Review Article - Der Pharma Chemica ( 2025) Volume 17, Issue 2
Review on : Microemulsion
Vishnudas K Lokhande*, MD Kshirsagar, MA Channawar and AV ChandewarVishnudas K Lokhande, Department of Pharmaceutics, Pataldhamal Wadhwani College of Pharmacy, Yavatmal, India, Email: lokhandev018@gmail.com
Received: 05-Dec-2023, Manuscript No. DPC-23-122150; Editor assigned: 08-Dec-2023, Pre QC No. DPC-23-122150; Reviewed: 26-Dec-2023, QC No. DPC-23-122150; Revised: 21-Feb-2025, Manuscript No. DPC-23-122150; Published: 28-Feb-2025, DOI: 10.4172/0975-413X.17.2.620-626
Abstract
Microemulsions have emerged as novel vehicles for drug delivery that allow sustained or controlled release for percutaneous, peroral, topical, transdermal, ocular, and parenteral administration of medicaments. Topical formulations are attractive alternatives to oral formulations and offer several advantages, such as avoiding first-pass hepatic metabolism and gastric degradation. Microemulsions are shown to be an effective dermal delivery mechanism for several active ingredients for pharmaceutical and cosmetic applications. Topical microemulsions allow rapid penetration of active molecules due to the large surface area of the internal phase, and their components reduce the barrier property of the stratum corneum. Microemulsions thereby enhance dermal absorption compared with conventional formulations and are therefore a promising vehicle due to their potential for transdermal drug delivery. They offer the advantage of spontaneous formation, ease of manufacturing and scale-up, thermodynamic stability, improved drug solubilization of hydrophobic drugs, and bioavailability. This review paper gives information about microemulsions.
Keywords
Microemulsion; Thermodynamically stable; Drug absorption; Bioavailibility; Applications
Introduction
Microemulsions are thermodynamically stable isotopically clear dispersion of two immiscible liquids, such as oil and water, stabilized by an interfacial film of surfactants along with co-surfactants. Microemulsions are having the advantages over both colloidal systems under investigation and conventional emulsions, suspensions and micellar solutions. Microemulsions provide alternative drug carriers. Microemulsions offer the advantages of spontaneous formation, easy of manufacturing and scale-up, thermodynamic stable, improved drug solubilization of hydrophobic drugs and bioavailability [1].
Microemulsions are three types based on their composition:
- Oil in water microemulsions where in oil droplets aredispersed in the continuous aqueous phase.
- Water in oil microemulsions where in water dropletsare dispersed in the continuous oil phase.
- Bi-continuous microemulsions where in microdomains of oil and water are interspersed withinthe systems.
In all three types of microemulsions, the interface is stabilized by an appropriate combination of surfactants and/or co-surfactants.
Topical drug delivery systems
Topical drug administration involves localised drug delivery to the body, for example, ophthalmic tissue, the vaginal epithelium, and skin for local or systemic effects. The main route of topical drug administration is the skin due to the fact that it is the largest human organ of the integumentary system [2].
Materials and Methods
Anatomy and physiology of the skin
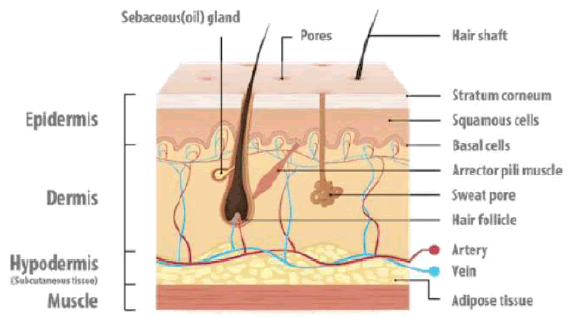
Skin is one of the most readily accessible organs on the human body for topical administration and is the main route of the topical drug delivery system. The total area of skin is about 20 square feet. The skin protects us from microbes, and the elements help regulate body temperature and permit the sensations of touch, heat, and cold. The pH of the leather varies from 4 to 5.6. Sweat and fatty acid secreted from sebum influence the pH of the skin surface (Figure 1). The skin is composed of three layers [3].
