boron-Catalyzed Arylthiooxygination (Figure 51) of NAllylamide: Synthesis of (Arylsulfanyl)oxazolines] [38,39,46].
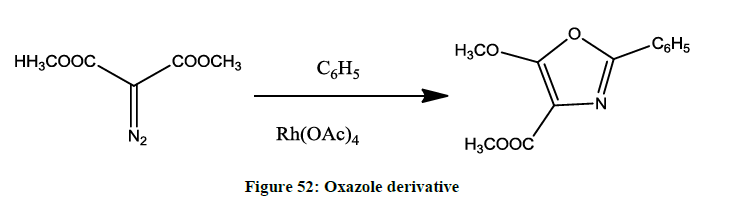
Organometallic reaction- (a) Rhodium carbene addition- Reaction between Nitrile and diazocarbonyl compound in presence of catalyst lewis acid (Figure 52).
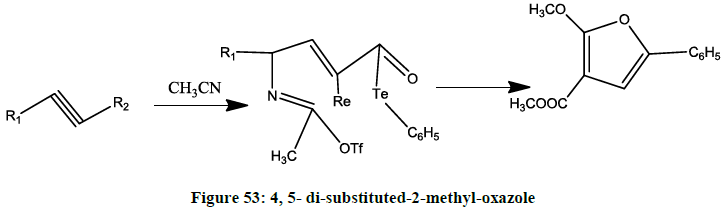
Organotellurium reagent Synthesis i.e. 4, 5-disubstituted-2-methyloxazole (Figure 53) from amidotellurinylation [46].
Few reactions of oxazole
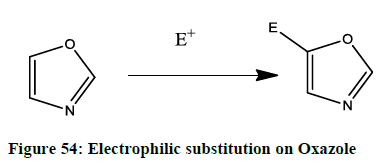
Electrophilic substitution
The preferred electrophilic action in 1, 3 oxazoles is taken place at position-5. (Figure 54) When an electron donating substituent activates the ring the electrophillic attack occurs very readily.
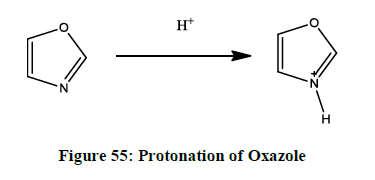
Protonation of oxazole [35]
The protonation of oxazole (Figure 55) is taken place in the 3rd position, which contains the nitrogen atom.
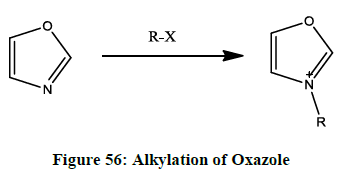
N-Alkylation of oxazole
Alkylation of oxazole (Figure 56) occurs in 3rd position, which shows high affinity for alkylation.
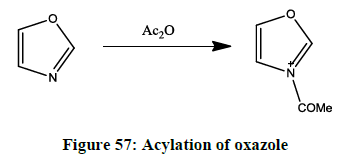
N-Acylation of oxazole [47]
The Acyl group attack takes place in the 3rd position as it shows high reactivity towards acylation of oxazoles (Figure 57).
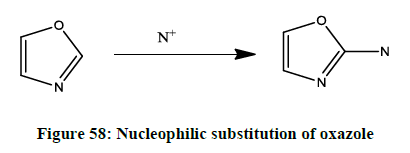
Nucleophilic substitution of oxazole
Nucleophilic substitution in the oxazole ring (Figure 58) is relatively uncommon. The ease of displacement of halogens on the oxazole ring is C2>C4>C5.
