Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 6
Shelf Life Determination of Picralima Nitida, Glibenclamide, Ciprofloxacin and Pefloxacin using UV Spectrometry Physicochemical Technique
Kenechukwu Keluo Onyechi1 and Chinenye Adaobi Igwegbe2*
1Department of Pharmaceutics and Pharmaceutical Technology, Nnamdi Azikiwe University, Awka, Nigeria
2Department of Chemical Engineering, Nnamdi Azikiwe University, Awka, Nigeria
- *Corresponding Author:
- Chinenye Adaobi Igwegbe
Department of Chemical Engineering
Nnamdi Azikiwe University
Awka, Nigeria
Abstract
Shelf life is one of the important property of a drug for safety and quality. The shelf life of Picralima nitida (herbal drug) and three orthodox drugs (glibenclamide, ciprofloxacin, and pefloxacin) has been investigated. The stability study was done using UV spectrometry method. Their shelf life was determined by accelerated stability studies on the basis of first-order degradation kinetics and t10% (the time required to degrade 10% of a drug at 27°C). The stability was studied at temperatures of 45°C, 60°C and 70°C during the course of one month at one-week interval (1, 2, 3 and 4 weeks). The initial concentration of 5, 2.5, 25 and 100 mg/ml was used for Picralima nitida, glibenclamide, ciprofloxacin, and pefloxacin, respectively in the study. Influence of storage time and temperature on the drug samples were investigated. The half-life was also evaluated. All experiments were carried out in the year 2012. The concentrations of the drug samples were found to decrease with increase in storage time and temperature. The shelf life of ciprofloxacin, pefloxacin, and glibenclamide were found to be 535.18, 298.17 and 134.31 weeks, respectively. The half-life of ciprofloxacin, pefloxacin and glibenclamide were also found to be 3553.85, 1980 and 891.89 weeks, respectively. The shelf life and half-life of Picralima nitida could not be determined using UV spectrometry technique because of the presence of complex metabolites, which results in the irregular increase in absorbance and instability. Storage time and temperature was found to have a great influence on the concentration of the drug substances.
Keywords
Accelerated stability study, Ciprofloxacin, Degradation kinetics, Glibenclamide, Pefloxacin, Picralima nitida, Shelf life determination
Introduction
Shelf life/stability studies are usually carried out on a drug substance in order to determine the degradation of its active ingredient [1]. Stability is defined as the capacity of a drug material to retain its specifications of its active ingredient at a period time to ensure its quality and safety. Stability testing of drug substances gives information of the drug variation with time under the influence of environmental conditions such as humidity, temperature, and light [2,3]. Stability of a drug product provides additional information on the possible storage conditions during the line processing of raw materials, the intermediate and finished product; and the shelf-life and expiration date of a pharmaceutical product.
The two common types of stability tests employed to estimate shelf life are real-time stability tests and accelerated stability tests [4]. In real-time stability testing, a product is stored at recommended storage conditions and monitored until it fails the specification. In accelerated stability tests, a product is stored at elevated stress conditions such as temperature, humidity, and pH [4].
Herbal drugs have been used in decades for the treatment of diseases and revitalization of body systems. WHO stated that about 80% of the world populates depend on herbal drugs [5], due to its economic importance and ease of reach. Therefore, the need for the study of their safety. Picralima nitida extracts have been reported to be effective as cough suppressant [6], antibacterial effect [7,8], treatment of viral infections [9], anti-inflammatory and antipyretic activity [10], analgesic activity [11,12] and anti-parasitic effect including malaria parasite [13,14]. Ciprofloxacin {1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3 quinolinecarboxylic acid} and Pefloxacin {1-ethyl-6-fluoro-7-(4- methyl piperazin-1-yl)-4-oxo-quinoline-3-carboxylic acid} are synthetic broad-spectrum antibiotic active against both gram-positive and gram-negative bacteria [15]. Pefloxacin, an analog of norfloxacin is a synthetic chemotherapeutic agent used to treat severe and life-threatening bacterial infections [16]. Glibenclamide {5-chloro-N-(4-[N-(cyclohexyl carbamoyl) sulfamoyl] phenethyl)-2-methoxybenzamide} also known as glyburide is an antidiabetic drug. Glibenclamide is one of only two oral antidiabetics in the World Health Organization model list of essential medicines [17,18]. Also, glibenclamide has been reported to improve the outcome in animal stroke models by preventing brain swelling [19].
