Research Article - Der Pharma Chemica ( 2019) Volume 11, Issue 1
Roles of Ionic Liquids in Medicines for the Treatment of Cancer and Tuberculosis
Kandasamy Govindsamy Moodley
Abstract
Some diseases that afflict humans can be cured or their effect on mortality date can be delayed. There are many diseases which lead to early deaths in spite of all the interventions and medications that modern medicines can provide. Although there is no irrefutable evidence for them, there are widely held views that diseases such as cancer may be caused by chemicals which enter the human body by various routes. Among the suspected sources, foods (both natural and synthetic) may be the carrier of carcinogens or potential carcinogens. Another source which may be implicated is environmental pollution involving air, water and earth. Whatever the sources of the disease-causing agents, it is alarming that an increasing number of humans are dying on account of being stricken with cancer or tuberculosis. Both of these diseases have debilitating effects on the persons who contract them. In spite of the fact that there are therapies available for the treatment of these afflictions, there is still much scope for development and/or discovery of newer medicines/medications/formulations which may cure or provide greater alleviation in the pain and suffering inflicted by these diseases. In terms of formulations, much research has been expended on the use of ionic liquids as solvents, and as components which can act against malignant cells. In the light of the above considerations, this paper will undertake a review of the research involving the medicinal applications of ionic liquids in the treatment of cancer and tuberculosis.
Keywords
Ionic liquid, Cancer medicine, Tuberculosis, Cell lines, Toxicity
Introduction
General background for the need of novel medicinal preparations
Almost 50% of the global mortality of humans may be attributed to common diseases, such as cancer and tuberculosis. Since the number of diseases, afflicting mankind, is increasing at an alarming rate, there is an urgency to discover cures for those that already exist. One of the most prevalent diseases is cancer; as it affects nearly 1 out of 2 people in the world. It is close behind cardiovascular diseases, as the main cause of human deaths. Cancer accounts for approximately 7 million deaths, globally, each year and the rate is expected to increase to over double that by 2020. The types of cancer causing the most deaths are breast cancer and lung cancer, affecting 63% of the population in developing countries [1]. Tuberculosis (TB) is a contagious and infectious disease caused by Mycobacterium tuberculosis and the disease accounts for, roughly, 2 million deaths per year out of almost 9 million patients who carry the infection. In fact, the disease is present in over 2 billion people but remains dormant for many years [2]. There appears to be a correlation between a number of diseases such as TB and cancer and political stability, in developing countries. There are also other external factors that seem to contribute to these cases; such as smoking or exposure to pollutants in the environment [3]. Thus, there is an urgent need to identify more reliable and powerful anti-cancer and anti-tuberculosis treatments.
Current cancer treatments involve extensive sessions of a debilitating chemotherapy drug placed into the human body; to effectively shrink the tumour enough so it can be removed surgically or kill the cancer cells all together. This method often involves long periods and side effects such as vomiting, low energy and the loss of hair. This explains the lack of selectivity to a type of cell. Thus it affects some of the healthy cells in the body putting a strain on the functioning of the body. Other methods of cancer treatment include immunotherapy, targeted therapy and precision medicine. Of these, precision medicine is the main objective of newer methods and drugs. For different reasons, the research battle against TB is driven by similar aims. TB is a bacterial disease that affects the lung. It presents mainly as two types, namely, latent TB and active TB.
The difference between the two is that latent TB can reactivate. To treat this disease, antibiotics must be taken for months to rid the body of these cells. Unfortunately, some of the chosen drugs do not work as the virus develops drug resistance. The main factors that need to be addressed are the time it takes for these methods to work and that the treatment should target only the unhealthy cells. This review is based on the premise that ionic liquids have promising roles in the design of medicines used in treating maladies such as cancer and TB.
Ionic liquids and their properties
Ionic liquids (ILs) are salts which exist in a liquid form at room temperature. It was discovered by Paul Walden in 1914; in the course of his investigation of ethyl ammonium nitrate. The first IL to be synthesized, namely, [EtNH3][NO3] was formed by neutralizing ethylamine with concentrated nitric acid [4]. ILs contain a minimum of one cation, which must be organic, and the cations are combined with a variety of anions which may have an inorganic or organic nature [5]. These ionic liquids are commonly known as room temperature ionic liquids (RTILs). RTILs have many interesting properties which allow them to be used for many different purposes. These properties can be enhanced by changing/tweaking the groups on the anion or cation [6]. Ionic liquids are mostly used as solvents due to their low vapour pressure. They have very extensive temperature range in which they exist as stable liquids [7].
Ionic liquids possess specific properties which give them the ability to be used in many applications in a wide variety of fields; since they are amenable materials which fit well into scientific research [8]. Many publications have appeared over the past 20 years on ionic liquids. They have covered a variety of topics in scientific research including synthesis of pharmaceutically important compounds [9] and materials for various medicinal applications [10]. On the contrary, there have not been many reports on the dangers of ionic liquids and the toxicity of ionic liquids [11]. Though ionic liquids can be used in many applications; which are mainly in the science and technology fields, it has been found that its use in the biomedical field is very significant.
Many of these uses exploit the toxicity of some ionic liquids towards biological cells. Studies on the biological activity of ionic liquids and their application in cancer and TB treatment are newly emerging and highly important areas [12], and which are the subject of this review. Prior to a discussion of the usefulness of ionic liquids in the above context, we need to be acknowledged that ionic liquids possess properties which are amenable to fine tuning by making systematic changes to the structures of both cations and anions. It is this latter property (namely, tunability) of ionic liquids which renders them suitable for applications such as antitumour, antiviral agents and for other therapeutic uses [13].
Toxicity of medicines as an essential feature of their properties
Toxins are generally biodynamic substances which have an adverse effect on the body [14]. However, a very pertinent claim made by Paracelsus, a surgeon and Professor of medicine [15] reads: “The poison is in the dose”. In this light, toxicity could be a desirable property. Some poisons which were created using these toxins were found [16] to have properties that could be used in medicine. A victim’s body which has been in contact with the toxins can change its biodynamic nature; thus indicating that toxins can be used as a medicine [17]. To give credence to these postulations and to create new drugs, it is important to understand the toxic nature of small molecules. This is no mean task to achieve [18]. To decrease the toxicity involved in chemotherapies in current medications and those targeted at finding a way to deal with tumor resistance to existing chemotherapies, it is important to analyze and develop new anticancer agents for drug discovery [19].
If and when a target property is found in a compound which exhibits potential to be a drug, the feasibility of using that compound as a drug, depends largely on its bioavailability [20]. There is, fortunately one commonly-used method, reported in the literature [20], which can be used to solve the solubility and stability problem of active pharmaceutical ingredients (APIs); namely, the formation of a salt. A variation of physicochemical properties of salts derived from many acids and bases are exploited for this purpose [21]. Cations and anions, which are pharmaceutically active, tend to combine to form a salt which has therapeutic properties of both parts [22,23].
Some reports on ionic liquids have discussed this specific synergistic effect observed by some ionic liquids [24]. Together with this and the properties that can be changed to alter the toxicity of ionic liquids, it is fair to assume that ionic liquids can be used as anti-cancer [25], antiviral [26] and other therapeutic agents [27]. It should be noted that the “therapeutic ionic liquids” contain different properties compared to anti-cancer and antiviral agents [27]. For therapeutic application, there is a need to balance the interference of toxicity with physicochemical and pharmacological properties to achieve the required response. Success for the above objective would herald a bright future for the role of ionic liquids in medicinal preparations.
Preamble to testing the toxicity of ionic liquids
In testing ionic liquids for their toxicity, researchers with interest in discovering new medicines incorporating ionic liquids have followed the traditions used in testing new medications. Thus many tests of the toxicity of ionic liquids have involved the use of non-human cells. Furthermore, the tests were conducted on cells outside the organism (in vitro) or within the organism (in vivo). In view of the fact the research has been conducted by a number of research groups with varied interests, it not possible to present this review under headings which show a clear-cut evolutionary trend in the use of ionic liquids as an important component of medication used in the treatment of cancer and tuberculosis [28,29]. However, every effort has been made to show how changes in the type of ionic liquids, based on the nature of cations and anions, affect toxicity of the ionic liquids and how this has been measured with particular reference to cancer cells.
It should be noted that there is a potential constraint to the broader use of lipid formulations in delivering BCS Class I and III drugs (Federal Drug Administration: BCS is acronym for Biopharmaceutics Classification System which places drugs into four classes based on criteria of solubility and permeability; Class I has high solubility and high permeability whereas class III has high solubility and low permeability). The limitation in use of formulations is attributed to the low solubility of drugs in commonly-used lepidic excipients and lipid formulations; such that the complete dose cannot be delivered in a single dosage unit by, for example: a capsule. In these instances, lipid suspensions can be viable alternative approaches; although there may also be scenarios where lipid-solution type formulations are preferable bio-pharmaceutically; for example, where a fast onset of action is required.
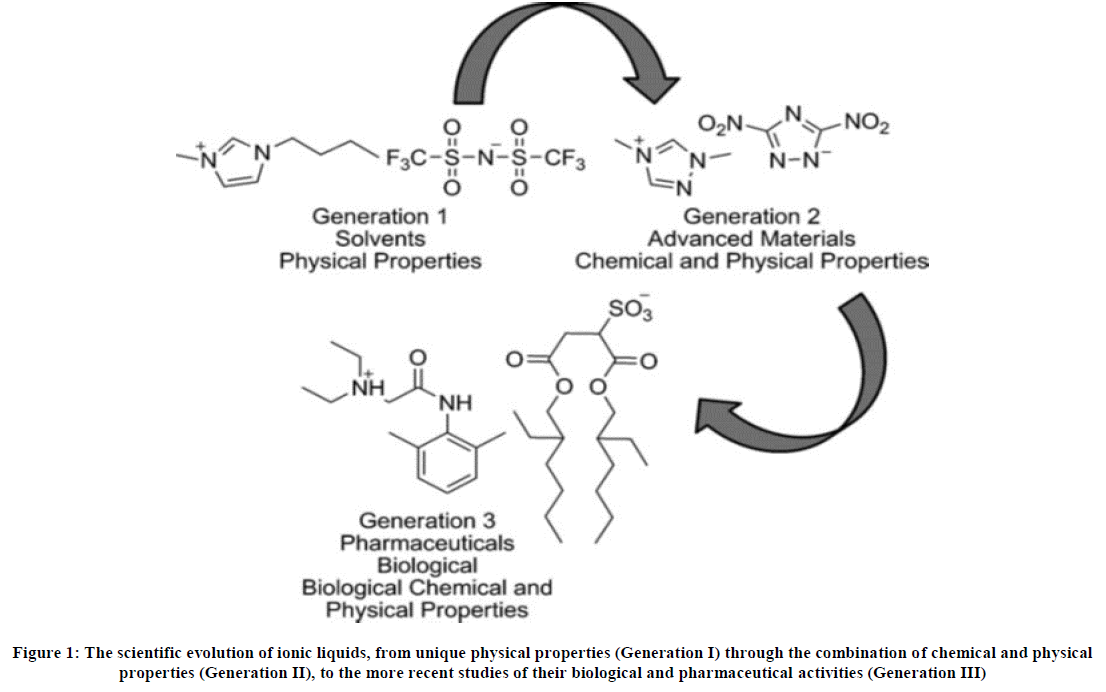
Approaches that improve drug solubility in lipid formulations are therefore desirable since they reduce the formulation drug ratio, and in turn, allow for a reduction in capsule size and or capsule [30]. Classification is however not confined to drugs only in the context of this review. In addition to being classified on the basis of the cations present, ionic liquids are also classed on the basis of their characteristics arising out of their chemical and physical properties into three ‘generations’ [31]. The first generation includes ionic liquids for which the accessible physical properties such as decreased vapour pressure and high thermal stability [32] are often unique (Figure 1, generation I).
Second-generation ionic liquids have potential use as functional materials such as energetic materials [33], lubricants [34] and metal ion complexing agents [35], (Figure 1, generation II). By taking advantage of their tunable physical and chemical properties, ionic liquids can produce a remarkable platform on which, at least potentially, the properties of both cation and anion can be independently modified and designed to enable the production of new useful materials while maintaining the main properties of an IL. Some room temperature ionic liquids (RTILs) have been used as reaction media to produce or improve on the preparation of various pharmaceuticals [31,36]. Recently, the third generation of ionic liquids [37] (Figure 1, generation III) has been identified as those using active pharmaceutical ingredients (APIs) to produce formulations with biological activity.
While a tremendous amount of research has been focused on the physical and chemical properties of ionic liquids, more recently the toxicity and biological behaviour of ionic liquids have been included as two of the most highly researched and/or debated topics in this field [37]. Biologically active ions have been used to develop novel ionic liquids; however, the primary drive behind the research into these materials has been focused on the use of well-known low-toxicity ions to obtain ionic liquids with the desired set of properties [38,39].
The pharmaceutical industry is unquestionably facing a series of challenges. While many of these challenges are related to the features of this industry and their current business models, there is also an urgent need for new scientific advances that yield innovative and effective drugs and therapies [40]. The classical strategies currently being followed are reaching a point at which it is difficult to discover effective and acceptable new chemical entities. Very few drugs (<10%) that are evaluated in clinical tests are selected for commercialization; thus decreasing the accessibility of efficient therapies for the patients who need them [41,42].
Roughly 50 % of available drugs are administrated as salts. The physicochemical and biopharmaceutical properties of a given drug can be finely tuned by synthesizing them with elected counter-ions. From a pharmaceutical point of view, the melting temperature and solubility are relevant parameters because of their routine measurement and due to their potential influence on drug processing and bioavailability [43-45]. This is an easy way to adjust the properties of a drug with ionizable functional groups to overcome undesirable features present in the parent drug [46]. The quality and safety with performance of a drug are of paramount importance. This is one of the reasons why regulatory authorities have begun to classify novel salts of a registered drug as a new chemical entity [47,48].
Ionic liquids have been increasingly exploited as solvents and/or co-solvents and/or materials in a wide range of applications, including biological applications [49-53]. These include biotechnology (biocatalysis, biomolecule purification and biofuel production), pharmaceutics (drug delivery and drug formulation) [54] and antimicrobial agents [55]. The technological utility of pharmaceutically active compounds (APIs) can be improved significantly by their use in ionic liquids rather than in pharmaceutically accepted organic solvents or their natural medium, water [56]. This is because ionic liquids favourably combine green properties with tailor-made chemical, physical and biological properties. Being very effective at dissolving many types of chemicals, including complex molecules and biologically active compounds such as proteins, nucleosides and amino acids, ionic liquids are considered to be promising candidates as useful components of novel formulations for pharmaceutical industries. In the light of the foregoing discussion, this review will focus on some recent technological developments based on ionic liquids that have proven to be very effective in drug delivery and drug formulation [57]. Although there may be some overlaps, sub-headings will be used to highlight the various aspects that need to be considered, in using ionic liquids in cancer therapy.