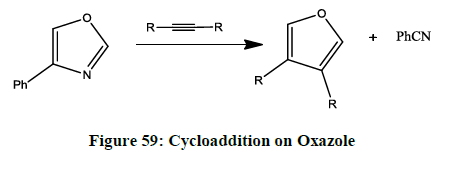
Cycloaddition (Figure 59) [48]
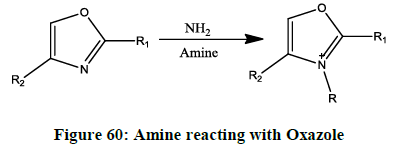
Amination (Figure 60) [48]
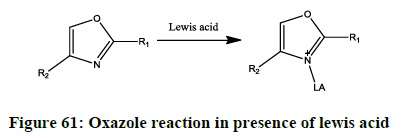
Lewis acids (Figure 61) [49]
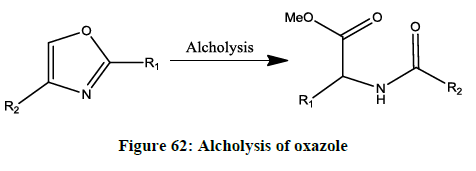
Alcholysis (Figure 62) [49]
Therapeutic review of oxazole derivatives (Table 1)
| S. No. | Structure and nomenclature | Therapeutic Activity | Author(s) Name/Year with cited references | Active Compound |
|---|---|---|---|---|
| 1 | 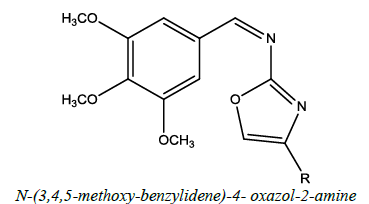 |
Antibacterial, Antifungal, Anthelmentic | Rawat, et al. [41] | R=H, Ph, PhCl, PhF, PhNO2 |
| 2 | 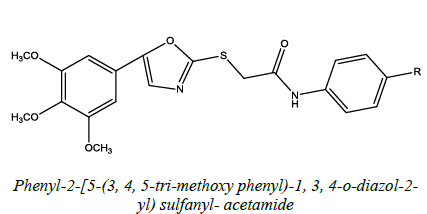 |
Antibacterial and Antifungal | Maharishi, et al. [50] | R=H, Cl, CH3, F |
| 3 | 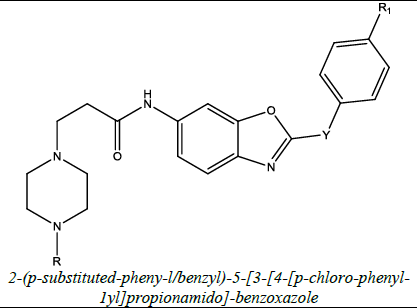 |
Antibacterial | Mustafa et al. [51] | Y=CH2, R1=Cl, X=CH2CH2, Y=CH2, R=Cl/CH3 |
| 4 | 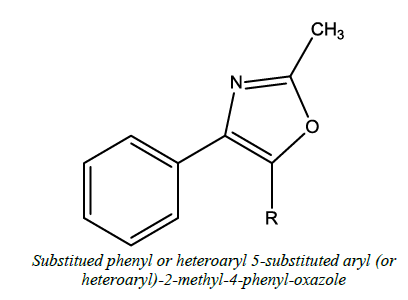 |
Antifungal, antibacterial, antimalarial | Singh et al. [52] | R=4-F-Phenyl, R=3-CF3 -Phenyl |
| 5 | 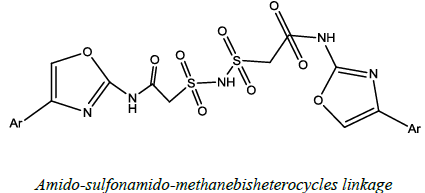 |
Antimicrobial, anticancer | Chokkappagari, et al. [53] | R=Aromatic group |
| 6 | 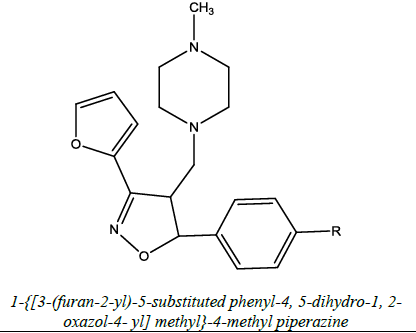 |
Antianxiety | Kumar et al. [54] | R= H/OH/ Cl. |
| 7 | 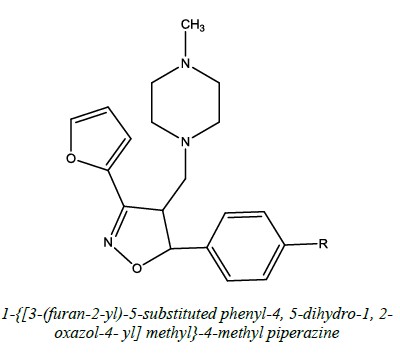 |
Antidepressant | Jagdish et al. [54] | R=H / 4-CH3/2Cl/ 4-Br/4-Cl, /4OH/ 4-OCH3 |
| 8 | 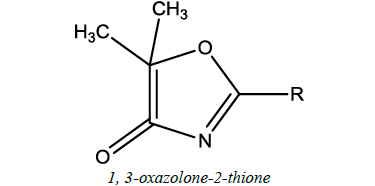 |
Anti-depressant, anticancer | Purohit et al. [55] | R=Sulphur (S) |