Epidermis: The multi layered epidermis varies in thickness, depending on cell size and number of cell layers of epidermis, ranging from 0.8 mm on palms and soles down to 0.06 mm on the eyelids. It consists outer stratum corneum and viable epidermis.
Dermis: The dermis is a 3 to 5mm thick layer composed of a matrix of connective tissue, which contains blood vessels, lymph vessels, and nerves. The cutaneous blood supply has an essential function in regulating body temperature. It also provides nutrients and oxygen to the skin while removing toxins and waste products.
Hypodermis: The hypodermis or subcutaneous fat tissue supports the dermis and epidermis. It serves as a fat storage area. This layer helps to regulate temperature provides nutritional support and mechanical protection. It carries principal blood vessels and nerves to the skin and may contain sensory pressure organs.
Drug absorption from topical formulations
The total amount of active ingredients absorbed in topical applications varies greatly depending on many factors, including the region of application, the frequency with which it is applied, and the viscosity or thickness of the involved vehicle. Other factors influencing drug absorption include the application location, age, and skin condition. An active ingredient can reach the dermis more quickly if not keratinized. The drug diffusion through the skin is managed in the best topical formulations by ensuring that the drug is only soluble enough in the vehicle to allow drug release at the desired rate. This is accomplished by assuring that the whole drug is dissolved in water [4].
Basic principle of permeation
It is well known that substances usually penetrate the skin by three different routes: Through the stratum corneum between the corneocytes (intercellular route); through these cells and the intervening lipids (intracellular route); or through the skin appendages, such as hair follicles and sweat glands. Molecules with adequate solubility in water and oil, with a log of oil/water partition co-efficient between 1 and 3 and a molecular weight lower than 0.6 kDa, may penetrate the skin [5].
Factors affecting topical absorption of drug
Physiological factors
- Thickness.
- Lipid content.
- The density of hair follicles.
- The density of sweat glands.
- Skin pH.
- Blood flow.
- Hydration of skin.
- Inflammation of skin.
Physicochemical factors
- Partition coefficient.
- The molecular weight (<400 Dalton).
- The degree of ionisation (only unionised drugs gets absorbed well).
- Effect of vehicles (Table 1).
| S. no | Macroemulsion | Microemulsion |
|---|---|---|
| 1 | They are lyophobic in nature. | They are the border between lyophilic and lyophobic. |
| 2 | Droplet diameter 1 to 20 mm. | Droplet diameter 10 to 100 mm. |
| 3 | Macroemulsion droplets exist as individual entities. |
Microemulsion droplets disappear within fraction of seconds. |
| 4 | Emulsion droplets are roughly spherical droplets of one phase dispersed into the other phase. |
Microemulsions are the structures of various droplets like bi-continous to swollen micelles. |
| 5 | Macroemulsions requires quick agitation for their formation. |
Microemulsions are obtained by gentle mixing of ingredients. |
| 6 | Most of the emulsions are opaque (white) in appearance. |
Microemulsions are transparent or translucent in nature. |
Table 1: Basic differences between macroemulsion and microemulsion.
Advantages
- Avoidance of first pass metabolism.
- Convenient and easy to apply.
- Avoidance of the risks and inconveniences of intravenous therapy and of the varied conditions of absorption, like pH changes, presence of enzymes, gastric emptying time etc.
- Achievement of efficacy with lower total daily dosage of drug by continuous drug input.
- Avoids fluctuation in drug levels, inter- and intrapatient variations.
- Ability to easily terminate the medications, when needed.
- A relatively large area of application in comparison with buccal or nasal cavity.
- Ability to deliver drug more selectively to a specific site.
- Avoidance of gastro-intestinal incompatibility.