Testing of the toxicity of ionic liquids on non-human cells
In toxicity tests using organisms, it was found that Vibrio fischeri and Daphnia magna showed increasing sensitivity in cases where the cation was changed from ammonium to imidazolium via pyridinium [55]. Some bacteria and algae are, however, somewhat less sensitive to cations with very long alkyl substituents [58]; for instance, the green alga Pseudokirchneriella subcapitata [C18mim] Cl has an EC50 (half maximal effective concentration) of 13 ppb; whereas the EC50 for [C12mim]Cl, it is around 1 ppb (parts per billion), with much more pronounced trends seen for phosphonium-based ionic liquids in the cases of Escherichia coli, Bacillus subtilis and other bacteria [59]. For algae the presence of an outer cell wall may be part of the explanation, making them less sensitive to the effects of surfactants; arising from incorporation into the cell membrane.
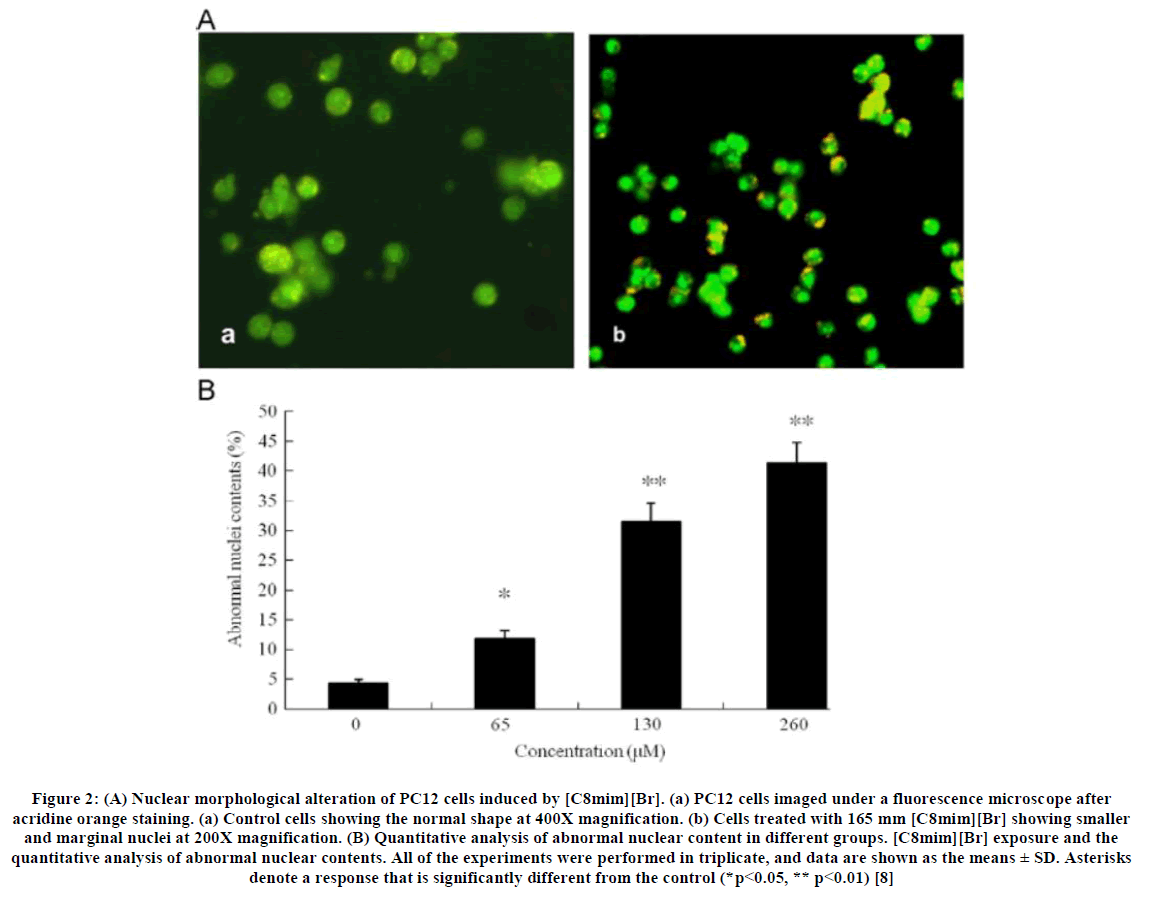
It has been shown, in-vitro, that imidazolium and pyridinium salts can inhibit the action of acetylcholinesterase, an enzyme present in bacteria, fungi, plants and higher organisms but not in yeasts [60]. Of interest is that the phosphonium salts tested in this study were less inhibitory than the imidazolium and pyridinium salts, contrary to what is often observed in vivo [61]; indicating that phosponium salts may act according to a pathway different to that used by imidizolium salts. Other studies have linked cytotoxicity and antimicrobial activity with lipophilicity, as estimated by measured or computed octanol water partition coefficients [58] and yet others have reported potential products formed by reactions of ionic liquids with cytochrome P450 [62]. As is often the case, the toxicity of ionic liquids is likely to occur through several different modes of action, with some more important than others; depending on the particular structure of the ionic liquid [63]. A study of the cytotoxicity of the alkylmethylimidazolium based ionic liquids was reported by Xiao-Yu Li et al., [8] in which ionic liquid was exposed to PC12 cell lines (Figure 2) (Rat pheochromocytoma cells). This was conducted by evaluating some factors such as cytotoxicity assays of ionic liquids with different alkyl chain lengths and MTT assay, caspase-3 activity (as an indicator of apoptosis), ROS (reactive oxygen species) level, DNA fragmentation and lactase dehydrogenase release (LDH). The ionic liquids used in this study were: 1-butyl-3-methylimidazolium bromide ([Bmim][Br]), 1-hexyl-3-methylimidazolium bromide ([Hmim][Br]), 1-octyl-methylimidazolium bromide ([Omim][Br]), 1-decyl-3-methyl-imidazolium bromide ([C10mim][Br]) and 1-dodecyl-3-methyl-imidazolium bromide ([C12mim][Br]).
Figure 2: (A) Nuclear morphological alteration of PC12 cells induced by [C8mim][Br]. (a) PC12 cells imaged under a fluorescence microscope after acridine orange staining. (a) Control cells showing the normal shape at 400X magnification. (b) Cells treated with 165 mm [C8mim][Br] showing smaller and marginal nuclei at 200X magnification. (B) Quantitative analysis of abnormal nuclear content in different groups. [C8mim][Br] exposure and the quantitative analysis of abnormal nuclear contents. All of the experiments were performed in triplicate, and data are shown as the means ± SD. Asterisks denote a response that is significantly different from the control (*p<0.05, ** p<0.01) [8]
The results of the tests described above indicated that cytotoxicity increases with increasing dosage of ionic liquid, cytotoxicity is enhanced if ionic liquids with longer side chains on the cations are used, the ionic liquid, 1-octyl-methylimidazolium bromide caused, inter alia, change in cell shapes from strips to spheres, shrinkage of the cell nucleus enhanced depolarization of mitochondria and DNA fragmentation, apoptosis of cells is attributable to the role of the ionic liquid in triggering the overproduction of ROS.
An important observation by researchers is that ionic liquids are akin to cationic surfactants and can cause easy permeability of biological membranes [64]. This is an excellent discovery for the world of medicine.
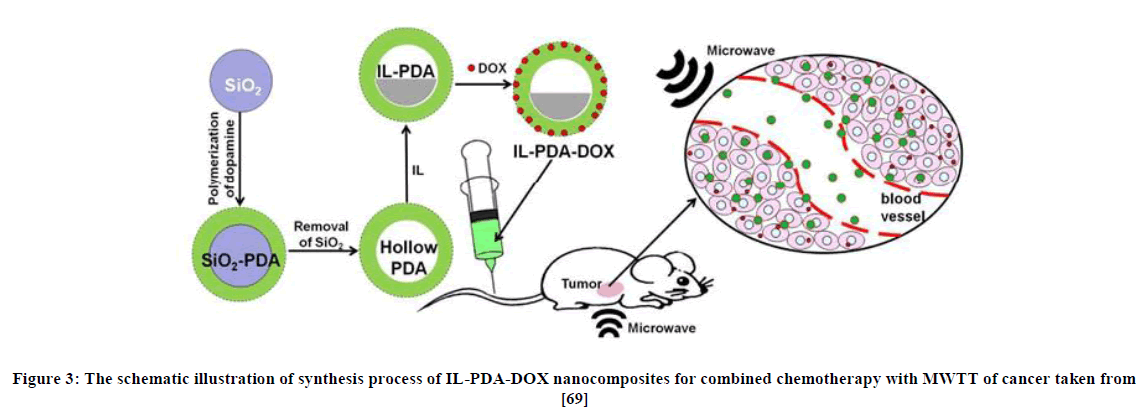
As described previously, anti-tumour activity and the cytotoxicity of the ionic liquids depend on the nature of the alkyl chains attached to the cations present in the ionic liquids. However the targeted properties of ionic liquids can be accessed in a different way. Recently, W. Tang et al., [65] reported a novel ionic liquid composite: doxorubicin loaded ionic liquid (IL-PDA-DOX). This composite was utilized in microwave thermal therapy and biocompatible delivery nanoplatforms for chemotherapy (Figure 3). The method of combinatorial therapy can be applied by packing the ionic liquid into the polydopamine nanoparticles which becomes a nano-carrier for chemotherapeutic drugs. The in-vitro and in vivo experiments of combined chemotherapy and microwave thermal therapy gave a positive result for doxorubicin (DOX)-loaded ionic liquid-polydopamine (IL-PDA) nanocomposites which displayed antitumor properties. This result allows a new door to be open to the world of cancer treatment; due to drug-loaded ionic liquid-polydopamine nanocomposites while combining chemotherapy and microwave thermal therapy [65].
Figure 3: The schematic illustration of synthesis process of IL-PDA-DOX nanocomposites for combined chemotherapy with MWTT of cancer taken from [69]
In the IL-PDA-DOX nanocomposites, it was observed that the loading rate for the DOX component reached about 10.79 %. It was also reported that this nanocomposite, when subjected to a low microwave power, showed a great microwave sensitization effect. They also showed another type of effect called an inhabitation effect when joined together with microwave (MW) irradiation. Without causing tissue toxicity, the IL-PDA-DOX allowed for tumor ablation while it behaves as MWTT (microwave thermal therapy) agent under the MW irradiation. The use of the composite (PDA-DOX) loaded with an ionic liquid in conjunction with chemotherapy and MWTT, resulted in a remarkable application for cancer treatment. When the loading rate of the nano composite (PDA-DOX) was altered, together with other materials such as nuclear magnetic materials, fluorescent materials and CT imaging materials, it is allowed for easy and effective tumor theranostics.
Effect of concentration of ionic liquids on toxicity on non-human and human cells
On account of the impact of ionic liquids on the environment and health, Marina Cvjetko et al., [66] investigated the effect of concentration on the cytotoxicity of imidazolium ionic liquids in CCO (channel catfish ovary cells) found in the ovarian fish cell line and on HeLa (Henrietta Lacks Immortal Cells) which is the cell line in human tumours with regard to viability of the cells. The range in concentration was from 0.1 mmolL-1 and 10 mmolL-1 for the following set of ionic liquids with commercial names given in square brackets: 1-n-butyl-3-methylimidazolium tetrafluoroborate; [Bmim][BF4], 1-n-butyl-3-methylimidazolium hexafluoro-phosphate; [Bmim][PF6], 1-n-butyl-3-methyl imidazolium bis (trifluoromethyl sulphonyl) imide; [Bmim][Tf2N], 1,3-dimethyl imidazolium hexaflourophpsphate; [Mmim][PF6]. The cytotoxicity of Ionic liquids was assessed by determining the viability of cells using MTT assay (a method which uses a yellow tetrazolium water soluble dye which is reduced by mitochondrial hydrogenases to give a purple product). As expected, the level of cytotoxicity increased with increasing concentration of the ionic liquids. However the cytotoxicity did not increase, in the same manner for the two sets of cells investigated; for example, at the concentration range of 0.1 mmolL-1 and 0.5 mmolL-1 of [BMIM][BF4], only the HeLa cells experienced a small cytotoxic effect, whereas the CCO cells were unaffected.
The order of cytotoxicity of the ionic liquids was found to be:
[BMIM][Tf2N] > [BMIM][BF4] > [BMIM][PF6] > [MMIM][PF6]
Furthermore, the toxicity of the ionic liquids and its effects on the cells are dependent on the type of anion used and the length of alkyl chain in the cation. This investigation not only showed that CCO cells in the ovarian fish cell line could be used in the initial testing of ionic liquids for toxicity, prior to use on humans, but it also validated a method for testing ionic liquids for toxicity towards other cell lines.
Changqin Jing et al., [67] investigated the effect of concentration changes of the IL, 1-octyl-3-methylimidazolium chloride ([C8mim][Cl]) on its response and cytotoxicity toward the QGY-7701 cell (human hepatocellular carcinoma cell line), which is the human hepato-carcinoma cell. Very specifically, the viabilities of QGY-7701 cells were tested under varied levels of concentrations. It was found that when the concentration of the IL group was over a certain value, the viability of the cell was lowered to a level which resulted in death of the cell (apoptosis). Another method indicating apoptosis is the Hoechst 33342 (bis-benzimide derivative that binds to Adenine and Thymine-rich sequences in the minor grove of double-stranded DNA) staining assay. This is also dependent on concentration. The cells were exposed for at least 24 hours at selected concentrations and the following aspects were assessed: cell shrinkage, fragmentation of the nuclei and condensation of the chromatin [68]. Another observation which adds credence to the above result is an increase of caspase-3 activity (which gives a measure of the apoptosis of tumour cells).