| 9 |  |
Anti-tubercular | Moura Kelly et al. [56] | R=H |
| 10 |  |
Anticancer | Dougal et al. [57] | R=R’=CH3 |
| 11 | 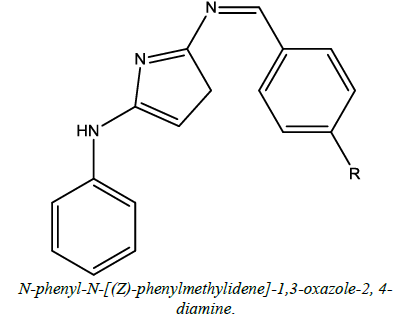 |
Anti-leprotic | Niraimathi [58] | R=CH3/Cl/ OCH3/ -N(CH3)2 |
| 12 | 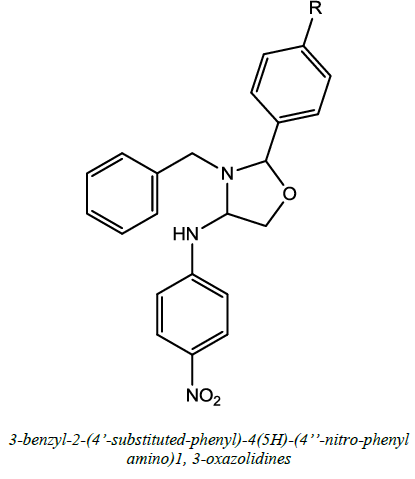 |
Anti-convulsant, Analgesics, Anti-imflamatory and Anti-tumor | Selvam et al. [59] | R=H/ OH/ Cl |
| 13 | 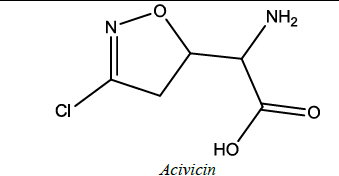 |
In leukemias, ovarian carcinoma and breast tumorxenograft. | Ghosh et al. [60] | |
| 14 | 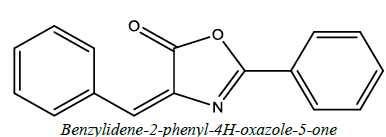 |
Pesticidal | Abdelaty et al. [61] | |
| 15 | 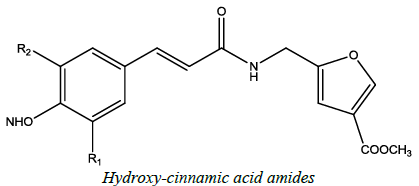 |
Antioxidant | Ivanka et al. [62] | R1=OCH3 /OH |
| 16 | 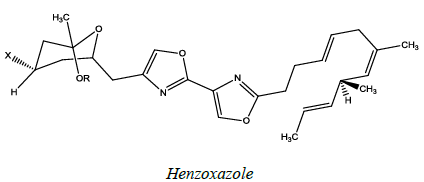 |
Anti-herpes | Delia et al. [63] | R=H, -CH3 |
| 17 | 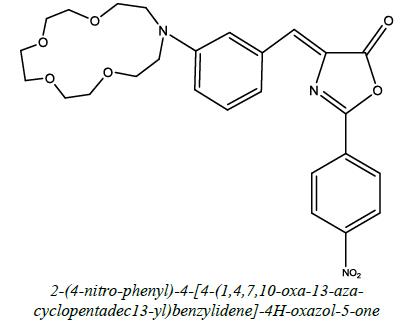 |
Photophysical properties | Ozturk et al. [64] | R=O, OCH3 |
| 18 | 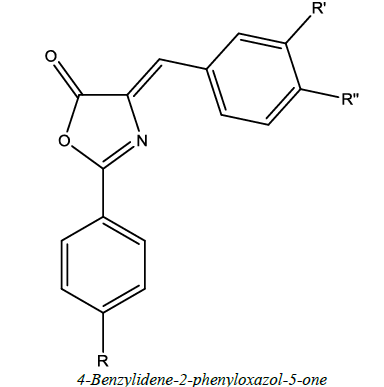 |
Antibacterial, Anticytotoxin Antifungal | Tandel et al. [65] | R=R’=R’’=H; R=R’=OH,R’’=H |
| 19 | 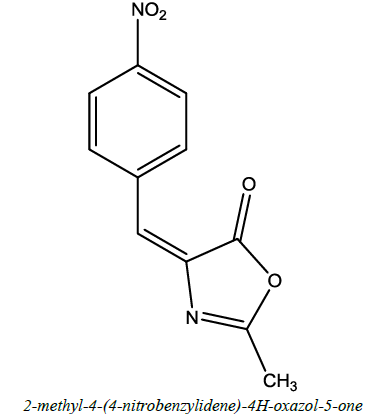 |
Immunomodulator | Mesaik et al.[66] | R=-NO2 R’= -CH3 |
| 20 |  |
Antiulcer | Kachwaha et al. [67] | |
| 21 | 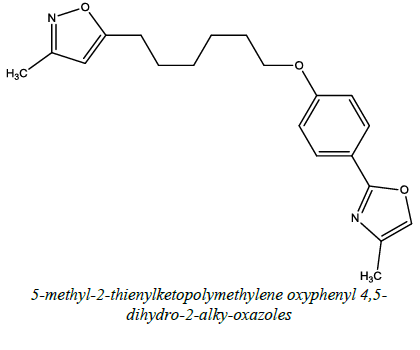 |
Anti-human picornavirus activity | Mar et al. [68] |
Table 1: Some therapeutic active oxazole compounds and their structure
Natural products containing oxazole moiety