- Providing utilization of drugs with short biological half-life, narrow therapeutic window.
Disadvantages
- Excessive irritability of the skin.
- The risk of an allergic response.
- Some drugs have a low permeability through the skin.
- Large-particle drugs are difficult to absorb via the skin.
- Contact dermatitis causes skin inflammation.
- The occurrence of a bubble during emulgel formulation.
Results
Microemulsions
Microemulsions, often in conjunction with a co-surfactant, are transparent, inert, isotropic liquid mixtures of oil, water and surfactant. Microemulsions are bicontinuous systems consisting of significant phases of separated water and oil by a surfactant/co-surfactant rich interfacial region. Such systems have advantages over traditional emulsions in that they are thermodynamically stable and spontaneously generated liquid systems [6].
Structure of microemulsion
Microemulsions or Micellar emulsions are dynamic systems in which the interface is continuously and spontaneously fluctuating. Structurally, they are divided into oil in water (o/w), water in oil (w/o), and bi-continuous microemulsions. In w/o microemulsions, water droplets are dispersed in the continuous oil phase, while o/w microemulsions are formed when oil droplets are distributed in the ongoing aqueous phase. In a system where water and oil are similar, the bi-continuous microemulsions may result. The mixture of oil, water, and surfactants can form a wide variety of structures and phases depending upon the proportions of the component [7].
Types of microemulsion system
According to Winsor there are four types of microemulsion phases exits in equilibria. These phases are called as Winsor phases.
They are:
- Winsor 1: With two phases, the lower (O/W) microemulsion phase is in equilibrium with excess oil.
- Winsor 2: With two phases, upper (W/O) microemulsion phase is in equilibrium with excess water.
- Winsor 3: With three phases, middle microemulsion phase (O/W plus W/O, called bio-continuous) in equilibrium with upper excess oil and lower excess water.
- Winsor 4: In single ‘n’ phases, with oil, water and surfactant homogenously mixed.
Characterization of microemulsions
Characterization of microemulsion is very difficult because of wide variety of structures. For this several techniques are required to characterize microemulsion. Various technique such as Nuclear Magnetic Resonance (NMR) spectroscopy, microscopy electrical conductivity been employed to characterize these systems.
- Microscopy: Polarizing microscopy which confirms the optical isometry but conventional optical isometry cannot be used because of small droplet whose size diameter is <150 nm. However, the sensitivity of microemulsion structure with respect of temperature causes problem in artificial results [8].
- Nuclear Magnetic Resonance (NMR) spectroscopy: Self diffusion is the random movement of molecules in the absence of any concentration gradient. Thus, it reflects the molecules are in this environment are localized. The molecules in this system are closed and thus they undergo self-diffusion. Therefore, in W/O microemulsions, self-diffusion of water molecules is slow, whereas the diffusion of O/W is high. This structure is obtained by proton-Fourier transform pulse gradient spin-echo NMR (PGSE-NMR) [9].
- Conductivity and viscosity: The nature of microemulsion and phase inversion which fall under the rheological properties. If viscosity of the microemulsion system is more than it is of good in nature in stability and also drug release. Whereas in conductivity the water phase of emulsion conducts electricity whereas oil phase does not conduct electricity [10].
Theories of micro emulsion formation
Historically, three approaches have been used to explain micro emulsion formation and stability. They are as follows:
- Interfacial or mixed film theories.
- Solubilization theories
- Thermodynamic treatments
The free energy of micro emulsion formation can be considered to depend on the extent to which surfactant lowers the surface tension of the oil water interface and change in entropy of the system such that,
Gf = γ a-T S
Where,
Gf=Free energy of formation
A=Change in interfacial area of micro emulsion
S=Change in entropy of the system
T=Temperature
γ=Surface tension of oil water interphase.