The cell growth of the QGY-7701 cell can be stopped and its viability in the oxygen dose- dependent conditions can be decreased by the IL compound [C8mim][Cl]. Apoptosis can be induced by exposing the IL to QGY-7701 cells. ROS (reactive species), SOD (superoxide dismutase) and CAT (catalase activities) are overly produced when [C8mim][Cl] is exposed which caused a decrease in the reduced glutathione (GSH) content. It also causes an increase in the malondialdehyde (MDA, an end-product of lipid peroxidation) cellular levels. It assumed that the ROS oxidative stress may be responsible for the death of the cell caused by [C8mim][Cl] in QGY-7701 cells.
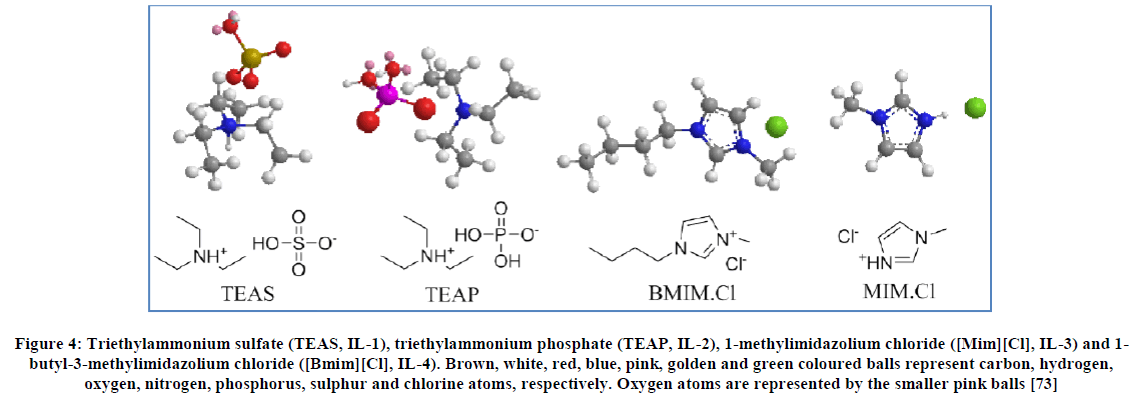
Kaushik et al., [69] conducted a study on ammonium and imidazolium based ionic liquids. The study focused on the factors affecting the toxicity on T98G (an anchorage-independent human tumor cell line that exhibits stationary phase G1 arrest in-vitro) brain cancer cells caused by ionic liquids as a function of the concentrations of the ionic liquids. Four ionic liquids having structures shown in Figure 4 were synthesized and applied on T98G brain cancer cells. The ionic liquids were found to be exhibit good toxicity in the concentration range of 0.78-0.09 mg/ml. As a control, the toxicity of the produced Ionic liquids were also exposed to the HEK (human embryonic kidney) cell line taken from normal brain cells.
Figure 4: Triethylammonium sulfate (TEAS, IL-1), triethylammonium phosphate (TEAP, IL-2), 1-methylimidazolium chloride ([Mim][Cl], IL-3) and 1-butyl-3-methylimidazolium chloride ([Bmim][Cl], IL-4). Brown, white, red, blue, pink, golden and green coloured balls represent carbon, hydrogen, oxygen, nitrogen, phosphorus, sulphur and chlorine atoms, respectively. Oxygen atoms are represented by the smaller pink balls [73]
Rates of cell proliferation and cell death were calculated using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) cell proliferation assay. It was found that ionic liquids 1, 2, 3 and 4 restrict the growth of both the HEK cells and T98G cells. When used in concentration range of 0.78-0.09 mg/ml it was found that after 1, 2 and 3 days of treatment, the ionic liquids specifically inhibit the proliferation of cancer cells. Ionic liquid 4 had the highest growth inhibiting effect on the T98G cells after 72 h. At the effective concentration, the ionic liquids seem to have a less damaging effect on the HEK cells. Another factor study in the investigation was the kinetic growth assay which is done after trypsinization and counting the total amount of cells per plate which involves a haemocytometer and trypan blue dye.
The growth kinetics assay data gathered in this study concludes that the Ionic liquids do inhibit the growth of the T98G cancer cells. IL-4 depends strongly on concentration and the length of time it is being exposed to show an inhibitory growth effect, whereas IL-1 only depends on the concentration. The size and density of cells were also distorted during this process. The ionic liquids were also subjected to a clogenic assay to determine the effect that they have on the colony forming capacity of cells. It is also used to confirm growth inhibition by the ionic liquids. Colony formation was found to be inhibited at all dosages of ionic liquids. To distinguish if a compound can be used as a drug and have great activity in a biological sense, it is required to have pharmacokinetic properties. There are certain tools that must be used to validate it against drugs available on the market. The OSIRIS (Open Source Independent Review and Interpretation System) is a program that can analyze the risk of toxicity that ionic liquids may have. The result pointed to ionic liquids being good therapeutic agents due to their low toxicity.
The above study gave insight into the usefulness of ionic liquids in aiding other drugs against cancer cells without having a toxic effect on humans and the environment due to its growth inhibition factors assessed by cell parameters for example: high activity against T98G brain cancer cell line, low cytotoxicity on HEK cells at effective concentrations; low toxicity risks in silico analysis; good oral bioavailability according to the Lipinski ‘rule of five’ [69]; and better drug likeness and drug-score values, nearly similar to commercial drugs. Ionic liquids seemed to have more effect on the T98G brain cancer cells than the HEK non-malignant cells. The most effective IL was IL-4 which had the most effect on the T98G brain cancer cell line than the HEK cell line. There are on-going investigations on the anti-proliferation effects that IL-4 has on the T98G brain cancer cell which is very beneficial to society.
The roles of cations and anions of the ionic liquids in imbuing toxicity
Several studies have found that the nature of the cations and anions affect the toxicity levels of ionic liquids. Raquel F.M. Frade et al., [70] assessed the changes in toxicity of the following types of ionic liquids: imidazolium, guanidinium, ammonium, phosphonium, pyridinium and pyrrolidinium. Since the anion was changed for an IL with a chosen cation, a large set of ionic liquids (over 80 in number) was involved in the study. The toxicity tests were effected on the human colon cancerous cell, CaCo-2 (a continuous cell of heterogeneous human epithelial colorectal adenocarcinoma cells) as these cancer cells can detach themselves from human erythrocytes.
By conducting toxicity assays on the ionic liquids themselves and combining these results with the toxicity results for the cancerous cells, the study was able to identify ionic liquids that are not harmful to human beings. The toxic effect of ionic liquids with the same cations were found to be greater when [DCA] (dicyanamide) and [NTf2] (bis trifluoromethane-sulfonyl amide) anions were involved. When the CaCo-2 monolayer cells contained [DCA] or [NTf2] anions, the monolayer appeared less harmful although the presence of [NTf2] did cause an increase in cellular metabolism in some cells which resulted in toxicity. When the anions (1-butyl-3-methylimidazolium)-[C4mim], (1-(2-hydroxyethyl)-3-methyl-imidazolium)-[C2OHmim] were present, the toxicity caused by the cation seemed to be negligible.
In summary the above study revealed that toxicity is not only affected by the length of the alkyl chain on the cation but also on the number of chains and the nature of the anion present. It was found that the longer the alkyl chain group, the greater the toxicity of the IL. A nontoxic IL does not seem to gain any toxicity through inclusion of a benzyl group although a COOH group causes great toxicity in such ionic liquids. By the addition of an ether function, the toxicity of a cation can be lowered. Guanidinium-based ionic liquids depend on the anion for their toxic nature as indicated by: [(di-h) 2dmg][Cl] (tetra-n-hexyl-dimethylguanidinium chloride) being toxic but [(di-h) 2dmg] [NTf2], tetra-n-hexyl-dimethylguanidinium bis(trifluoromethyl sulfonyl)amide and [(di-h) 2dmg] [DCA] tetra-n-hexyl-dimethylguanidinium dichloroacetic acid were not considered to be toxic indicating that the Cl anion is imbuing the ionic liquid with toxicity.
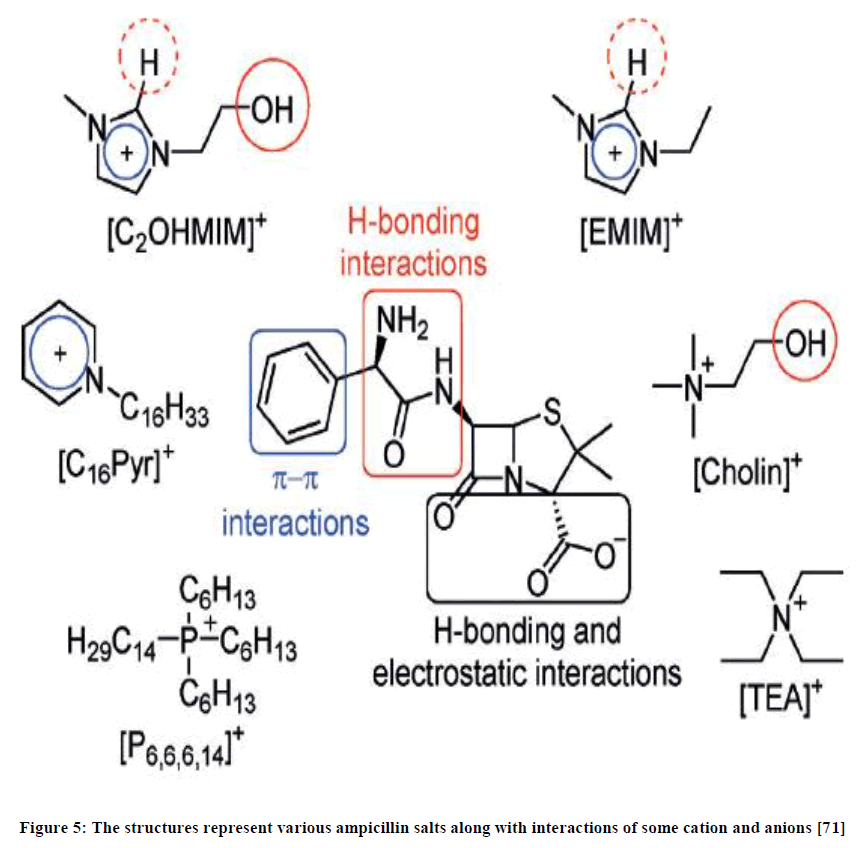
With regard to third generation of ionic liquids, one of the most important applications is their ability to conjoin with APIs (Active Pharmaceutical Ingredients) to create API-Ionic liquids. This type of composite has been the basis of a study, by Ricardo Ferraz et al., [71] involving ampicillin as the anion and the following four organic cations: ammonium, imidazolium, phosphonium, or pyridinium. This was prepared by using procedures that are controlled by buffers. The above compound is said to have great anti-proliferation effects on tumour cells lines. Treatment of tumours with these API-Ionic liquids, showed effects on the following cells: PC3 (prostate), T47D (breast), HepG2 (liver), MG63 (osteosarcoma) and RKO (colon). The presence of sodium salt of ampicillin (Figure 5) in API-IL compounds caused a high toxicity which is attributed to the amphipathic nature of the salt.
Figure 5: The structures represent various ampicillin salts along with interactions of some cation and anions [71]
The control cells used, in the above investigation, were skin and gingival fibroblasts. These were held in a MEM (minimal essential membrane) medium which consisted of 10% foetal bovine serum, penicillin, streptomycin, amphotericin B and ascorbic acid. Cells became detached in the temperature range of 70-80 degrees. After standing for 24 h at ambient temperature, the cells attach again and the renewed and supplemented medium was established at different concentration of ampicillin-based ionic liquids. The product obtained after removing the culture medium was stained and dissolved in DMSO so that its absorbance could be determined. The results for IC50 (half maximal inhibitory concentration) and LD50 (Lethal Dose, 50%) were obtained by a nonlinear regression of the concentration effects. The IC50 is used to check the inhibiting factor of biological activity of a compound.
IC50 values, of some of the API-IL compounds tested, were found to be in the range of 5 and 45 nm, which are considered to have little or no cytotoxic activity. The result also showed a decreased in cytotoxicity for the control cells, namely, skin and gingival fibroblasts. This supports the assumption that these compounds have little or no toxic nature toward human cell lines. The best result was obtained for the API-IL pair of 1-hydroxyethyl-3-methylimidazolium-ampicillin ([C2OHmim][Amp]) which had both low cytotoxicity and antitumour effects, making this compound eligible for further testing and screening on its potential as a recommended drug.
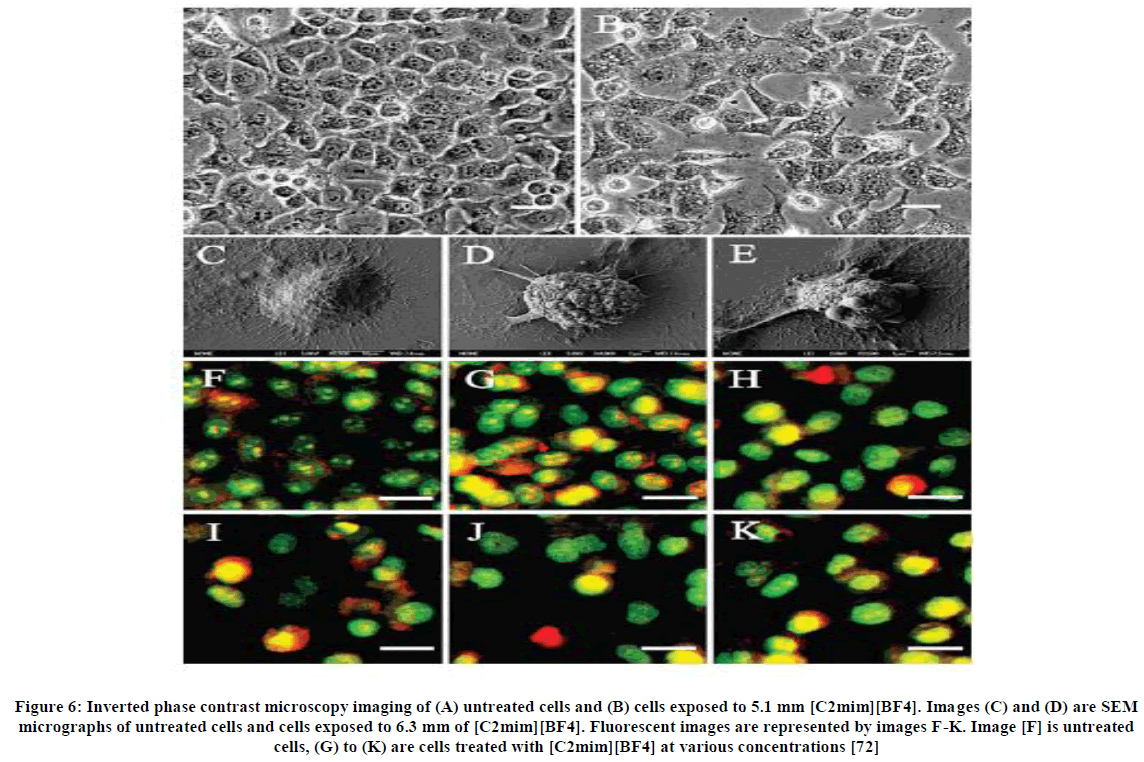
Wang et al., [72] investigated the toxicity of ethyl-, butyl-, octyl-, benzyl- and allyl-substituted 1-alkyl-3-methylimidazolium, alkyl pyridinium, N-alkyl-N, N-dimethyl-N-(2-hydroxyethyl) ammonium (choline derivatives) and alkyl-triethylammonium ionic liquids on HeLa cells present in humans. The toxicity was measured by using the MTT assay method with and without foetal bovine serum; for exposure periods of 24 and 48 h. To determine the source of toxicity, microscopy (inverted contrast light microscopy, scanning electron microscopy and fluorescence microscopy), was employed to determine the intracellular calcium concentration, the presence of reactive oxygen species (ROS) by staining cells and analysing with a flow cytometer and mitochondrial membrane potential in (by staining and analysing) cell cultures which were exposed to ionic liquids. The MTT assay technique was used to measure the number of viable cells left after exposure to the cell culture. The images for the tests are shown in Figure 6.