Natural products always have uses extremely in medicinal field since the very advent of mankind. Huge number of bioactive natural products have been isolated or extracted from various sources like bacteria, plant and marine sources containing moities like oxazole. These products show various vital biological activities like antileprotic, analgesics, antifungal, antibacterial, antitubercular, and anti-inflammatory properties. Studies have shown several medicinally recognised chemical compounds containing poly substituted oxazole moiety in natural products which have been found also to contain N and O.
Oxazole isolated from marine sources
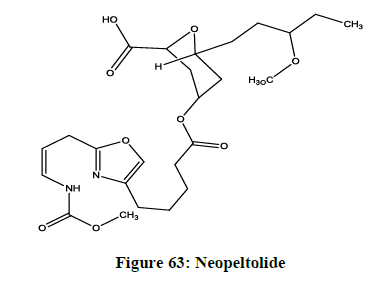
Neopeltolide
A newly found marine derived macrolide has successfully been isolated from a deep-water sponge family named as neopeltolide (Figure 63). It has been seen that this compound is very effective in stopping or inhibiting the in vitro proliferation of the human lung adenocarcinoma, the ovarian sarcoma causing cancer and murine leukemia cell lines.
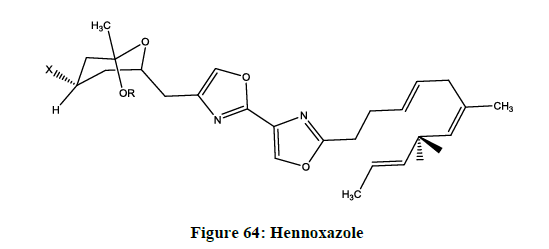
Hennoxazole
Hennoxazole (Figure 64) is isolated from a marine source, active against herpes and a peripheral analgesics, containing a bisoxazole unit muscoride [60].
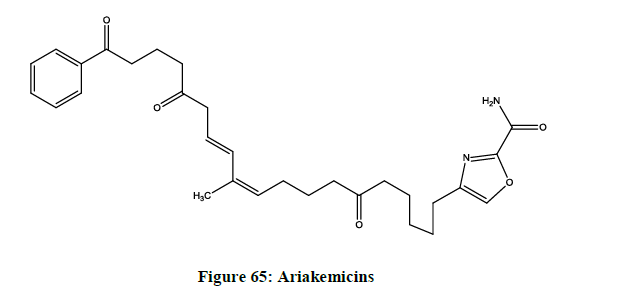
Ariakemicins A (Figure 65)
An atypical linear hybrid polypeptide or non-ribosomal peptide antibiotics, which was discovered by fermenting an extract of the marine gliding bacterium Rapidithrix sp.