When micro emulsion is formed the change in A is very large due to the large number of very small droplets formed. In order for a micro emulsion to be formed (transient) negative value was required, it is recognized that while value of A is positive at all times, it is very small and it is offset by the entropic component. The dominant favorable entropic contribution is very large dispersion entropy arising from the mixing of one phase in the other in the form of large number of small droplets. However, there are also expected to be favorable entropic contributions arising from other dynamic processes such as surfactant diffusion in the interfacial layer and monomer-micelle surfactant exchange. Thus a negative free energy of formation is achieved when large reductions in surface tension are accompanied by significant favorable entropic change. In such cases, micro emulsion is spontaneous and the resulting dispersion is thermodynamically stable [11].
Discussion
Method of formulation
- Phase inversion method
- Phase titration method
Phase inversion method
In the phase inversion method phase inversion of microemulsions occurs by addition of excess amount of the dispersed phase. During phase inversion quick physical changes occur including changes in particle size that can affect drug release both in vivo and in vitro. For non-ionic surfactants, this can be completed by changing the temperature, forcing a transition from oil in water microemulsion at low temperatures to water in oil microemulsion at higher temperatures (transitional phase inversion). During cooling, the system crosses a point of zero spontaneous curvature and minimal surface tension, promoting the formation of finely dispersed oil droplets. This method is also known as Phase Inversion Temperature (PIT) method. Other than temperature, other parameters such as pH value or salt concentration may be considered more effectively instead of the temperature alone.
Additionally, a transition in the spontaneous radius of curvature can be obtained by changing the water volume fraction. By successively adding water into oil, initially water droplets are formed in a continuous oil phase. By increasing the water volume fraction changes, the spontaneous curvature of the surfactant from initially stabilizing a w/o microemulsion to an o/w microemulsion at the inversion point [12].
Phase titration method
Microemulsions are formulated by the spontaneous emulsification method (phase titration method) and can be shown with the help of phase diagrams. A mixture of fatty acid and oil is added to a caustic solution to prepare a microemulsion, then after it is titrated with a cosurfactant, an alcohol, until the system turned clear. Microemulsions are formed along with various association structures (including emulsion, micelles, lamellar, hexagonal, cubic, and various gels and oily dispersion) depending on the chemical composition and concentration of each component. It is found that as the chain length of the surfactant increased, microemulsions with significant transmittances by visible spectrum can be formed with oils of longer chain lengths. It is also found that different alcohols affect the formation of microemulsions in different ways. The best results, in terms of the greatest percent transmittance coupled with the widest range of oil (dispersed in water) concentration, are obtained from short or branched alcohols [13].
Evaluation
Appearance: The microemulsions were checked for optical transparency and homogeneity by visually observing against the light. The monophasic systems were also checked for the presence of any undissolved drug or solid particulates [14].
Zeta potential: The assessment of the physical stability of colloidal dispersions is characterized by measuring zeta potential of microemulsions. This was measured using Malvern Zetasizer.
Viscosity: The viscosity of microemulsion was measured using a Brookfield viscometer with spindle LV-S63.
PH: pH was determined using pH meter.
Percent transmittance test (Limpidity Test): The Percent Transmittance Test of the micro emulsion can be measured spectrophotometrically using spectrophotometer.
Measurements of droplet size: Size examination of micro-emulsion can be obtained by Dynamic-Light-Scattering experiments or electron microscopy. The polydispersity can be done by the similar Instrument.
In-Vitro drug release: The diffusion study is often disbursed on a changed Franz-Diffusion cell, among capacity of 20 mL. The Receptor section was occupied with of Buffer. The donor section was secure with plastic wrap membrane, holding the micro-emulsion preparation and also the basic drug solution, distinctly. At prearranged time intermission trials were reserved from the receptor section and examined for drug content, employing an ultra violet spectrophotometer at definite wavelength.