Figure 6: Inverted phase contrast microscopy imaging of (A) untreated cells and (B) cells exposed to 5.1 mm [C2mim][BF4]. Images (C) and (D) are SEM micrographs of untreated cells and cells exposed to 6.3 mm of [C2mim][BF4]. Fluorescent images are represented by images F-K. Image [F] is untreated cells, (G) to (K) are cells treated with [C2mim][BF4] at various concentrations [72]
The results showed that choline-derivatives and alkyl-trimethyl ammonium salts were less toxic than those of the imidazolium and pyridinium precursors, giving more than enough reason to use these salts in further and future investigations. Toxicities of salts were seen to be higher when foetal bovine serum was absent than in cases where it was present. Salts that contained ethyl groups are assumed to function by different method of action than salts that contain longer alkyl groups.
An overproduction of ROS in the mitochondrial membrane caused by the opening of the PTP (permeability transition pores) in the membrane is a sign of apoptosis of a cell, indicated by the term “loss of mitochondrial membrane potential”. This releases into cytosol, species such as calcium and cytochrome c. Staining and analysing of the cells through flow cytometry showed that mitochondrial activity had been lowered in cells that were so treated. Increase in ROS production was determined by fluorescence intensity. In some exposed cells radiometric fluorescence measurements were taken and there was an increase in intracellular calcium. The cells were exposed to [C2mim][BF4] and the above tests signified that apoptosis was possible when cells were exposed.
If apoptosis or necrosis of cells is caused by an ionic liquid, then the ionic liquid involved is not contributing to a green process. The more that is known about ionic liquids and their effect on cells, the better the understanding of how it is possible to reduce the toxicity of them. This will be ground breaking in the drug delivery world to invent new drugs or improve old ones.
Kumar et al., [58] reported on the difference in cytotoxicities of ionic liquids that have various cations and anions with the use of MCF7 human breast cancer cell line. The MCF7 cell line is used to avoid the in-vitro experiments as it is a well-characterized cell line which is used in the study of safety and toxicity. The ionic liquids, used in this study, contain pyridinium, pyrrolidium, piperidinium or imidazolium cations, with various types of alkyl chains of varying lengths in conjunction with anions such as bromide, bis(trifluoromethanesulfone)imide (Tf2N), trifluoromethylsulfonate (TfO) or nonafluoromethylsulfonate (NfO). Their cytotoxic effects were measured.
The cytotoxic strengths of the ionic liquids were determined from their interactions with the MCF7 cell line. A graph was plotted of dose versus response for each IL. IC50 values, defined as the concentration of a test substance that results in 50% growth inhibition, were calculated from a modified Hill equation [73]. IC50 values of specific ionic liquids and its effect on the MCF7 cells ranged from 8-44 mm. The IC50 values show the toxicity of these specific ionic liquids varied between 10 m to few tens of mm. The result obtained from this test was that the untreated cell shows no cell death whereas exposure to [MPPyrro][Br] resulted in apoptosis. The study showed that the longer the alkyl chain the greater the cytotoxicity increases with increasing chain length.
Other strategies for changing toxicity of ionic liquids
Egorova et al., [74] suggested that natural amino acids can be used to decrease the toxicity of ionic liquids conveniently; allowing them to be used as solvents, reagents or catalysts. The study involved various amino acids-based ionic liquids called AA ionic liquids, where the amino acids take the role of the cation and anions. These compounds were exposed to the NIH/3T3 (mouse embryo fibroblast) and CaCo-2 cell cultures. The result obtained was compared to the toxicity of an imidazolium IL.
The experiment conducted using many types of amino acid based ionic liquids and the results showed that the amino acid seemed to decrease the toxicity. However, when ionic liquid with the amino acid acting as a cation was tested, the result showed a remarkable increase in toxicity. The imidazolium based ionic liquids caused the NIH/3T3 cells to experience apoptosis. This implies that an amino acid can be included to increase the biological activity in ionic liquids. It is thus clear that ionic liquids have a possible place in medical applications instead of being used as a solvent.
Methods of assessing toxicity of medications involving ionic liquids
The lipophilicity parameters of ionic liquids and how they affect the cytotoxicity of IPC-81 leukaemia cells was investigated by Johannes Ranke et al., [75] to ascertain what factors and how these factors impact on the toxicity of ionic liquids. To this end many different types of ionic liquids were tested in this study. This included pyrrolidinium, pyridinium, quinolinium, quaternary phosphonium and quaternary ammonium based cations and smaller anions such as chloride and bromine. The focus of the study was on the effect of the cation on the lipophilicity threshold range; on the apparent premise that anions may have little or nothing to contribute to the cytotoxicity of the ionic ligand.
To measure lipophilicity of the cation, a reversed phase gradient HPLC was used to give k (retention factor) values. A photometric 96 well plate assay was also conducted on these ionic liquids and the results of the two tests were correlated with each other to give a result of the cytotoxicity of the IL regarding the rat lymphoma cell line, IPC 81. The results of the study showed that, due to the structural variation of the IL, the lipophilicity dominates over the toxicity. The same result is expected to occur in the whole organism. Kinetics, thermodynamics and the metabolic rate are seen to modulate this result in the organism.
The application of ionic liquids sometimes requires the use of the lipophilicity factor which can be used in multiphase systems for organic synthesis or for extractions, because it is linked to low miscibility of ionic liquids with water. This gives a positive result for ionic liquids to be used in the treatment of cancer or drug related systems; although an environmental factor is still to be of concern. In order to synthesize medical products, a comparison between the risk and benefits must be made to assess the capability of the product and its usefulness to society.
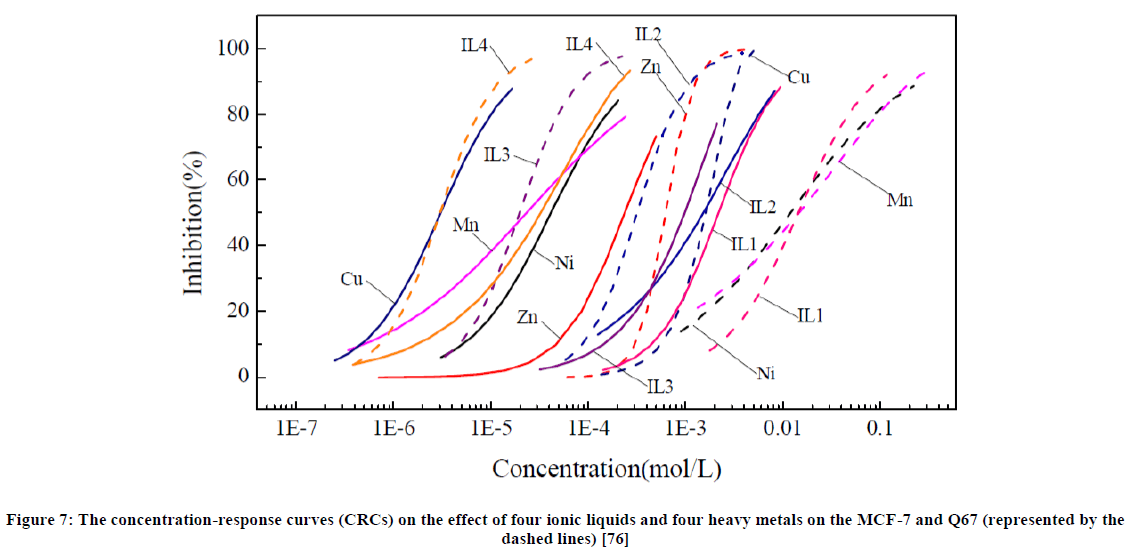
Using the MTS assay method and microplate toxicity analysis, Chenglin Wang et al., [76] assessed the effect that the toxicities of four ionic liquids and four heavy metals have on MCF-7 (human breast cancer cells) and photobacteria Q67 (Vibrio qinghaiensis sp). The toxicity of the selected ionic liquids and heavy metals towards the chosen human cells and bacteria were determined using MTS assays. The latter involved the addition of ionic liquids to a culture of cells in well plates. The inhibition ratio was calculated using the equation:
E=(OD0-OD)/OD0 × 100%
The toxicity was obtained using data from a plot of concentration versus response data.
An alkyl chain effect is caused by the toxicity of ionic liquids on the cell MCF-7 and Q67 (Figure 7). These cells seem to have a more sensitive effect when they are exposed to heavy metals than to ionic liquids. The study showed that Cu (heavy metal) was the most toxic compound to the MCF-7 cell culture. The least toxic to the cancer cells was IL-1. The order of toxicity of ionic liquids on the cancer cells was found to be [C12mim][Cl] > [omim][Cl] > [hmim][BF4] >[emim][BF4]. The toxicity of ionic liquids seems to be directly proportional to the alkyl chain length. Ionic liquids resulted in lower toxicity values compared to that of the heavy metals. This suggests that ionic liquids have a bright future in the field of medicine.
Figure 7: The concentration-response curves (CRCs) on the effect of four ionic liquids and four heavy metals on the MCF-7 and Q67 (represented by the dashed lines) [76]
Mohammad H. Fatemi and Parisa Izadiyan [77] considered 227 ionic liquids in their study to accurately establish the cytotoxicity of each specific compound based on the structural design of the ionic liquids. The total of 227 ionic liquids which were distributed based on cation source as follows: 94 imidazolium, 53 pyridinium, 23 pyrrolidinium, 22 ammoniums, 15 piperidinium, 10 morpholinium, 5 phosphonium and 5 quinolinium cations in conjunction with 25 different types of anions. These ionic liquids were tested against the Leukaemia Rat Cell Line (IPC-81).
The toxicity was evaluated from structural parameters obtained by employing Quantitative Structure Toxicity Relationship (QSTR) methods. The latter involved the use of Genetic Algorithms (GA), Multiple Linear Regressions (MLR) and Multilayer Perceptron Neutral Network (MLPNN) techniques. QSTR models which were used to estimate the cytotoxicity, were subjected to the principles of the Organization for Economic Co-operation and Development (OECD). Furthermore, the results predicted by using the models had very high correlation with the experimental results obtained by these researchers who have made a useful contribution to theoretical framework required to understand the effect of structure on the cytotoxicity of an ionic liquid.
The factors that were considered when evaluating the two QSTR models created were robustness, goodness of fit and the ability to predict the cytotoxicity. In the study, the factors which were found to contribute to cytotoxicity were:
 Symmetry of the cations of the ionic liquids
 Nature of the substituents on the cations of the ionic liquids
 Distribution of charge of the cation of the molecule including
 Increase in the number of heavy atoms in the anions.
This was found to be the case for cytotoxicities which were obtained using the F12 (Ham's F12 (F-12 Nutrient Medium)) rat cell line. Using experimental methods, these researchers succeeded in determining the toxicities of several ionic liquids by implementing the conditions listed as follows: using cations having high symmetries and fewer substituents with heteroatoms such O and N; choosing smaller anions with lesser F atoms and lipophilic fragments: The general findings of the study were:
 The cytotoxicity was largely determined by the nature of the cation than the anion
 Imidazolium-based ionic liquids were more toxic than pyridinium-based ones.
There are several studies on the effect of the side chain on the cation on the toxicity to ionic liquids. One such investigation by J. Ranke et al., [77] involved studying the biological effects of selected methyl- and ethyl-imidazolium based ionic liquids (Figure 8) on luminescent bacteria and two cell lines from rats; namely, IPC-81(leukaemia cells) and C6 (glioma cells).
The alkyl chains attached to the two nitrogen atoms of the imidazolium ring which constitutes the cation were designated as R1 and R2.
R1 is either methyl or ethyl, R2 is one of the C3–C10 n-alkanes. Anions, A–, studied were chiefly chloride, tetrafluoroborate, and hexafluorophosphate, but in some cases also bromide and toluenesulfonate [78] were also used.
The number of carbon atoms on R1 and R2 ranged from 3 to 10. For the bacterium standard, 30 min bioluminescence inhibition assay was performed on Vibrio fisheri treated with water- soluble ionic liquids. The rat cells (IPC-81 and C6 glioma) in cell cultures were exposed to varying concentrations of ionic liquids. Prior tests were done to ascertain that the concentrations of solvents used did not have a toxic effect on the cells being tested.
Linear-regressions were performed on logarithms of EC50 values obtained experimentally, was used to determine the structure-activity relationship. It was ascertained that elongated n-alkyl chain in both the R1 and R2 positions caused an increase in toxicity. R1-methylimidazolium ionic liquid displayed less toxicity than the R1-ethylimidazolium ionic liquids when tested against the cell lines and was therefore preferred. EC50 values of short chains were usually lower and therefore more likely to be used in applications. The ionic liquids with longer alkyl chain seemed to show higher toxicity in all test systems conducted. The role of anions in contributing to eco-toxicity of the ionic liquids tested, were found to be minimal.