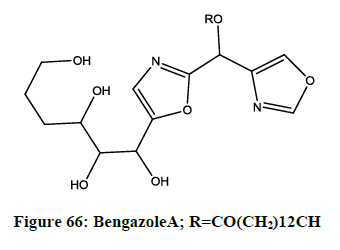
Bengazole A
It is a new natural source of bengazole A (Figure 66), which is extracted from the bioassay guided fractionation of extract of the sponge Dorypleressplendens. This compound shows growth inhibitory activity to seven murine and human cancer cell lines [63].
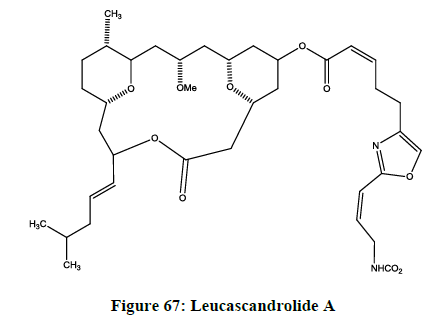
Leucascandrolides
Leucascandrolides A (Figure 67) and B were isolated by Pietra and coworkers from a calcareous sponge leucascandracaveolata, it was collected from the east coast of New Caledonia, Coral Sea [69].
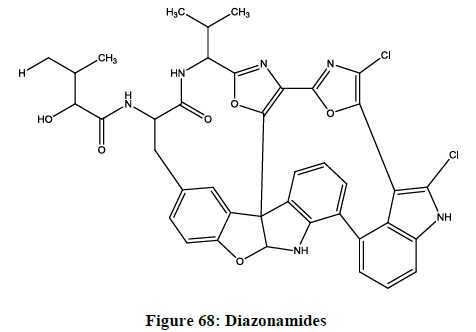
Diazonamides
The cytotoxic activity of diazonamide A (Figure 68) was evaluated against a panel of three human tumour cell lines, which included lung (A549), colon (HT29), and breast [70], which was isolated from the ascidian Diazona sp. Collected from Indonesia.
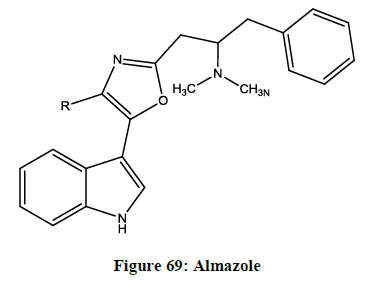
Almazole (Figure 69)
It is isolated from the red seaweed called Haraldiophylum found in the Dakar coast [71].
Oxazole compounds isolated from microbes [71]
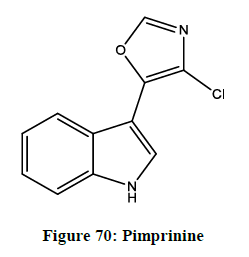
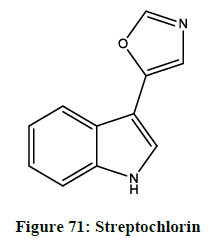
5-(indol-3-yl) oxazole
It exists in diversity of natural products ranging from the comparatively simple pimprinine (Figure 70) and streptochlorin (Figure 71) to the complex diazonamide A. Pimprinine has a variety of activities, as antibiotics, fungicidal, anti-epilepsy effects and monoamide oxidase inhibition. The diazoamides A has activity against HCT-116 human carcinoma and B-16 murine melanoma cell lines.
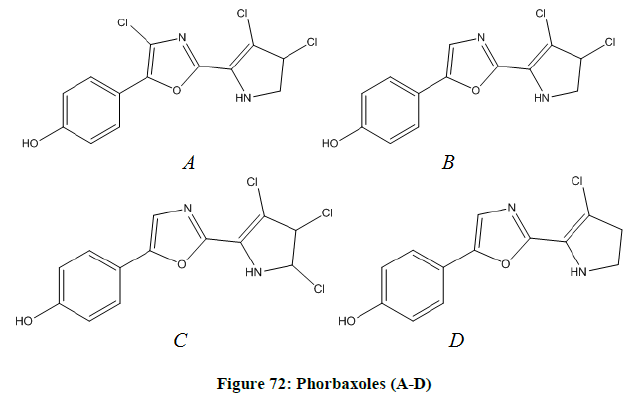
Pyrrolyl-oxazoles
The 2-(pyrrole-2-yl) oxazoles known as phorbaxoles A-D (Figure 72 A-D) are tyrosine- proline dipeptide derivative, chlorinated on the pyrrole. On the 4th position of the oxazole, phorbazole A is substituted as like as diazonamides [60].