Determination of residual drug remaining in the skin on topical administration: The skin in the above permeation studies can be used to determine the amount of drug in the skin. The skin cleaned with gauze soaked in 0.05% solution of sodium lauryl sulfate and shall bathed with distilled water. The permeation area shall be cut and weighed and drug content can be determined in the clear solution obtained after extracting with a suitable solvent and centrifuging.
Estimation of skin irritancy: As the formulation is intended for dermal application, skin irritancy should be tested. The dorsal area of the trunk is shaved with clippers 24 hours before the experiment. The skin shall be scarred with a lancet. 0.5 ml of product is applied and then covered with gauze and a polyethylene film and fixed with a hypoallergenic adhesive bandage. The test should be removed after 24 hours, and the exposed skin is graded for the formation of edema and erythema. Scoring is repeated 72 hours later. Based on the scoring, the formulation shall be graded as “non-irritant,” “irritant,” and highly irritant [15].
Conclusion
Conclusion drawn from the exhaustive study of microemulsion that these novel carriers are a superior alternative tool for drug delivery than the simple conventional as well as from the many other novel drug delivery carriers because of its small globular size, high penetration power, increased dissolution rate, improved bioavailability, ease of preparation, stability, capacity to deliver hydrophilic and lipophilic drugs. It has potential to provide both lipophilic as well as hydrophilic the drugs of all the categories like anti-cancerous, anti-tubercular, antiinflammatory, anti-pyretic, anti-psychotic, anti-depressant, anti-anginal and many more. Microemulsion has also gained the importance because of their versatile nature and successful delivery by various routes like oral, nasal, ocular, topical and parenteral but researchers and industries need to focus their study on patents and marketed products of microemulsions which few are very and require more concentration. After reviewing all the research articles, we came to a favorable inference that by barring all the challenges microemulsion emerges as a promising carrier system to cross the skin barrier and potentially delivers the drug inside.
References
- Mamatha GT, Kumar P. World J Pharm Res. 2015; 11: p. 866-881.
- Sujatha B, Himabindu E, Bttu S, et al. J Pharm Sci. 2020;12(6): p. 750-753.
- Gull A, Ahmed S, Ahmad FJ, et al. J Drug Deliv Technol. 2020; 57: p. 101641.
- More SK, Pawar AP. EJBPS. 2020; 155: p.105539.
[Crossref] [Google Scholar] [PubMed]
- Talianu MT, Dinu-Pîrvu CE, Ghica MV, et al. Nanomaterials. 2020; 10(11): p.2292.
[Crossref] [Google Scholar] [PubMed]
- Kumar RS, Mukherjee J. JDDT. 2019; 9(4-A): p.908-910.
- Jadhav A, Daundkar A, Morale D, et al. Int J Pharm Sci Rev Res. 2018;52(2): p.60-65.
- Singh PK, Iqubal MK, Shukla VK, et al. J Pharm Chem Biol Sci. 2014;1(1): p.39-51.
- Jantrawut P, Boonsermsukcharoen K, Thipnan K, et al. Nanomaterials. 2018; 8(7): p.545.
[Crossref] [Google Scholar] [PubMed]
- Karayıldırım �K. CBUJOS. 2017; 13(4): p.943-947.
- Shaaban HA, Sadek Z, Edris AE, et al. J Oleo Sci. 2015; 64(2): p.223-232.
[Crossref] [Google Scholar] [PubMed]
- Sevcikova Petra, Kasparkova Vera, Haurlandpva Iva, et al. Euro Fed Lipid. 2014; 116(4): p.448-457.
- Ghosh V, Saranya S, Mukherjee A, et al. Colloids Surf B Biointerfaces. 2013; 105: p.152-157.
[Crossref] [Google Scholar] [PubMed]
- Vijayalakshmi Ghosh, Amitava Mukherjee, N Chandrasekaran, Int J Pharm Pharm Sci. 2012; 4(4):497-500.
- Badawi AA, Nour SA, Sakran WS, et al. Aaps Pharmscitech. 2009; 10: p.1081-1084.
[Google Scholar] [PubMed]