The above summary of results suggests that the toxicity potential of an ionic liquid can be reduced by designing ionic liquids by avoiding substituents which have shown to enhance cytotoxicity of the ionic liquid. Furthermore, the purities of the ionic liquids as well as the purities of all solvents used in experimental toxicity assessments should be known in order to be assured that the reported toxicities are attributable only to the ionic liquid used. Thus the risk potential of ionic liquids should not be confined to toxicity of the ionic liquid alone, but also to exposure to impurities in the ionic liquids. This testing can push it further into API and other cancer related therapies to improve them. A broader set of ionic liquids should be used to test the health factor and risk factor before an investigation such as the above is conducted.
Hsiu-Liang Chen et al., [79] considered the properties of ionic liquid, which allow ionic liquids to be used in many processes. Before ionic liquids can be used in industrial processes, the toxicity of the molecule and its effect on environment had to be evaluated. The study conducted involved 21 imidazolium based IL and their cytotoxicities was assessed using a colorimetric assay in conjunction with cell counting kit-8 (CCK-8) and WST-8,2-(2-methoxy-4-nitro-phenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium-mono-sodium salt) against the human lung carcinoma A549 cell line. The ionic liquids used were synthesized using various cations and anions.
The toxicity of the 1-alkyl-3-methylimidazolium and 1-vinylimidazole cation showed that longer alkyl chains resulted in higher toxicities. The anion species also had a dominating factor on the cytotoxicity of these IL compounds. The result showed C9(Vim)2(NTf2)2 < C6NTf2 < C9NTf2 < C12NTf2 when the ionic liquids contained the NTf2- anion although the cation seemed to follow the opposite trend in terms of toxicity:
C9(Vim)2(NTf2)2 < C6NTf2 < C9NTf2 < C12NTf2
The EC50 values are largest for tetrafluoroborate, whereas bis(trifluoromethylsulfonyl)imide results in the lowest EC50 value. It can be said that some ionic liquids influence human health depending on the side chain and anion comprising the ionic liquids. In order to use ionic liquids against certain cells, the properties and structural features should be adjusted to accommodate such specifications.
Gouveia et al., [80] reported on the use of imidazolium-based, pyridinium-based and choline based ionic liquids and their toxic effect on biomaterials present in different organisms; such as human cell HeLa, A salina from the crustacean family and bacteria. The anions of the ionic liquids were bromide ions and several amino acids. The ionic liquids produced were all water soluble and RTILs liquids (room temperature ionic liquids). Methods to evaluate the toxicity of the compounds, included toxicity assays called MTT assays which measure the metabolic activity of a cell. Antibacterial activity was also evaluated on the bacteria cells.
The toxicity of the three organisms were measured namely the HeLa cell, A. salina and the bacteria cells. The result of the crustacean cell, A. salina shows that the toxicity of ionic liquids on such cells is dependent on the cation and anion of the IL. The study showed that when the biomaterials choline and amino acids are present in the ionic liquids the cytotoxicity was lowered approximately 10 times than for imidazolium and pyridinium ionic liquids. This suggests that these ionic liquids are promising compounds that can be used in many applications.
It is clear from the foregoing that different types of ionic liquids can be used to create drugs that may resist cancer cells. Another study to illustrate this involves the assessment of toxicity of the following ionic liquids: 1-alkoxymethyl-3-hydroxypyridinium chloride, saccharinate and acesulfamates when exposed to the IPC81 rat promyelocytic leukaemia cell line. This investigation was conducted on a cellular and molecular level by Stasiewicza et al., [81].
Results of the above study indicated that the enzyme, acetylcholinesterase (Ache) activity was only inhibited by the long chain alkoxy methyl chains at specific concentrations. On account of the interaction between the anionic site in the enzyme and the quaternary nitrogen in the IL cation (caused by the hydroxyl group present in the pyridinium ring), the Ache activity is lowered. During the enzymatic assay method, the anionic component was varied. It was seen by this investigation that the regardless of the anion present, the cation that contains the longer chain inhibits the growth of cells more effectively. In summary it can be said that these compounds decreased the biological activity and toxicity of the ionic liquids.
Ionic liquids in topical applications
Dobler et al., [82] reported that ionic liquids can be used in topical drug delivery systems. This is based on the premise that ionic liquids have the ability to increase the solubility of drugs and allow for increased topical and transdermal delivery. In this study two emulsions which contain hydrophobic and hydrophilic ionic liquids, namely, [Bmim][PF6] and [Hmim][Cl] were synthesized and tested in vitro skin penetration. A fluorescent dye was used to show that in the presence of ionic liquids drug penetration is enhanced.
Ionic liquids in anti-tumour agents
Francesco P. Ballistreri et al., [83] designed and synthesized 10 derivatives of ionic liquids (Figure 9). The products obtained were tested against two cancer cell lines, namely, MCF7 and LNCap (breast cancer cells and prostate cancer cells respectively).
Figure 9: In vitro biological evaluation of some derivatives [83]
Log GI50 (concentration of drug to cause 50% reduction) values indicated that anti-proliferative activity was shown by derivatives 1, 2, 3, 5, 6, 8 and 9. Furthermore compounds 5 and 6 exhibited higher activity than others in the list. They thus have potential to be used as antitumour agents.
Ana Rita Dias et al., [84] engaged in a study which considered ways of imbuing ionic liquids with antitumour properties. They synthesized ionic liquids, with this potential, by varying the length of the cation side chain, the functional group on the side chain and the type of anion used. These IL compounds were also tested on various cancer cell lines and their toxicities were tested using the MTT assay which involves the reduction of tetrazolium dye MTT by the enzyme of the cells. These researchers found that toxicity could be manipulated by switching cation and anion pairs in the sets of chosen ionic liquids.
Uses of Ionic liquids in tuberculosis medications
In comparison to the extensive applications of ionic liquids in medical formulations for treatment of cancer, there are relatively few reports on the uses of ionic liquids in anti-tuberculosis medications. The uses of ionic liquids in treatment of tuberculosis may be classified as follows:
 As catalysts for the synthesis of anti-tuberculosis agents
 As solvents for antibiotics against tuberculosis
 As solvents and catalysts in synthesis of anti-tuberculosis drugs
 As a component in the synthesis of compounds for delivery of drugs needed in the human body for combatting the effects of tuberculosis.
Ionic liquids in anti-tuberculosis studies
A green protocol of 6-(adamantan-1-yl)-2-substituted imidazo[2,1-b][1,3,4]thiadiazoles (AITs) was reported [25] using 1-(adamantan-1-yl)-2-bromoethanone and 5-alkyl/aryl-2-amino-1,3,4-thiadiazoles on a nanomaterial base in ionic liquid media. The anti-tuberculosis activity of imidazothiadiazoles against M. tuberculosis using H37Rv strain was ascertained. Among the tested compounds, 6-(adamantan-1-yl)-2-(4-methodylphenyl)imidazo[2,1-b][1,3,4]thiadiazole showed potent inhibitory activity with an MIC (Minimum Inhibitory Concentration) value of 8.5 μM. Interestingly, the inhibitory effect of this molecule against M. tuberculosis was comparable to standard drugs such as Pyrazinamide, Streptomycin and Ciprofloxacin. In the above report, replacement of organic solvents with ILs significantly improved yields of the product to greater than 90%. In particular, [BMIM]+[BF4]- and [BMPY]+[PF6]- were found to be better ILs and the chosen [BMIM]+[BF4]- for the preparation of compounds due to its solubility in water.
The method was found to be green protocol for the preparation of alkyl or aryl substitution on thiadiazoles ring. The in vitro Alamar Blue assay was employed to determine the anti-tuberculosis activity of AITs against the M. tuberculosis H37Rv strain. Most AITs showed inhibitory activity towards the M. tuberculosis H37Rv strain suggesting that AITs possess significant anti-TB activity. Compounds: 6-(adamantan-1-yl)-2-phenylimidazo[2,1-b][1,3,4]thiadiazole, 6-(adamantan-1-yl)-2-(4-methoxyphenyl) imidazo[2,1-b][1,3,4]thiadiazole and 6-(adamantan-1-yl)-2-(trifluoro methyl)imidazo[2,1-b][1,3,4]thiadiazole displayed relatively low MIC values of 10.5, 8.5 and 12.5 μM respectively when compared to the other structurally related compounds. Compounds with electron-donating, phenyl, 4-methoxy phenyl and methyl substituents attached to the imidazo-thiadiazole scaffold were favourable for activity against M. tuberculosis.
A high solubility of antituberculosis antibiotic drugs was reported recently [85]. Ammonium ionic liquids containing isoniazid and pyrazinecarboxamide demonstrated promising perspectives in processing the drug. Solid-liquid equilibrium (SLE) measurements have been made using a dynamic (synthetic) method. The solid-liquid phase equilibria were described using six different correlation equations which revealed a relatively good description with an acceptable standard deviation temperature range. In this work, ammonium ionic liquids containing two methyl groups were studied. In particular, didecyldimethylammonium nitrate [DDA][NO3]; ethyl(2-hydroxyethyl)dimethylammonium [C2][NTF2] and (benzyl)dimethyl alkylammonium nitrate [BA][NO3]. The aim of the work was to improve the solubility of isoniazid and pyrazinecarboxamide, the two most common antibiotics against tuberculosis, in alternative solvents namely ammonium based ionic liquids. The (solid + liquid) phase diagrams for isoniazid and pyrazinecarboxamide in series of ammonium ionic liquids have been measured by a dynamic method from 292.15 K to 418.48 K.
From the data reported, it could be concluded that, for both drugs, the solubility trend is identical. Thus, the highest solubility for both isoniazid and pyrazinecarboxamide was observed in [DDA][NO3] and was followed by [C2][NTf2]. The lowest solubility was noticed for [BA][NO3]. Comparing both nitrate ionic liquids, the higher solubility of drugs in [DDA][NO3] than in [BA][NO3] might be caused principally by the interactions that both cations might create with the examined drugs. Thus, ammonium ionic liquids showed to be the best solvents for antibiotic antituberculosis drugs. Among studied ILs, the didecyldimethylammonium nitrate is the best solvent for isoniazid and pyrazinecarboxamide. The studied ionic liquids are appropriate solvents for the drug manufacturing and such solvents can be suitable for pharmaceutical processing [86,87]. The binary systems composed of isoniazid or pyrazinecarboxamide and ionic liquids form the solid-liquid phase equilibria with a eutectic point. The favourable solubilities in alternative solvents obtained within this study for the investigated drugs, accompanied by the above mentioned beneficial properties of ILs, can allow ionic liquids to compete in a safer mode with solvents routinely used in pharmaceutical industry.
A series of rac-(2S)-[(R)-(4-substituted phenyl){[4-(4-substituted phenyl)-1,3-thiazol-2-yl]amino}methyl]cyclohexanone derivatives were recently synthesized and studied for their antitubercular activities [88]. The potential anti-TB activity against clinically isolated Mycobacterium tuberculosis and virulent H37Rv strains were studied. All the synthesized compounds showed potent activity against M. tuberculosis strains at MIC ranging from 50 μg/ml to 6.25 μg/ml of concentration. The compounds were screened against the clinically isolated M. tuberculosis and virulent strain of H37Rv and displayed pronounced anti-mycobacterial activity at concentrations 200 g/1 ml, 100 g/1 ml, 50 g/1 ml of DMSO. Few of the compounds showed inhibition of growth of organisms at 50 μg/ml even after 6 weeks of incubation. The compound, rac-(2S)-2-[(R)-(4-hydroxyphenyl){[4-(4-hydroxy-phenyl)thiazol-2-yl]amino}methyl] cyclo-hexanone displayed maximum antibacterial, antifungal and anti-tubercular activity at 6.25 μg/ml due to –OH substitution on ring at para positions which enhanced the effects leading to compounds having excellent activity in comparison to other compounds either with unsubstituted or substituted aromatic ring. Moderate anti-tubercular activities have been observed for compounds which possess mycobacterial activity due to presence of –OCH3, -Cl, -NO, and -F.
Anti-tubercular activity was investigated for pyridinium and benzimidazolium chloride ionic liquids [89] followed by antimicrobial activities. Among them, N,N’-bis[3-(1-alkoxymethyl)pyrdinium chloride]methylenediamines, 1-undecyloxymethyl-3-(1-benzimid-azolmethyl-amino)-pyridinium, 1-undecyloxy-methyl- and 1-dodecyloxymethyl-3-[1(benzotriazole-1-yl)methylamino]pyridinium chlorides exhibited strong activity against a wide range of pathogenic bacteria including M. tuberculosis strain H37Rv. It was found, in the TAACF (Tuberculosis Antimicrobial Acquisition and Coordinating Facility) screening program, that they are active (MIC < 6.25 μg/ml and Inhibition > 92%) and had potential for use in the treatment of mycobacterial infections. It is known that compounds that possess two quaternary ammonium moieties in the molecule are strongly active against bacteria. The new observation was that pyridinium salts of formula weight, higher than 445 with four and five nitrogen atoms were potential new biocides [89].