The objective of this study is to estimate the shelf life of Picralima nitida (herbal drug) and three orthodox drugs (glibenclamide, ciprofloxacin and pefloxacin) at different storage times and temperatures using UV spectrometry method.
Materials and Methods
2.1 Collection and Preparation of the Drug Samples
Picralima nitida (Apocynaceae) seeds known as akuamma seed in Ghana and Osuigwe in Igboland (Eastern, Nigeria) were collected from Ihembosi, Anambra state, Nigeria. The pods were washed with clean water to remove dirt, dried and dissected longitudinally to expose the interior component. The seeds were isolated from the pulp into a clean stainless steel bowl. The seeds obtained from the pods were allowed to dry at room temperature for one week and the testa manually removed. Further drying of the cotyledons was done until they turned dark brown and hard. The seeds were pulverized into coarse particles using a manual grinder and further size reduction was done manually in a porcelain mortar. The resulting powder was screened through a 0.25 μm sieve and packed in an air tight bottle. 200 g of the seed powder was defatted by maceration in n-hexane and dried. The powder was then packed in a glass container and methanol of 1 l added to cover two thirds the volume of the container. The maceration was allowed to continue for 48 h with occasional agitation. The mixture was filtered through a filter paper into a glass beaker and the filtrate allowed to dry at room temperature. The resinous extract obtained was weighed, packed, covered and stored in a refrigerator for further usage.
The orthodox drugs used in the study were: (1) Ciprofloxacin hydrochloride (antibacterial drug) tablets USP 500 mg (gecip®) manufactured in 2010 by MICRO lab limited, Bangalore, India and marketed by Geneith Pharm LTD, Oshodi, Lagos State, Nigeria. (2) Glibenclamide (hypoglycaemic drug) tablets BP 5 mg (diatab®) manufactured in 2009 by Mayer and Baker, Nigeria PLC, Ikeja, Lagos State, Nigeria. (3) Pefloxacin (antibacterial drug) tablets 400 mg (peflomed®) manufactured in 2011 by Bharat Parenterals LTD, Barode Gujarat, India and marketed by Evans medical PLC, Agbara, Ogun State, Nigeria.
All drugs (tablets) were obtained from a pharmaceutical store in Awka, Anambra State, Nigeria on February 19, 2012.
2.2 Stability Study using UV Absorption Spectra
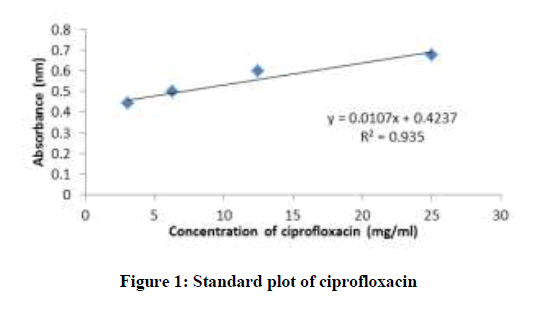
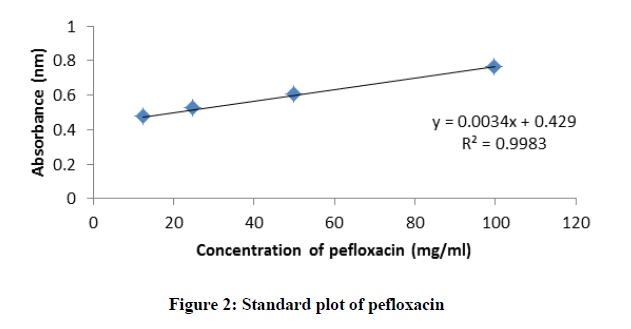
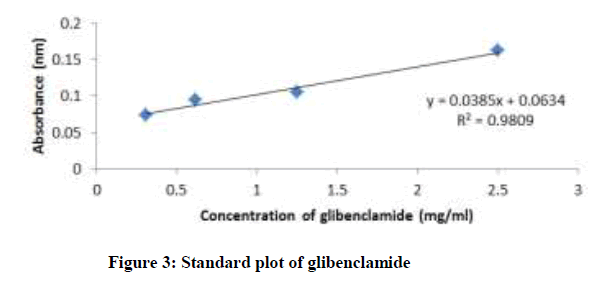
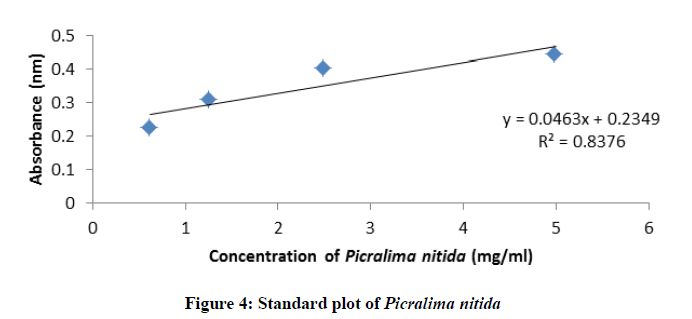
Standard plot of absorbance against concentrations for Picralima nitida, ciprofloxacin, pefloxacin, and glibenclamide were determined using UV-Vis spectrophotometer (Shimadzu model 1800) by two-fold serial dilution of the stock solutions (ciprofloxacin hydrochloride-25 mg/ml in distilled water, pefloxacin-100 mg/ml in distilled water, glibenclamide-2.5 mg/ml in methanol and Picralima nitida seed extract-5 mg/ml in methanol) into four different concentrations. Their absorbance at different concentrations was obtained at their various characteristic wavelengths of 429, 433, 370 and 425 nm for ciprofloxacin, pefloxacin, glibenclamide, and Picralima nitida respectively. All reagents used in the study were of analytical grade.
The stock solutions of the drugs were then subjected to different temperatures (45°C, 60°C and 70°C) and the samples collected at one-week interval for one month to obtain their absorbance in which their coresponding final concentrations were evaluated from the regression equations of their standard plots. All experiments were carried out in the year 2012.
Results and Discussion
4.1 Standard Plots of Ciprofloxacin, Pefloxacin, Glibenclamide, and Picralima nitida
The standard plots (absorbance against concentration) of ciprofloxacin, pefloxacin, glibenclamide, and Picralima nitida at different dilution concentrations with their respective absorbance are presented in figures 1-4. The regression equations of their standard plots were used further to evaluate the final concentrations of the drug samples after the accelerated stability studies at different storage times and temperatures.
4.2 Effect of Storage Time and Temperature on Drug Concentration
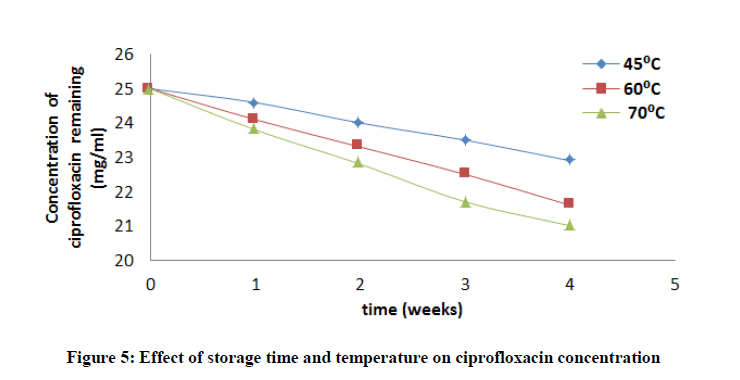
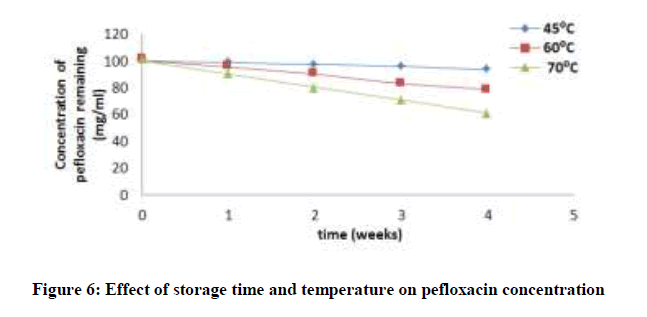
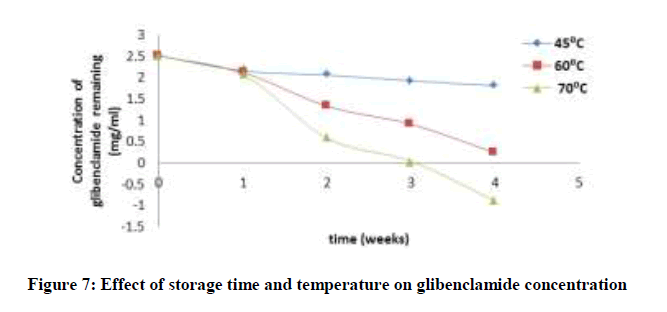
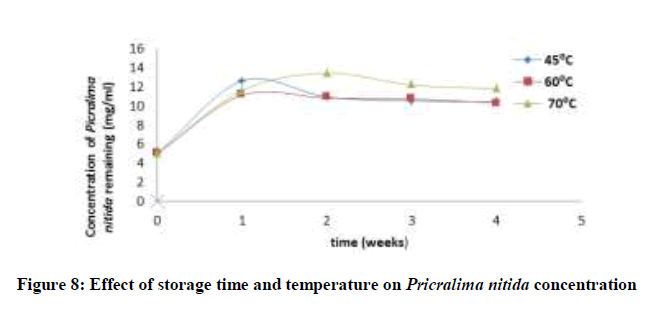
Accelerated stability tests was investigated by stressfully conditioning the drug for a period of time at high or elevated temperatures. The concentration of ciprofloxacin, pefloxacin, glibenclamide, and Picralima nitida remaining after storage times of 0, 1, 2, 3 and 4 weeks at different storage temperatures of 45, 60 and 70°C was determined using an initial concentration of 5, 2.5, 25 and 100 mg/ml for Picralima nitida, glibenclamide, ciprofloxacin, and pefloxacin, respectively. The concentration of the drug substances remaining was plotted against time (Figures 5-8). From figures 5-8, it can be seen that the concentrations of ciprofloxacin, pefloxacin, glibenclamide, and Picralima nitida decreased with increase in storage time. Increase in temperature increased the decomposition of ciprofloxacin, pefloxacin, and glibenclamide since the molecules tend to move faster with increased kinetic energy [20] but for Picralima nitida sample, its concentration was increased. This implies that the storage time and temperature have a great influence on the concentration of the drug substances.
4.3 Kinetics of Degradation