Conclusion
Two important heterocycles Furan and Oxazole and their derivatives are discussed in details including the preparation procedures along with their wide range of medicinal importance as broad spectrum antimicrobial, antileishmanial agents, genotoxic, and CNS active agents both centrally and peripherally, fungicides, herbicides, antioxidant agent etc., which will be helpful in conducting of further research works in relevant field of synthetic chemistry.
References
- R. Dua, S. Shrivastava, S.K. Sonwane, S.K. Srivastava, Biol. Res., 2011, 5, 120-144.
- T. Eicher, S. Hauptmann, The chemistry of herocycles: structure, reactions, syntheses, and applications, Wiley-Vch, 2nd (Edn.), 2003, 215-254.
- J.W. Dobereiner, Reports of the German Chemical Society, 1822, 3, 121-146.
- C.F. Meotti, D.O Silva, A.R.S. Santos, G. Zeni, J.B.T. Rocha, C.W Nogueira, Environ. Toxicol. Pharmacol., 2003, 37, 37-44.
- I.L. Finar, Organic Chemistry (Stereochemistry and Chemistry of Natural Product), 5th (Edn.), Pearson education (Singapore) pvt. Ltd., (Indian branch) Delhi, 2001, 2, 428, 828.
- S. Kramer, J.L.H. Madsen, M. Rottlander, T. Skrydstrup, Org. Lett., 2010, 12, 2758-2761.
- Z. Chen, G. Huang, H. Jiang, H. Huang, X. Pan, J. Org. Chem., 2011, 76, 1134-1139.
- Z.P. Zhang, X.B. Cai, S.P. Wang, J.L. Yu, H.J. Liu, Y.Y. Cui, J. Org. Chem., 2007, 72, 9838-9841.
- T. Wang, X.I. Chen, Z.P. Zhan, Org. Lett., 2011, 13, 3324-3327.
- J.T. Li, X.H. Zhang, Z.P. Lin, B. J. Org. Chem., 2007, 3, 1860-1871.
- N. Zanatta, H.S. Alves, S.H. Coelho, M. Deise, Borchhardt, P. Mahado, M. Kelen Flores, F.M da Silva, B.T. Spader, J.M. Santurio, H.G. Bonacorso M.A.P. Martins, Bioorg. Med. Chem., 2007, 15, 1947-1958.
- F. Karipcin, M. Atis, B. Sariboga, H. Celik, M. Tas, J. Molec. Struc., 2013, 24, 69-77.
- M.N.A. El Hady, R.R. Zaky, K.M Ibrahim, E.A. Gomaa, J. Molec. Struc., 2012, 1016, 169-180.
- C.A. Obafemi, P.O. Adaleni, O.A. Fadare, D.A. Akinpelu, O. Famuyiwa, J. Molec. Struc., 2013, 1049, 429-435.
- M. Rani, M. Yusuf, S.A. Khan, J. Saudi Chem. Soc., 2012, 16, 431-436.
- S. Chandrappa, C.V. Kavitha, M.S. Shahabuddin, K. Vinaya, C.S.A Kumar, S.R. Ranganatha, S.C Raghavan, K.S. Rangappa, Bioorg. Med. Chem., 16, 2009, 2576-2584.
- P. Quillardet, V. Michel, X. Arrualt, M. Hufnung, E. Touati, Toxicol. Environ. Mutagene., 2000, 470, 177-188.
- V. Srivastava, A.S. Negi, J.K. Kumar, U. Faridi, B.S. Sisodia, M.P. Darokar, S. Luqman, S.P.S Khanuja, Bioorg. Med. Chem. Lett., 16, 911-914.
- E. Andrey, V.A. Glazunova, L.G. Dezhenkova, E.K. Shevtsova, V.F. Traven, J. Balzarini, H.S Huang, A.A Shtil, M.N. Preobrazhenskaya, Eur. J. Med. Chem., 2011, 46, 423-428.
- P. Diana, A. Carbone, P. Barraja, G. Kelter, H.H Fiebig, G. Cirrincione, Bioorg. Med. Chem., 2010, 18, 4524-4529.