A class of functionalized pyridinium and alkoxymethyl hydrophobic ionic salts were studied for their anti-microbial and anti-tubercular activities [90]. Some of them exhibited strong activity against bacterial strains. It was found that, ammonium halides such as 1-alkylpyridinium halides exhibited strong bacteriostatic activities against gram positive bacteria rather than those against gram negative bacteria. The activity of the studied compounds depends on the length of the substituent. The most favourable alkoxymethyl group was that which contains 10-12 carbon atoms in 1-alkoxymethyl(carbamoyl)pyridinium compounds. The synthesized compounds were tested against Mycobacterium tuberculosis H37Rv (ATCC 27294). It was found in the preliminary screening for anti-tubercular activity, according to an international program with the TAACF, that few of the pyridinium and alkoxymethyl compounds are active with MIC >6.25 μg/ml and inhibition of 40%. In summary, compounds containing quaternary ammonium moieties in the molecule, pyridinium and alkoxymethyl ionic salts which have strong anti-microbial activities. Table 1 shows a list of ionic liquids which have the potential to act against cancer and tuberculosis cell lines.
| S. No | Ionic liquids used | Activity | Ref |
|---|---|---|---|
| 1 | Imidazolium based Cations: 1-(2-Hydroxyethyl)-3-methylimidazolium (C2OHMIM) 1-(2-(2-Methoxyethoxy) ethyl)-3-methylimidazolium (C5O2MIM) Choline: 1-n-Butyl-3-methylimidazolium (C4MIM), 1-n-Butyl-2,3-dimethylimidazolium (BDMIM), 1-n-Octyl-3-methylimidazolium (C8MIM), 1-n-Decyl-3-methylimidazolim (C10MIM), Tetra-n-hexyldimethylguanidinium (DMG), Tri-n-hexyl-n-tetradecylphosphonium (P66614), Tri-n-octylmethylammonium (Aliquat). Anions: Tetrafluoroborate (BF4), Hexafluorophosphate (PF6), Dicyanoamide, Acesulfame, Sacharin, Bistrifluoromethane-sulfonimidate (NTf2). | Antitumor agent: In this study, two colon carcinoma HT-29 and CaCo-2 cell lines were used to evaluate the toxicity of ILs. In both cells, C4MIM, C2OHMIM, C5OHMIM and cholines were the least toxic cations independently of the anion. Increasing the length of the substituent chain contributed to a significant increasing of MIM toxicity and thus, C8MIM and C10MIM had a strong toxic effect on the cells. The most toxic ILs were Aliquat acesulfame, Aliquat saccharin, Aliquat DCA and DMG PF6. The results suggested that the presence of the NTf2 anion decreases the toxicity to a large extent, independently of the cation and for both cell types. | [91] |
| 2 | Ethyl-, butyl-, octyl-, benzyl-, and allyl substituted 1-alkyl-3-methyl imidazolium, alkyl pyridinium, N-alkyl-N,N-dimethyl-N-(2-hydroxyethyl) ammonium (choline derivatives) and alkyl-triethylammonium salts (1-butyl- ([C4mim]+), 1-hexyl- ([C6mim]+) and 1-decyl-3-methylimidazolium ([C10mim]+) chloride, hexafluorophosphate and tetrafluoroborate salts) | Antitumor agent: The toxicity of ionic liquids and precursor salts on HeLa cells was studied, showing that choline-derivatives and alkyl-triethylammonium salts were less toxic than their pyridinium and imidazolium analogues. It was found that the length of the alkyl-substituents on the cations had a large effect on the toxicity. The observed toxicities were higher when cells were culture in the absence of foetal bovine serum with the exception of salts incorporating the ethyl group, suggesting that these salts may have an additional mode of action different from salts with longer alkyl substituents. The investigation provided evidence indicating that [C2mim][BF4] induces apoptosis in cervical cancer HeLa cells. | [92] |
| 3 | Ionic liquids containing the cations pyridinium, pyrrolidinium, piperidinium, or imidazolium with various alkyl chain lengths, and the anions bromide, bis(trifluoromethanesulfone)imide (Tf2N), trifluoromethylsulfonate (TfO), or nonafluoromethylsulfonate (NfO). Three new hydrophobic and task-specific ILs: 1-(3-cyanopropyl)-1-methylpiperidinium [(CF3SO2)2N], 1-methyl-1-[4,5-bis(methylthio)-pentyl]piperidinium [(CF3SO2)2N] and 1-methyl-1-[4,5-bis(methylthio)-pentyl]pyrrolidinium [(CF3SO2)2N] | Antitumor agent: Both water-soluble and hydrophobic ionic liquids, an increase in the alkyl chain length of the substituents in the cation resulted in increased toxicity, and that the length of alkyl chain substituent influenced the toxicity of the cation ring. The TSIL with functionalized side chains have significantly lower toxicity compared to the ionic liquids with ‘‘simple” alkyl side chains. All the ionic liquids tested in this work have higher toxicity than that of sodium bromide with 1-methyl-1-propylpyrrolidinium bromide being least toxic or the ‘‘greenest’’ among the panel of other ionic liquids. In vitro cytotoxicities were measured for ionic liquids using the MCF7 human breast cancer cell line IC50 values of the ionic liquids toward the MCF7 cells ranged from 8 µM to 44 mM. The toxicity depended significantly on the nature of the cations and anions, especially when the cations contained a long side chain. | [58] |
| 4 | 7 Phosphonium and ammonium-based ionic liquids | Antitumor agent: A grouping consisting of breast (MDA-MB-231), non-small cell lung (NCI-H23 and NCI-H522), colon (SW-620 and COLO 205), melanoma (UACC-62, MDA-MB-435 and LOXIMVI), ovarian (OVCAR-5 and OVCAR-3) and CNS (U251 and SF-295) cell lines was used to screen in vivo activity. The anti-tumor activity of phosphonium and ammonium-based ionic liquids has been determined using NCI 60 human tumor cell lines. With increase in alkyl chain length significant improvement in anti-tumor activity can be achieved. In general, phosphonium-based ILs were found to be more active then ammonium ILs. Results clearly show that the ‘tunability’ of ionic liquids (changing the cation/anion combination and modifying the cation with different substituents) can control their biological activity and cytotoxicity. This property of ILs could play a major role in their therapeutic applications, such as in cancer therapy. Further investigation into the mechanism of action with more extensive screening of these compounds may lead to their potential utility as drugs. | [73] |
| 5 | 80 ILs from different classes of cations and anions: Imidazolium (IM), dimethyl-guanidinium (dmg) and tetramethyl-guanidinium (tmg), methyl-pyrrolidinium (MPyr), 2-methyl-1-ethyl-pyridinium (2-MEPy), quaternary ammonium (benzyltriethyl-ammonium- BzTEA; phenyltrimethyl-ammonium–PhTMA; tri-n-octyl-methylammonium-Aliquat) and tri-n-hexyl-tetra-n-decylphosphonium (P6,6,6,14). Dicyanoamide-[DCA] and bis(trifluoromethanesulfonyl)-amide-[NTf2] | Antitumor agent: The work was performed on confluent human colon cancerous cells (CaCo-2). The toxicity of the cation increases when longer alkyl chains are present. The presence of a benzyl group does not seem to contribute to non-toxic [MIM] based ionic liquids, however, introduction of a COOH group lead to a great reduction of [C10MIM] toxicity. In addition, introduction of ether functionality in the dimethyl-guanidinium lowered toxicity of this cation. The type of anion can affect strongly toxicity of the ionic liquid, and some seem to have a bigger impact in the overall toxicity than others, as [NTf2] and [DCA]. The results concluded that [C4MIM], [C2OHMIM] and [C4MPyr] are potential good candidates for building human friendly ionic liquids, but some combinations with different cations exist that were very promising, as well: [C5O2MIM][PF6], [C10O2HMIM][PF6/DCA], [2-MEPy] [EtSO4], [BzTEA][DCA], [BzIMBz][Cl], [BzMIM][Cl], [PhTMA][Cl/DCA], [(di-h)2dmg][DCA/NTf2], [(eb)(mb)dmg][Cl/BF4], [(eb)2dmg] [BF4] and [(C3O)4dmg][Cl]. | [13] |
| 6 | Imidazolium based ionic liquids: 1-dodecyl-3-methylimidazolium chloride, 1-dodecyl-3-methylimidazolium tetrafluoroborate, 1-hexadecyl-3-methylimidazoium chloride, 1-octadecyl-3-methylimidazolium chloride, 1-octadecyl-3-methylimidazolium hexafluorophosphate, 1-octadecyl-3-methylimidazolium bis(triflic)imide and 1-octadecyl-3-methylimidazolium tris(pentafluoroethyl) trifluorophosphate. | Antitumor agent: The anti-tumor activity of imidazolium-based ionic liquids has been determined using NCI 60 human tumor cell lines (Leukemia, Non-small Lung cancer, CNS cancer, Melanoma, Breast cancer, Ovarian cancer, Renal cancer, Prostate cancer cells (US National Cancer Institute’s 60 Tumor Cell Line Panel)). Structure-activity relationship showed that chain length of N-3 alkyl substitution plays a significant role in the anti-tumor activity and cytotoxicity. With increase in alkyl chain length from C-8 to C-12 significant improvement in antitumor activity was observed, while cytotoxicity towards the tumor cell lines remained low as evident by higher LC50 (>100 µM) values in most of the cases. Further increase in alkyl chain length enhanced both the anti-tumor activity and the cytotoxicity of these compounds towards the tumor cell lines. These results clearly showed that the ‘tunability’ of ionic liquids (by changing the cation/anion combination and by modifying the cation with different substituents) can control their biological activity and cytotoxicity and could play a major role in their therapeutic applications such as in cancer therapy. | [93] |
| 7 | Ionic liquids based on betulinic acid: Cholinium salt of betulinic acid-glycine [cholinium][BA-Gly], benzalkonium salt of betulinic acid-glycine [benzalkonium][BA-Gly], [benzalkonium][betulinate], [cholinium][betulinate]. | Antitumor agent: Ionic derivatives of betulinic acid have exhibited high cytotoxicities toward several cancer cell lines. These ionic derivatives have shown much higher inhibitory effects against different cancer cell lines such as melanoma A375, neuroblastoma SH-SY5Y and breast adenocarcinoma MCF7. For A375 cell lines, the derivative [cholinium][betulinate exhibited a low IC50 value of 36 µM (vs 154 µM for betulinic acid); for MCF7 cell lines, the [cholinium][betulinate also exhibited a low IC50 value of 25 µM (vs 112 µM for betulinic acid). The high cytotoxicity of these new derivatives can be linked to their greatly improved water solubility. The cell viability studies based on both MTT and LDH assay methods have confirmed the high inhibitory effect of our ionic derivatives of betulinic acid (particularly 4and5) against different cancer cells. | [94] |
| 8 | 1-octyl-3-methylimidazolium chloride ([C8mim][Cl]) | Antitumor agent: In this study, cytotoxicity and responses of the cellular antioxidant system of 1-octyl-3-methylimidazolium chloride ([C8mim][Cl]) on human hepatocarcinoma QGY-7701 cells was evaluated. The results showed that [C8mim][Cl] can inhibit QGY-7701 cell growth and decrease their viabilities in a dose-dependent manner. The results also revealed that [C8mim][Cl] exposure can induce apoptosis in the [C8mim][Cl]-treated QGY-7701 cells. In addition, the results of biochemical assays showed that [C8mim][Cl] exposure causes overproduction of reactive oxygen species (ROS), inhibits superoxide dismutase (SOD) and catalase (CAT) activities, decreases reduced glutathione (GSH) content, and increases the cellular malondialdehyde (MDA) level. These results suggested that ROS-mediated oxidative stress may be responsible for the apoptosis induced by [C8mim][Cl] in human hepatocarcinoma QGY-7701 cells. | [95] |
| 9 | The combination of anionic ampicillin with appropriate ammonium, imidazolium, phosphonium and pyridinium cations yielded active pharmaceutical ingredient ionic liquids (API-ILs) | Antitumor agent: The combination of ampicillin with organic cations in place of sodium has yielded ionic liquids that showed low IC50 and LD50 values against five different cancer cell lines: T47D (breast), PC3 (prostate), HepG2 (liver), MG63 (osteosarcoma), and RKO (colon). Furthermore, very low toxicity against two primary cell lines-skin (SF) and gingival fibroblasts (GF) indicated that the majority of these compounds are nontoxic to certain healthy human cells. Outstanding cytotoxic and antiproliferative activities against several tumor cell lines, with IC50 and LD50 values in the low micromolar range, associated with low activity against primary fibroblasts was observed for[C2OHMIM][Amp]. On the other hand, [TEA][Amp], [Cholin][Amp], and [EMIM][Amp]showed IC50 values in the nanomolar or low micromolar range against some of the cancer cell lines tested, as well as very low toxicity toward normal cells. The selection of the appropriate counter-ions can explain these antitumor activities due to the stronger and more stable cation-anion interactions relative to the sodium analogue. | [96] |
| 10 | 1-methyl-3-octylimidazolium bromide | Antitumor agent: In the study, [C8mim]Br exerted cytotoxicity on hepatocarcinoma HepG2 cells via inducing apoptosis of the cells. Meanwhile, caspases probably played a key role in the initiation and execution of apoptosis of HepG2 cells induced by [C8mim]Br. The results suggested that [C8mim]Br induced apoptosis of HepG2 cells may be mediated by the mitochondrial pathway. | [97] |
| 11 | 1-n-butyl-3-methylimidazolium hexafluorophosphate 1-n-butyl-3-methylimidazolium tetrafluoroborate 1-n-butyl-3-methylimidazolium chloride 1-n-butyl-3-ethylimidazolium chloride 1-n-hexyl-3-methylimidazolium chloride 1-n-decyl-3-methylimidazolium chloride | 1-n-butyl-3-methylimidazolium hexafluorophosphate 1-n-butyl-3-methylimidazolium tetrafluoroborate 1-n-butyl-3-methylimidazolium chloride 1-n-butyl-3-ethylimidazolium chloride 1-n-hexyl-3-methylimidazolium chloride 1-n-decyl-3-methylimidazolium chloride | [98] |
| 12 | Methyl and ethyl imidazolium ionic liquids with chloride, PF6 and BF4 | Antitumor agent: The results suggested that the use of methyl imidazolium derivatives should be preferred to ethyl-imidazolium compounds because of their lower toxicity toward V. fischeri and the mammalian cell lines. IPC-81 (leukemia cells) and C6 (glioma cells) rat cell lines were also studied. Generally short-chain derivatives seemed to be favourable because they exhibit higher EC50 values. The ionic liquids with the longest alkyl chain length showed higher toxicity in all test systems used. | [99] |
| 13 | 74 ionic liquids with imidazolium, pyrrolidinium, pyridinium, quinolinium, quaternary phosphonium and quaternary ammonium cations with chloride, bromide, BF4 and PF6. | Antitumor agent: The cytotoxicity of ILs performed on C6 rat glioma cells and IPC-81 rat leukemia cells. The cytotoxicity as estimated by the tetrazolium reductase assay was increasing with the lengths of the 1-alkyl chains from C4MIM to C10MIM. Consistently, cell proliferation rates were decreasing with increasing lengths of the 1-alkyl chains. The results revealed the correlations between lipophilicity, cellular sorption, and cytotoxicity. | [79] |