The accelerated degradation kinetics was done at elevated temperatures of 45°C, 60°C and 70°C. The degradation kinetic constant, k was evaluated by using the first order rate equation:
ln C= ln C0 - kit (1)
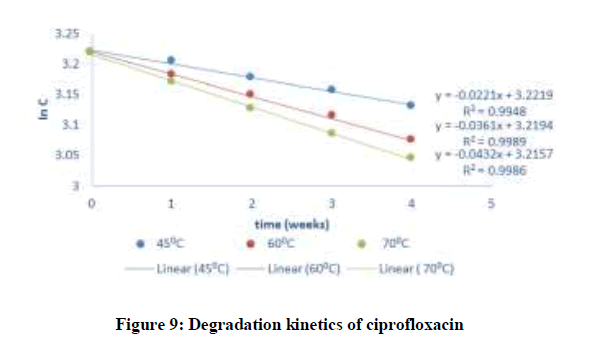
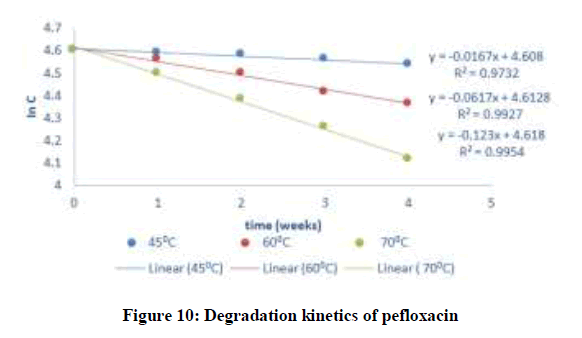
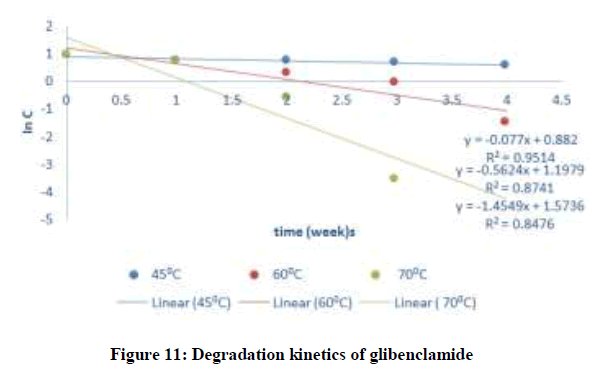
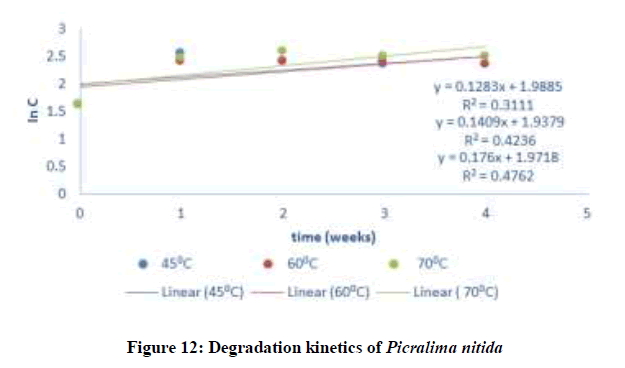
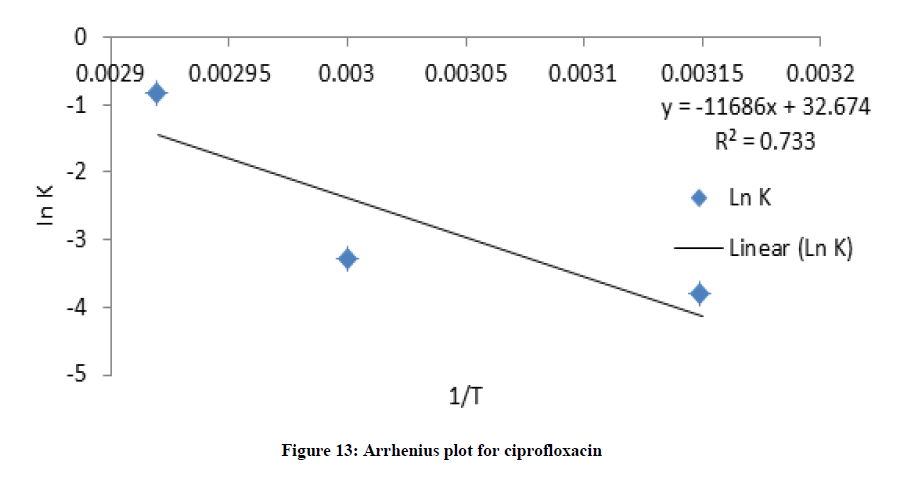
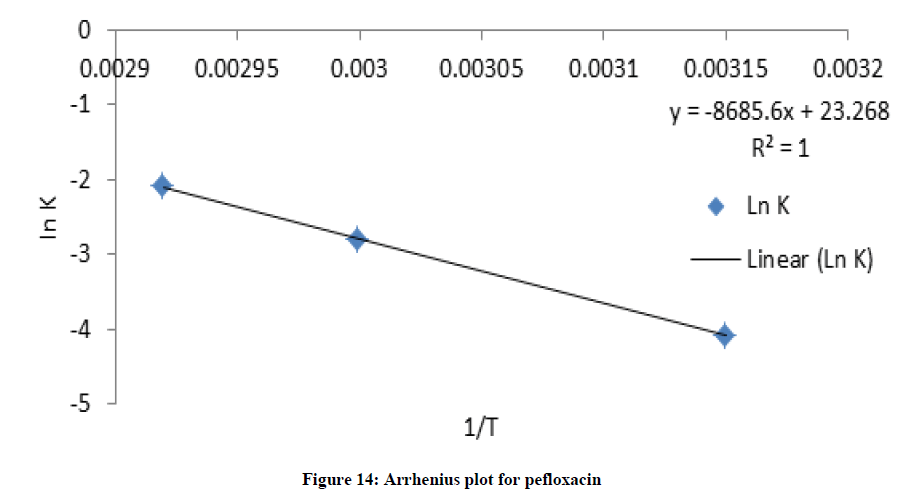
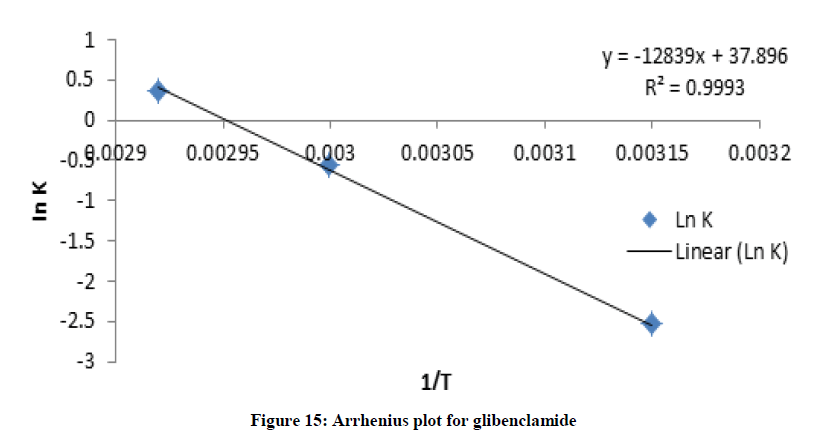
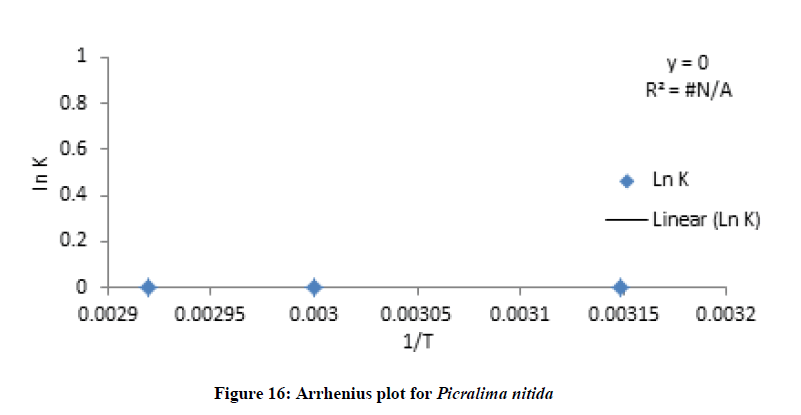
Where C and C0 is the concentration of drug remaining at a time (t = t) and drug concentration at time (t = 0), respectively. ki is the degradation rate constant which can be evaluated from the slopes of the linear plot In C of and t (Figures 9-12). The average k can be calculated ki at each week at temperatures of 45, 60 and 70°C. The degradation rate constant, k obtained at various temperatures for the drug substances are presented in Table 1. The correlation coefficients (R2 > 0.95) shows that the degradation experimental data of ciprofloxacin and pefloxacin fitted into the first-order plot at all temperatures (Figures 9,10) but that of glibenclamide fitted the plot only at 45°C (Figure 11). From, Figure 12, it can be seen that the experimental data gotten from the degradation of Picralima nitida didn’t fit into the first-order model at all temperatures.