- C.H. Tseng, Y.L. Chen, S.H. Yang, S.I. Peng, C.M, Cheng, Han, S.R. Lin, C.C. Tzeng, Bioorg. Med. Chem., 2010, 18, 5172-5182.
- P. Diana, A. Carbone, P. Barraja, G. Ketler, H.H. Fiebigg, G. Cirrincione, Bioorg. Med. Chem., 2010, 18, 4524-4529.
- E.S. Lee, B.C. Park, S.H. Paek, Y.S. Lee, Basnet, D.Q. Jin, H.G. Choi, C.S. Yong, J.A. Kim, Biol. Pharm. Bull., 2006, 29, 361-364.
- M.M. Alam, A. Husain, S.M. Hasan, S, Khanna, M. Sahquiquazzaman, J. Enzyme Inhibit. Med. Chem., 2010, 25, 323-330.
- Y.L. Chen, Y.L. Zhao, C.M. Lu, C.C. Tzeng, J.P. Wang, Bioorg. Med. Chem., 2006, 14, 4373-4378.
- K. Wieckowski, K. Salat, J. Bytnar, M. Bajda, B. Felipek, J.P. Stables, B. Malawska, Bioorg. Med. Chem., 2012, 20, 6533-6544.
- Drizin, R.J. Gregg, M.J.C. Scanio, L. Shi, M.F. Gross, R.N. Atkinson, Bioorg. Med. Chem., 16, 2008, 6379-6386.
- G. Zeni, D.S. Lῡdtke, C.W. Nogueira, R.B. Panatieri, Tetrahedron Lett., 2001, 42, 8927-8930.
- C.B. Mishra, S.K. Barodia, A. Prakash, J.B.S. Kumar, P.M. Luthra, Bioorg. Med. Chem., 2010, 18, 2491-2500.
- S.Y. Choi, Y.O. Kim, D. Son, J. Lee, S. Kim, H. Kim, S.Y. Kim, J. Hura, Brain Res., 1472, 2012, 32-37.
- S. Manfredini, S. Vertuani, B. Manfredi, G. Rossoni, G. Calveillo, P. Palozza, Bioorg. Med. Chem., 2000, 8, 2791-2801.
- K. Nishio, A. Fukuhara, Y. Omata, Y. Saito, S. Yamaguchi, H. Kato, Y. Yoshida, E. Niki, Bioorg. Med. Chem., 16, 2008, 10332-10337.
- G. Mathew, A. Jacob, E. Durgashivaprasad, N.D. Reddy, M.K. Unnikrishnan, Int. Immunopharmacol., 15, 2013, 182-189.
- C.Y. Liew, K.W. Lam, M.K. Kim, H.H. Harith, C.L. Tham, Y.K. Cheah, M.R. Sulaiman, N.H. Lajis, D.A. Israf, Int. Immunopharmacol., 11, 2011, 85-95.
- T. Eicher, S. Hauptmann, The chemistry of heterocycles: Structure, reactions, synthesis and applications, John Wiley and Sons, 2003, 2nd (Edn.), 545-550.
- C. Ainsworth, J. Am. Chem. Soc., 67th (Edn.), 1965.
- D.C. Palmer, Oxazole Synthesis, Reaction and Spectroscopy, John Wiley and Sons, 2003, 391-392.
- I.J. Turchi, Review on Oxazoles, John Wiley and Sons, 1986, 18-20.
- S. Bala, M. Saini, S. Kamboj, Int. J. Chem. Tech. Res., 2011, 3, 1102-1118.
- A. Ehsani, Ind. J. Chem. Technol., 2016, 23, 289-295.
- B.S. Rawat, S.K. Shukla, World J. Pharm. Pharmaceut. Sci., 2016, 5, 1473-1482.
- R.K. Singh, A. Bhatt, R. Kant, P.K. Chauhan, J. Chem. Biol. Interface. 2016, 6th (Edn.), 4, 263-269.
- I.H.R. Tomi, J.H. Tomma, A.H.R. Al-Daraji, J. Saudi Chem. Soc., 2015, 19, 392-398.
- M.R. Reddy, G.N. Reddy, U. Mehmood, I.A. Hussein, S.U. Rahman, Eur. J. Org. Chem., 2015, 47, A-F.