| 14 | Imidazolium-based ionic liquids with ethyl, butyl and hexyl alkyl side chains and 27 different anions, as well as lithium and sodium as a counter ion in combination with those 27 different anions | Antitumor agent: The results demonstrated using the IPC-81 rat leukemia cell line and most of the commercially available anions investigated showed no or only marginal cytotoxic effects. The anionic moieties with lipophilic and hydrolysable structural elements seem to be of considerable relevance with respect to the observed cytotoxic effects. | [100] |
| 15 | Seven different head groups with varying alkyl side chains and functionalized side chains, anions, chloride, bromide and iodide. | Antitumor agent: WST-1 cytotoxicity assay was used for a preselection of toxicologically favourable structural elements for a large number of ILs. The results confirmed a linear correlation between IL cation lipophilicity and cytotoxicity using an HPLC derived lipophilicity parameter. Considering the structural design of ionic liquids cations, the side chain was the main effector to alter cytotoxicity. According to their relatively low lipophilicity, short functionalised side chains could diminish the observed cytotoxicity compared to non-polar alkyl chains. The used acute cell viability assay and the tested IPC-81 leukemia cell line provided a useful tool for the screening of a large number of ILs, allowing the identification of possible toxicophore substructures. | [101] |
| 16 | 1-decyl-3-methylimidazolium chloride ([C10mim][Cl]), 1,3-dimethylimidazolium methyl sulfate ([C1mim][MSO4]). | Antitumor agent: The imidazolium derived ILs tested for their biological activity in the Caco-2 human colon adenocarcinoma cell line. The results indicated that the cytotoxicity of ILs was dependent on their chemical structure. In this respect, the fact that methylimidazolium alkyl sulfate derivatives have a relatively low cytotoxic effect can be considered as a positive attribute. | [102] |
| 17 | 1-Alkoxymethyl-3-hydroxypyridinium acesulfamates, 1-Alkoxymethyl-3-hydroxypyridinium saccharinates, 1-Alkoxymethyl-3-hydroxypyridinium chlorides | Antitumor agent: The results showed that the postulated molecular modifications at both cationic and anionic sites result not only in a lower overall biological activity, and hence a lower potential toxicity of the new type of ILs, but also in enhanced biodegradability. The cytotoxicity data with IPC-81 rat leukaemia cell lines showed EC50 for acesulphamates and saccharinates to be higher than the values for the chloride analogues. Also, a slight alkoxymethyl chain length effect on the overall cytotoxicity was discernible. | [103] |
| 18 | 3-butyl-1-methyl-1H-imidazolium tetrafluoroborate | Antitumor agent: The cell toxicity of IL to Chinese hamster lung fibroblast cells (V79 cell line) was studied. The half inhibition concentration (EC50) of [bmim]BF4 was around 5 mM. The cytotoxicity of [bmim]BF4 at a concentration of less than 6 mM was reversible for the V79 cell line, because cell morphology and proliferation rate returned to normal after the removal of the IL from the culture medium. This finding suggested that the IL [bmim]BF4 could be used as a tool to control mammalian cell proliferation rate. | [104] |
| 19 | N,N,N-trialkylammonioundecahydrododecaborates | Antitumor agent: The toxicity against V79 mammalian cells, reproduction inhibition of Scenedesmus vacuolatusalgae, and inhibition of acetylcholinesterase were studied. EC50 values for the toxicity against V79 cells range between 9.1 mM for the trimethylammonio derivative and 0.19 mM for the trihexyl derivative. The data demonstrate that increasing hydrophobicity leads to higher toxicity and inhibition for straight alkyl chains. The cytotoxicity strongly increases with the length of the alkyl chain and consequently with lipophilicity. | [105] |
| 20 | 1-butyl-3-methylimidazolium tetrafluoroburate | Antitumor agent: The binding study of curcumin with various surfactant solutions and quenching of curcumin by o-nitrophenol clearly predicted electrostatic interaction of head group of surfactant molecule and deprotonated form of curcumin, while curcumin having greatest affinity for cationic than non-ionic and finally anionic surfactant solution. The cationic or anionic aqueous surfactant solutions suggested hydrophobic interaction of IL [bmim][BF4]. These findings further enhanced potential application of IL as a modulator in solubilization in the micellar system, association of drug-surfactant during drug delivery, micellization and chemistry. | [106] |
| 21 | Poly(ionic liquid-co-N-isopropylacrylamide) | Antitumor agent: The potential practical applications as drug delivery carriers were demonstrated using doxorubicin (DOX) as a model drug. With a lower pH (pH 5.2) and higher temperature (above 37°C), structural collapse of the INPs occurred as well as release of DOX owing to protonated DA departure from the INPs (ionically assembled nanoparticles) and a lower LCST (lower critical solution temperature) at the pathological conditions. The result shows that 80% of DOX molecules were released from INPs within 48 hours at pH 5.2, 43°C, but only 30% of the drug was released within 48 hours at 37°C and pH 7.4. Moreover, drug-loaded INPs exhibited an inhibitory effect on cell growth. | [107] |
| 22 | 1-butyl-3-methylimidazolium bromide | Antitumor agent: Results of ex vivo skin permeation studies through mice skin indicated that the selected IL/o (ionic liquid-in-oil) ME (micro emulsion) exhibited 4-fold enhancement in percent drug permeation as compared to aqueous solution, 2.3-fold as compared to hydrophilic ointment, and 1.6-fold greater permeation than water in oil (w/o) ME. The results of in vivo studies against dimethylbenz(a)anthracene (DMBA)/12-O-tetradecanoylphorbol-13-acetate (TPA)-induced mice skin carcinogenesis demonstrated that the IL/o ME could effectively treat skin cancer in 4 weeks. In addition, the side effects such as erythema and irritation associated with the conventional formulations were not observed. Histopathological studies showed that the use of IL/o ME caused no anatomic and pathological changes in the skin structure of mice. These studies suggested that the use of IL-based ME system can efficiently enhance the solubility and permeability of 5-FU (5-fluorouracil) and hence its therapeutic efficacy. | [108] |
| 23 | 1-butyl-3-methylimidazolium hexafluorophosphate | Antitumor agent: The ex vivo drug permeation studies through rat skin was performed using Franz-diffusion cell and the IL/w based ME showed maximum mean cumulative percent permeation of 99.030 ± 0.921% in comparison to oil-in-water (o/w) ME (61.548 ± 1.875%) and oily solution (48.830 ± 2.488%) of ETO (etodolac). Histopathological studies also demonstrated that IL/w based ME caused no anatomical and pathological changes in the skin. | [109] |
| 24 | bis(trifluoromethylsulfonyl)amide ionic liquids trifluoromethanesulfonate ionic liquid (119) | Antitumor agent: The results reported that the ILs were appropriate solvents for the drug manufacturing and such solvents can be suitable for pharmaceutical processing. Analysing solubilities obtained in the case of the bis(trifluoromethylsulfonyl)amide ionic liquids, it can be noticed that the solubility of the studied antitubercular drug decreases with an increase of the alkyl chain in the cation of the ionic liquid. | [110] |
| 25 | isoniazid and pyrazinecarboxamide in ammonium ionic liquids | Antitumor agent: Ammonium ionic liquids showed to be the best solvents for antibiotic antituberculosis drugs. Among studied ILs, the didecyldimethylammonium nitrate is the best solvent for isoniazid and pyrazinecarboxamide. The studied ionic liquids were appropriate solvents for the drug manufacturing and such solvents can be suitable for pharmaceutical processing. The favourable solubilities in alternative solvents obtained within this study for the investigated drugs, accompanied by the abovementioned beneficial properties of ILs can allow ionic liquids compete in a more “safer” mode with solvents routinely used in pharmaceutical industry. | [111] |
| 26 | Acyclovir, methotrexate and 1-[(5-(p-nitrophenyl) furfurylidene) amino] hydantoin sodium) | Anticancer agent: The investigation reported IL-assisted nonaqueous microemulsion prepared with pharmaceutical accepted components, which can be effectively used in solubilization of a number of drugs molecules that are insoluble or poorly soluble in water and most of organic liquids. This nonaqueous microemulsion may have potential applications a drug delivery carrier. | [86] |
| 27 | Pyrazine-2-carboxamide in bis(trifluoromethyl sulfonyl)amide ionic liquids, 1-Decyl-3-methylimidazolium trifluoromethanesulfonate ionic liquid, Bis(trifluoromethylsulfonyl)amide and trifluoromethanesulfonate based ionic liquids | Antituberculosis agent: The obtained solubility data are in the acceptable range of solubility allowing us to state that the studied ILs are sufficient solvents for the drug and can be suitable for the pharmaceutical processing. Among studied ILs 1-decyl-3-methylimidazolium trifluoromethane sulfonate IL was found to be the best solvent for a carboxamide drug. Considering the solubilities obtained in the case of the bis(trifluoromethylsulfonyl)amide ILs, it can be concluded that the solubility of the studied antitubercular drug decreases with an increase of the alkyl chain in the cation of the IL. | [112] |
| 28 | N-acetyl-L-cysteine, isoniazid, pyrazine-2-carboxamide (pyrazine-2-carboxamide), coumarin, 4-hydroxycoumarin, 4-isobutylacetophenone, ibuprofen and thymoquinone, was tested in a hydrophobic ionic liquid (trihexyl(tetradecyl)phosphonium bis[(trifluoro methyl)sulfonyl]amide). Trihexyl(tetradecyl)phosphonium, bis[(trifluoromethyl)sulfonyl]amide, pyrazinecarboxamide. | Antitumor agent: The most hydrophobic compounds were the most soluble in the ionic liquid and the solubility decreased in the following order 4’-isobutylacetophenone > thymoquinone > coumarin > ibuprofen. This allows to conclude that the studied ionic liquid can be an appropriate solvent for drug manufacturing and suitable for pharmaceutical processing. | [113] |
| 29 | N-acetyl-L-cysteine, isoniazid, pyrazine-2-carboxamide, coumarin, 4-hydroxycoumarin, isobutylacetophenone, ibuprofen and thymoquinone, was tested in trihexyl(tetradecyl)phosphonium chloride | Antitumor agent: Solubility data for several pharmaceutical and bioactive compounds was provided in a hydrophobic trihexyl(tetradecyl) phosphonium chloride ionic liquid. The solubility of most of the compounds was in satisfactory ranges. Pyrazinecarboxamide has the lowest solubility, and thymoquinone exhibited the best solubility, while 4’-isobutylacetophenone was found to be completely miscible in the IL. The most soluble pharmaceutical and bioactive compounds were found for [P6,6,6,14][Cl]. The solubilities of all drugs in trihexyl(tetradecyl)phosphonium chloride were approximately 2 times higher than in the trihexyl (tetradecyl)phosphonium bis[(trifluoromethyl) sulfonyl]amide ionic liquid. | [114,115] |
Table 1: list of ionic liquids
Conclusion
This review has shown that ionic liquids which have a wide range of properties, can be used to synthesize medications which can so speak help “to fight fire with fire’’; that is the toxicities of ionic liquids can be fine-tuned to give the level of toxicity which will perform the required task of destroying malignant cells (in the case of diseases which mutate cells). This fine-tuning is possible because the number of combinations of cations and anions, which constitute ionic liquids, runs into several millions. In simple terms these changes can be made by changing the charges on the ions and the structures of the ions. Considering the fact that a study which involved 227 ionic liquids used only imidazolium, pyridinium, pyrrolidinium, ammonium, piperidinium, morpholinium, phosphonium and quinolinium cations in conjunction with 25 different types of anions, it can be said that the research on the use of ionic liquids as part of medical formulations, is still in its early stages of establishment as viable alternatives to other medications. However, the reported research on the utilization of ionic liquids can make a significant impact on the search for new medical formulations for diseases such as cancer and tuberculosis.
Future Scope
Since new diseases are constantly adding to the older ones which have been wiped or are well-controlled by medicines and other medical treatments, there is a growing need to increase research efforts to make new medications. The latter are needed to replace medicines which are no longer effective due to development of immunity by the target bacteria or other agents which cause the illness. There is also the dire need to replace medicines which have side effects which are so severe that many patients stop using such medications.
From the point of view of resources for research, it is noted that many medical research units have been established, globally, by governments, charitable trusts and non-government institutions. In terms of person-power to do the research, it is worthy of note that an ever-increasing number of graduates are opting for multi-disciplinary projects for their doctoral degrees. This trend is also driven by the scarcity of job opportunities in certain disciplines. The points mentioned above show that there is bright future for researchers in the field of synthesis and testing of new medicines.
Acknowledgment
The author is grateful to Eskom-Holdings and Durban University of Technology for funding.
References
- A.B. Mariotto, K. Robin Yabroff, Y. Shao, E.J. Feuer, M.L. Brown, J. Natl. Cancer Inst., 2011, 103, 117.
- A. Matteelli, A.C. Carvalho, K.E. Dooley, A. Kritski, Future Microbiol., 2010, 5, 849.
- W.W. Nazaroff, B.C. Singer, J. Exposure Sci. Environ. Epidemiol., 2004, 14, 71.
- N.V. Plechkova, K.R. Seddon, Chem. Soc. Rev., 2008, 37, 123.
- S.A. Forsyth, J.M. Pringle, D.R. MacFarlane, Aust. J. Chem., 2004, 57, 113.
- I.J. Villar-Garcia, K.R. Lovelock, S. Men, P. Licence, Chem. Sci., 2014, 5, 2573.
- S. Sowmiah, V. Srinivasadesikan, M.C. Tseng, Y.H. Chu, Molecules., 2009, 14, 3780.
- D. Neto, A. Brenno, J. Spencer, J. Braz. Chem. Soc., 2012, 23, 987.
- I.M. Marrucho, L.C. Branco, L.P.N. Rebelo, Annu. Rev. Chem. Biomol. Eng., 2014, 5, 527.
- M. Smiglak, J.M. Pringle, X. Lu, L. Han, S. Zhang, H. Gao, D.R. MacFarlane, R.D. Rogers, Chem. Commun., 2014, 50, 9228.
- T.P.T. Pham, C.W. Cho, Y.S. Yun, Water Res., 2010, 44, 352.
- K.S. Egorova, E.G. Gordeev, V.P. Ananikov, Chem. Rev., 2017, 117, 7132.
- V. Kumar, S.V. Malhotra, Bioorg. Med. Chem. Lett., 2009, 19, 4643.
- P.B. Tchounwou, C.G. Yedjou, A.K. Patlolla, D.J. Sutton, Heavy metal toxicity and the environment, In Molecular, clinical and environmental toxicology, Springer, Basel, 2012, 133.
- P. Ball, Chem. World., 2007, 4, 34.
- N. Tamilselvan, T. Thirumalai, P. Shyamala, E. David, J. Acute. Disease., 2014, 3, 85.
- A.L. Harvey, Toxicon.,2014, 92, 193.
- W. Zhou, Y. Wang, A. Lu, G. Zhang, Int. J. Mol. Sci., 2016, 17, 246.
- G. Housman, S. Byler, S. Heerboth, K. Lapinska, M. Longacre, N. Snyder, S. Sarkar, Cancers., 2014, 6, 1769.