| Drug substance | Temperature (°C) | k (week-1) | Absolute Temperature (K) | 1/T | In k | Activation energy, Ea ( kJ/mol.K) | Shelf-life at 27°C (weeks) | Half-life at 27°C (weeks) |
|---|---|---|---|---|---|---|---|---|
| Ciprofloxacin | 27 | 0.000195 | 300 | 0.00333 | -6.24038 | 97.1574 | 535.18 | 3553.85 |
| 45 | 0.0221 | 318 | 0.00315 | -3.81218 | ||||
| 60 | 0.0361 | 333 | 0.003 | -3.32146 | ||||
| 70 | 0.432 | 343 | 0.00292 | -0.83933 | ||||
| Pefloxacin | 27 | 0.00035 | 300 | 0.00333 | -5.655048 | 72.21208 | 298.17 | 1980 |
| 45 | 0.0167 | 318 | 0.00315 | -4.09235 | ||||
| 60 | 0.0617 | 333 | 0.003 | -2.78547 | ||||
| 70 | 0.123 | 343 | 0.00292 | -2.09557 | ||||
| Glibenclamide | 27 | 0.000777 | 300 | 0.00333 | -4.85787 | 106.74345 | 134.31 | 891.89 |
| 45 | 0.077 | 318 | 0.00315 | -2.56395 | ||||
| 60 | 0.5624 | 333 | 0.003 | -0.57554 | ||||
| 70 | 1.4549 | 343 | 0.00292 | 0.374937 | ||||
| Picralima nitida | 27 | - | 300 | 0.00333 | - | - | - | - |
| 45 | -0.1282 | 318 | 0.00315 | - | ||||
| 60 | -0.1409 | 333 | 0.003 | - | ||||
| 70 | -0.176 | 343 | 0.00292 | - |
Table 1: Degradation rate constants calculated at different temperatures, shelf life and half-life of ciprofloxacin, pefloxacin, glibenclamide, and Picralima nitida
4.4 Shelf-Life Determination
The effect of temperature on the degradation kinetics was also determined using Arrhenius equation. Arrhenius establishes a relationship between temperature and rate of reaction. Arrhenius plot was made by plotting ln k values against 1/T (Figures XIII-XVI). The value of k at 27°C (k27) was extrapolated from the Arrhenius plot using equation 2 [21]:
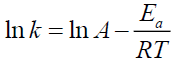 (2)
(2)
Where k is the degradation rate constant, A is the frequency of molecular collisions occurring between the molecules or Arrhenius factor, Ea is the energy of activation (kJ/mol.K), T is the absolute temperature (K) and R is the ideal gas constant (8.314 J/mol.K).
The degradation rate also depends on the activation energy of the chemical reaction [4]. The activation energy was evaluated from the slope of the plot (Figures 13-16) using:
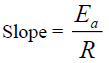 (3)
(3)
The activation energies Ea for the degradation of each drug samples were found to be high (Ea > 50 kJ/mol.K), which were calculated as shown in table 1. The higher Ea is, the less the degradation reaction is influenced by temperature; the result indicated that temperature contributed to the degradation of the drugs [22].
The shelf-life of the drug substances were estimated by substituting the values of k27 in the equation:
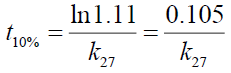 (4)
(4)
Where t10% is the shelf life (the time required for 10% degradation of the drug) and k27 is the degradation rate constant at 27°C. The shelf life of the drug substances is presented in Table 1. The shelf life of ciprofloxacin, pefloxacin, and glibenclamide were found to be 535.18, 298.17 and 134.31 weeks, respectively. The shelf life of Picralima nitida (herbal drug) could not be determined because of the presence of complex metabolites, which results in the irregular increase in absorbance and instability.
Half-life, t1/2 of the drug substances (the period of time required for the concentration of drug to be reduced by one-half of a given concentration) were also estimated at 27°C as follows:
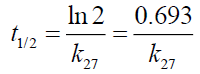 (5)
(5)
The half-life of the drug substances is stated in Table 1. The half-life of ciprofloxacin, pefloxacin and, glibenclamide were found to be 3553.85, 1980 and 891.89 weeks, respectively while that of Picralima nitida could not be determined because of its complexity.