- A.K. Shakya, A. Kaur, B.O. Al-Najja, R.R. Naik, Saudi Pharmaceut. J., 2015, 1-9.
- S. Bresclani, N.C.O. Tomkinson, Transition Metal Mediated Synthesis of Oxazoles, Published by West Chem, 2015, 1-2.
- Y. Hu, X. Xin, B. Wan, Tetrahedron Lett., 2015, 56, 32-52.
- K.D. Rynearson, S. Dutta, K. Tran, S.M. Dibrov, T. Hermann, Eur. J. Org. Chem., 2013, 7337-7342.
- R. Kharb, J. Sharma, A.K. Sharma, Int. J. Pharm. Biol. Chem., 2013, 2nd (Edn.), 2, 390-400.
- M.B. Shukla, J.B. Mahyavanshi, K.A. Parmar, Ind. J. Chem., 2016, 55B, 374380.
- M. Arisoy, O.T. Arpaci, F.K. Onurdag, S. Ozgen, Ind. J. Chem., 2016, 55B, 240-247.
- A.M. Lincoff, M.D. Jain, C. Tardif, G.G. Schwartz, S.J. Nicholls, L. Ryden, Effect of aleglitazar on CV outcomes after acute coronary syndrome in patients with type 2 diabetes mellitus, 15th (Edn.), 2014, 1515-1522.
- C. Premakumari, A. Muralikrishna, A. Padmaja, V. Padmavathi, S.J. Park, G.D. Reddy, Arab. J. Chem., 2014, 7, 385-395.
- J. Kumar, G. Chawla, M. Akhtar, K. Sahu, V. Rathore, S. Sahu, Arab. J. Chem., 2013, 1-10.
- P. Shriram, D. Kumar, I. Sudhir, K. Asif, Unique Res. J. Chem., 2013, 1st (Edn.), 1, 16-29.
- K.C.G. Moura, P.F. Carneiro, M.C. Pinto, J.A. Silva, V.R.S. Malta, G. Dias, Bioorg. Med. Chem., 2012, 20, 6482-6488.
- D.J. Ritson, J.E. Moses, Tetrahedron., 2012, 68, 197-203.
- V. Niramathi, A.J. Suresh, T. Latha, Int. J. Pharm. and Pharmaceut Sci., 2011, 4th (Edn.), 3, 152-153.
- T.P. Selvam, Synthesis, Characterization and Biological Activity of novel 3-benzyl-2-(4’-substitutedphenyl)4(5H)-(4”-nitrophenylamino)-1, 3-oxazolidines, 2011, 10, 344-350.
- A. Ghosh, Synthesis and Antitumor Activity of Oxazole and Isooxazole Derivative, Rajiv Gandhi University of health Sciences, 2010.
- A. Aty, World J. Agri. Sci., 5th (Edn.), 2009, 1,105-113.
- I. Stankova, M. Spasova, Hydroxycinnamic Acid Amides with Oxazole-Containing Amino Acid: Synthesis and Antioxidant Activity, Z. Natural. Forest., 2009, 64c, 176-178.
- D. Hernández, E. Riego, F. Albericio, M. Álvarez, Eur. J., 2008, 3389-3396.
- G. Ozturk, H. Karabryuk, M. Aygun, S. Alp, S. Ozcelik, Tuning photoinduced intermolecular electron transfer by electron accepting and donation substituents in oxazolones, Springer publications, 2008, 1-13.
- R.C. Tandel, D. Mammen, Indian J. Chem., 2008, 47B, 932-937.
- M.A. Mesaik, J. Bioorg. Med. Chem., 2004, 12th (Edn.), 9, 2049-2057.
- S. Kachwaha, S. Varghese, D. Gupta, D. Mashke, S.R. Daneshwar, Indian J. Pharmaceut. Sci., 2002, 64(6), 545-549.
- A. Mai, M. Artico, S. Massa, R. Ragno, A.D. Montis, P.L. Colla, Antivir. Chem. Chemother., 1996, 7th (Edn.), 4, 213-220.
- T.H. Graham, The Synthesis of Oxazole Containing Natural Products, University of Pittsburgh, 2006, 1-3.
- D. Davyt, G. Serra, Mar. Drugs., 2010, 8, 2755-2780.
- D. Kumar, N.M. Kumar, M.P. Tantak, J. Chem. Biol., 2012, 2nd (Edn.), 5, 331-338.