- P. Khadka, J. Ro, H. Kim, I. Kim, J.T. Kim, H. Kim, J.M. Cho, G. Yun, J. Lee, Asian J. Pharm. Sci., 2014, 9, 304.
- A. Karagianni, M. Malamatari, K. Kachrimanis, Pharmaceutics., 2018, 10, 18.
- J.H.Jr. Davis, Chem. Lett., 2004, 33, 1072.
- A.E. Visser, R.P. Swatloski, W.M. Reichert, R. Mayton, S. Sheff, A. Wierzbicki, J.H.Jr. Davis, R.D. Rogers, Chem. Commun., 2001, 1, 135.
- C. Zeng, X. Xu, N. Zhou, Y. Lin, Spectrochim. Acta. Part A., 2012, 94, 48.
- A.R. Dias, J. Costa‐Rodrigues, M.H. Fernandes, R. Ferraz, C. Prudêncio, Chem. Med. Chem.,2017, 12, 11.
- D.A. Judd, J.H. Nettles, N. Nevins, J.P. Snyder, D.C. Liotta, J. Tang, J. Ermolieff, R.F. Schinazi, C.L. Hill, J. Am. Chem. Soc., 2001, 123, 886.
- A.L. Kubo, L. Kremer, S. Herrmann, S. Mitchell, O. Bondarenko, A. Kahru, C. Streb, Chem. Plus. Chem., 2017, 82, 867.
- R. Ferraz, J. Costa-Rodrigues, M.H. Fernandes, M.M. Santos, I.M. Marrucho, L.P.N. Rebelo, C. Prudêncio, J.P. Noronha, Z. Petrovski, L.C. Branco, Chem. Med. Chem., 2015, 10, 1480.
- S. Cherukuvada, A. Nangia, Cryst. Growth Des., 2013, 13, 1752.
- S. Gupta, R. Kesarla, A. Omri, ISRN pharmaceutics., 2013, 2013, 848043.
- R. Ferraz, L.C. Branco, C. Prudencio, J.P. Noronha, Z. Petrovski, Chem. Med. Chem.,2011, 6, 975.
- M. Villanueva, A. Coronas, J. García, J. Salgado, Ind. Eng. Chem. Res., 2013, 52, 15718.
- Q. Zhang, J.N.M. Shreeve, Chem. Rev., 2014, 114, 10527.
- H. Xiao, Tribol. Trans., 2017, 60, 20.
- X.Q. Sun, B. Peng, J. Chen, F. Luo, Talanta.,2008, 74, 1071.
- (a) N. Seo, Y.R. Lee, H.S. Park, Q.K. Truong, J.Y. Lee, H.K. Chung, Arch. Pharmacal. Res., 2017, 40, 364.
- (b) K. Park, J. Controlled Release., 2014, 190, 3.
- D. Zhao, Y. Liao, Z. Zhang, Clean-soil, air, water., 2007, 35, 42.
- W. Kunz, E. Maurer, R. Klein, D. Touraud, D. Rengstl, A. Harrar, S. Dengler, O. Zech, J. Dispersion Sci. Technol., 2011, 32, 1694.
- M. Saraiva, S. Costa, P. Pinto, A. Azevedo, Chem. Sus. Chem., 2017, 10, 2321.
- S.S. Mitkare, K.G. Lakhane, P.U. Kokulwar, Res. J. Pharm. Technol., 2013, 6, 1274.
- R. Ferraz, V. Teixeira, D. Rodrigues, R. Fernandes, C. Prudêncio, J.P. Noronha, Z. Petrovski, L.C. Branco, RSC Adv., 2014, 4, 4301.
- H. Rodríguez, K. Bica, R.D. Rogers, Trop. J. Pharm. Res., 2008, 7, 1011.
- P. Censi, P. Di Martino, Molecules., 2015, 20, 18759.
- S.N. Black, E.A. Collier, R.J. Davey, R.J. Roberts, J. Pharm. Sci., 2007, 96, 1053.
- N. Papaiconomou, N. Yakelis, J. Salminen, R. Bergman, J.M. Prausnitz, J. Chem. Eng. Data., 2006, 51, 1389.
- O.A. Cojocaru, K. Bica, G. Gurau, A. Narita, P.D. McCrary, J.L. Shamshina, Med. Chem. Comm., 2013, 4, 559.
- P.L. Gould, Int. J. Pharm., 1986, 33, 201.
- T.S. Wiedmann, A. Naqwi, Asian J. Pharm. Sci., 2016, 11, 722.
- P. Kubisa, Prog. Polym. Sci., 2004, 29, 3.
- J.P. Hallett, T. Welton, Chem. Rev., 2011, 111, 3508.
- K. De Winter, K. Verlinden, V. Křen, L. Weignerová, W. Soetaert, T. Desmet, Green Chem., 2013, 15, 1949.
- X. Xu, C. Li, K. Pei, K. Zhao, Z.K. Zhao, H. Li, Sens. Actuators., B 2008, 134, 258.
- D.D. Patel, J.M. Lee, The Chemical Record., 2012, 12, 329.
- N. Adawiyah, M. Moniruzzaman, S. Hawatulaila, M. Goto, Med. Chem. Comm., 2016, 7, 1881.
- J.N. Pendleton, B.F. Gilmore, Int. J. Antimicrob. Agents.,2015, 46, 131.
- K. Kumari, P. Singh, G.K. Mehrotra, Int. J. Green Nanotechnol., 2012, 4, 262.
- X. Wang, C.A. Ohlin, Q. Lu, Z. Fei, J. Hu, P.J. Dyson, Green Chem., 2007, 9, 1191.
- L. Salvatella, Educ. Quim., 2017, 28, 232.
- L.C. Tome, N.H.C.S. Silva, H.R. Soares, A.S. Coroadinha, P. Sadocco, I.M. Marrucho, C.S.R. Freire, Green Chem., 2015, 17, 4291.
- F. Yurt Lambrecht, K. Ocakoglu, S. Gokhan Colak, O. Alp Ersoz, O. Er, Chem. Biol. Drug Des., 2017, 90, 141.
- E. Rutkowska, K. Pajak, K. Jóźwiak, Acta Pol. Pharm., 2013, 70, 3.
- S.V. Tiwari, J.A. Seijas, M.P. Vazquez-Tato, A.P. Sarkate, K.S. Karnik, A.P.G. Nikalje, Molecules., 2018, 23, 440.
- X.Y. Li, C.Q. Jing, W.L. Lei, J. Li, J.J. Wang, Ecotoxicol. Environ. Saf., 2012, 83, 102.
- A. Benedetto, Biophys. Rev., 2017, 9, 309.
- W. Tang, B. Liu, S. Wang, T. Liu, C. Fu, X. Ren, L. Tan, W. Duan, X. Meng, RSC Adv., 2016, 6, 32434.
- M. Cvjetko, K. Radošević, A. Tomica, I. Slivac, J. Vorkapić-Furač, V. Gaurina Srček, Arh. Hig. Rada Toksikol., 2012, 63, 15.
- B. Zhang, C. Jing, X. Li, J. Wang, Toxin Rev., 2015, 34, 161.
- S. Elmore, Toxicol. Pathol., 2007, 35, 495.
- N.K. Kaushik, P. Attri, N. Kaushik, E.H. Choi, Molecules., 2013, 18, 4917.
- R.F. Frade, C.A. Afonso, Hum. Toxicol., 2010, 29, 1038.
- R. Ferraz, J. Costa Rodrigues, M.H. Fernandes, M.M. Santos, I.M. Marrucho, L.P.N. Rebelo, Chem. Med. Chem.,2015, 10, 1480.
- R.A. Kumar, N. Papaïconomou, J.M. Lee, J. Salminen, D.S. Clark, J.M. Prausnitz, Environ. Toxicol., 2009, 24, 388.
- D.A. Volpe, S.S. Hamed, L.K. Zhang, The AAPS Journal., 2014, 16, 172.
- K.S. Egorova, M. Seitkalieva, A.V. Posvyatenko, V.P. Ananikov, Toxicol. Res., 2015, 4, 152.
- S. Stolte, M. Matzke, J. Arning, A. Böschen, W.R. Pitner, U. Welz-Biermann, B. Jastorff, J. Ranke, Green Chem., 2007, 9, 1170.
- C.L. Wang, X.W. Zhu, S.S. Liu, Trans Tech Publications, 2013, 610, 721.
- M.H. Fatemi, P. Izadiyan, Monatshefte für Chemie-Chemical Monthly, 2011, 142, 1111.
- J. Ranke, K. Mölter, F. Stock, U. Bottin-Weber, J. Poczobutt, J. Hoffmann, B. Ondruschka, J. Filser, B. Jastorff, Ecotoxicol. Environ. Saf., 2004, 58, 396.
- H.L. Chen, H.F. Kao, J.Y. Wang, G.T. Wei, J. Chin. Chem. Soc., 2014, 61, 763.
- W. Gouveia, T.F. Jorge, S. Martins, M. Meireles, M. Carolino, C. Cruz, Chemosphere., 2014, 104, 51.
- M. Stasiewicz, E. Mulkiewicz, R. Tomczak-Wandzel, J. Kumirska, E.M. Siedlecka, M. Gołebiowski, Ecotoxicol. Environ. Saf., 2008, 71, 157.
- D. Dobler, T. Schmidts, C. Zinecker, P. Schlupp, J. Schäfer, F. Runkel, AAPS Pharm. Sci. Tech., 2016, 17, 923.
- F.P. Ballistreri, V. Barresi, P. Benedetti, G. Caltabiano, C.G. Fortuna, M.L. Longo, G. Musumarra, Bioorg. Med. Chem., 2004, 12, 1689.
- S. Anusha, C.D. Mohan, J. Mathai, S. Rangappa, S. Mohan, PLoS ONE, 2015, 10, 0139798.
- C.I. Melo, R. Bogel-Łukasik, M.N. da Ponte, E. Bogel-Łukasik, Fluid Phase Equilib., 2013, 338, 209.
- H. Mizuuchi, V. Jaitely, S. Murdan, A.T. Florence, Eur. J. Pharm. Sci., 2008, 3, 326.
- D.J.C. Constable, C. Jimenez-Gonzalez, R.K. Henderson, Org. Process Res. Dev., 2007, 11, 133.
- A.M. Ghatole, K.R. Lanjewar, M.K. Gaidhane, K.M. Hatzade, Spectrochim. Acta Part A, 2015, 151, 515.
- J. Pernak, J. Rogoża, I. Mirska, Eur. J. Med. Chem., 2001, 36, 313.
- J. Pernak, J. Kalewska, H. Ksycińska, J. Cybulski, Eur. J. Med. Chem., 2001, 36, 899.
- R.F. Frade, A. Matias, L.C. Branco, C.A. Afonso, C.M. Duarte, Green Chem., 2007, 9, 873.
- R.F. Frade, A.A. Rosatella, C.S. Marques, L.C. Branco, P.S. Kulkarni, N.M. Mateus, C.A. Afonso, C.M. Duarte, Green Chem., 2009, 11, 1660.
- S.V. Malhotra, V. Kumar, Bioorg. Med. Chem. Lett., 2010, 20, 581.
- C. Suresh, H. Zhao, A. Gumbs, C.S. Chetty, H.S. Bose, Bioorg. Med. Chem. Lett., 2012, 22, 1734.
- C. Jing, X. Li, J. Zhang, J. Wang, J. Biochem. Mol. Toxicol., 2013, 27, 330.
- R. Ferraz, J. Costa‐Rodrigues, M.H. Fernandes, M.M. Santos, I.M. Marrucho, L.P.N. Rebelo, C. Prudêncio, J.P. Noronha, Z. Petrovski, L.C. Branco, Chem. Med. Chem., 2015, 10, 1480.
- X. Li, J. Ma, J. Wang, Ecotoxicol. Environ. Saf., 2015, 120, 342.
- P. Stepnowski, A.C. Skladanowski, A. Ludwiczak, E. Laczyńska, Hum. Exp. Toxicol., 2004, 23, 513.
- J. Ranke, M. Cox, A. Müller, C. Schmidt, D. Beyersmann, Toxicol. Environ. Chem., 2006, 88, 273.
- S. Stolte, J. Arning, U. Bottin-Weber, M. Matzke, F. Stock, K. Thiele, M. Uerdingen, Green Chem., 2006, 8, 621.
- S. Stolte, J. Arning, U. Bottin-Weber, A. Müller, W.R. Pitner, U. Welz-Biermann, Green Chem., 2007, 9, 760.
- A. García-Lorenzo, E. Tojo, J. Tojo, M. Teijeira, F.J. Rodríguez-Berrocal, M.P. González, V.S. Martínez-Zorzano, Green Chem., 2008, 10, 508.
- M. Stasiewicz, E. Mulkiewicz, R. Tomczak-Wandzel, J. Kumirska, E.M. Siedlecka, M. Gołebiowski, J. Gajdus, M. Czerwicka, P. Stepnowski, Ecotoxicol. Environ. Saf., 2008, 71, 157.
- W. Qiu, X. Zeng, Anal. Bioanal. Chem., 2008, 392, 203.
- T. Schaffran, E. Justus, M. Elfert, T. Chen, D. Gabel, Green Chem., 2009, 11, 1458.
- D. Patra, C. Barakat, Spectrochim. Acta. Part A, 2011, 79, 1823.
- W. Cui, X. Lu, K. Cui, L. Niu, Y. Wei, Q. Lu, Langmuir., 2012, 28, 9413.
- S. Goindi, P. Arora, N. Kumar, A. Puri, AAPS Pharm. Sci. Tech., 2014, 15, 810.
- S. Goindi, R. Kaur, R. Kaur, Int. J. Pharm., 2015, 495, 913.
- A. Forte, C.I. Melo, R. Bogel-Łukasik, E. Bogel-Łukasik, Fluid Phase Equilib., 2012, 318, 89.
- M. Moniruzzaman, N. Kamiya, M. Goto, J. Colloid Interface Sci., 2010, 352, 136.
- C. Lourenço, C.I. Melo, R. Bogel-Łukasik, E. Bogel-Łukasik, J. Chem. Eng. Data., 2012, 57, 1525.
- R.A. Faria, M.N. da Ponte, E. Bogel-Łukasik, Fluid Phase Equilib., 2015, 385, 1.
- R.A. Faria, E. Bogel-Łukasik, Fluid Phase Equilib., 2015, 397, 18.