Conclusion
The shelf life of Picralima nitida (herbal drug) and three orthodox drugs (glibenclamide, ciprofloxacin, and pefloxacin) have been investigated. The stability/shelf life study was done using UV spectrometry. The shelf life, t10% (the time required to degrade 10% of a drug at 27°C) was determined by accelerated stability studies. The stability studies were done at temperatures of 45°C, 60°C and 70°C at 1, 2, 3 and 4 weeks. The initial concentration of 5, 2.5, 25 and 100 mg/ml was used for Picralima nitida, glibenclamide, ciprofloxacin, and pefloxacin, respectively for the stability study. The effects of storage time and temperature on the concentration of the drug samples were also investigated. First-order degradation kinetics constant, k and half-life, t1/2 were also evaluated. All experiments were carried out in the year 2012. The concentrations of the drug samples were found to decrease with increase in storage time and temperature. The shelf life of ciprofloxacin, pefloxacin, and glibenclamide were found to be 535.18, 298.17 and 134.31 weeks, respectively. The half-life of ciprofloxacin, pefloxacin and, glibenclamide were also found to be 3553.85, 1980 and 891.89 weeks, respectively. The shelf life and half-life of Picralima nitida (herbal drug) could not be determined because of its complexity (presence of complex metabolites, which results in the irregular increase in absorbance and instability). Storage time and temperature were found to have a great change in the concentration of the drugs.
Acknowledgement
The authors wish to acknowledge the Department of Pharmaceutics and Pharmaceutical Technology, Nnamdi Azikiwe University, Awka, Nigeria and National Agency for Food and Drug Administration and Control (NAFDAC), Agulu, Nigeria.
References
- W. Liu, J.C. Hsu, F. Bretz, A.J. Hayter, Y. Han, Journal of Applied Statistics., 2014, 41(9), 1989-2000.
- A. Uzunović, E. Vranić, Bosnian Journal of Basic Medical Sciences., 2008, 8(1), 93-97.
- E. Koleva, T. Paneva, V. Tzotchev, Science, Engineering & Education., 2016, 1(1), 106–112.
- R.T. Magari, BioPharm International., 2003, 16(11), 36-48.
- WHO, Final report of the seminar on the use of medicinal plants in health care, WPRO Publication, Tokyo, 1996.
- H.M. Burkhill, The useful plants of West Tropical Arica. Royal botanic gardens, United Kingdom, 1985, 1(2), 28.
- C.K. Nkere, C.U. Iroegbu, Afr. J. Biotech., 2005, 4(6), 522-526.
- T.O. Fakeye, O.A. Itiola, H.A. Odehla, Phythother. Res., 2000, 14, 368-370.
- E.J. Akpan, I.B. Umoh, Nig. Soc. Exptl. Bio., 2004, 16(2), 72-78.
- I.C. Ezeamuzie, M.C. Ojinnaka, E.O. Uzogara, S.E. Oji., J. Ethanopharmacol., 1994, 54(2-3), 113-117.
- H. Arens, H.O. Borde, B. Illorich, J. Stockight, Planta Med., 1982, 46, 210-214.
- M. Duwiejua, E. Woode, D.D. Obiri, J. Ethnopharmacol., 81(1), 2002, 73-79.
- M.M. Iwu, D.L. Klayman, J. Ethanopharmacol., 1992, 36(2), 133-135.
- J. Bickii, G.R.F. Tchouya, J.C. Tchouankeu, E. Tsamo, Afr. J. Trad. Comp. Alter. Med., 2007, 4(1), 107 - 111.
- E.C. Ibezim, C.O. Esimone, P.O. Nnamani, I.V. Onyishi, S.A. Brown, C.E. Obodo, Afr. J. Biotechnol., 2006, 5(19), 1781-1784.
- J.P. Gonzalez, J.M. Henwood, Drugs., 1989, 37(5), 628-68.
- WHO Expert Committee, The selection and use of essential medicines, WHO Tech Rep Ser., 2011, 965, 1-249.
- L.E. Taha., S.M.A. Bakhit., J.A.A. Al-Sa’aidi, A.B.O. Uro, AL-Qadisiya J. Vet. Med. Sci., 2014, 13(2), 113-117.
- H. Kunte, S. Schmidt, M. Eliasziw, G.J. de Zoppo, J.M. Simard, F. Masuhr, M. Weih, U. Dirnagl Stroke., 2007, 38(9), 2526-2530.
- A.H. Moreno, H.R.N. Salgado, Adv. Anal. Chem., 2016, 2(1), 1-5.
- V. Agrahari, S. Putty, C. Mathes,, J.B. Murowchick, B.C. Youan, Drug Test. Anal., 2015, 7(3), 207-213.
- X. Lianga, Z. Liub, H. Shia, Y. Zhanga, S. Wanga, K. Bia and X. Chen, J Pharm Anal., 2017, 7, 118-122.