Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 6
Silica-Chitosan Xerogel Composite Nanospheres for Controlled Release of Lisinopril
Somayeh Sadighian1,2,3*, Seyyed Aliasghar Arjmandi4 and Sahar Najaflou3
1Cancer Gene Therapy Research Center, Zanjan University of Medical Sciences, Zanjan, Iran
2Zanjan Pharmaceutical Nanotechnology Research Center, Zanjan University of Medical Sciences, Zanjan, Iran
3Department of Pharmaceutical Biomaterials, School of Pharmacy, Zanjan University of Medical Sciences, Zanjan, Iran
44Department of Engineering, University of Zanjan, Zanjan, Iran
- *Corresponding Author:
- Somayeh Sadighian
Cancer Gene Therapy Research Center
Zanjan University of Medical Sciences, Zanjan, Iran
Abstract
Single- and double-layered nanocomposites prepared by encapsulation of lisinopril-loaded silica xerogels with the biodegradable polymer chitosan. The in vitro drug release profile from both the nanocomposites and bare nanospheres investigated. The single-layered nanocomposites (silica xerogel) showed an initial burst, followed by two sustained release stages lasting for 13 days. In comparison with the silica xerogel nanocomposites, the silica xerogel/ Chitosan nanocomposites released a reduced initial burst together with three sustained release stages. The silica xerogel/chitosan nanocomposites can provide additional advantages in the localized drug delivery applications, as compared to the silica xerogel.
Keywords
Silica, Chitosan, Xerogel, Lisinopril, Drug delivery
Introduction
Silica-based xerogels, obtained using the sol-gel transition process have attracted a remarkable attention in recent decade as potential carriers for controlled drug delivery owing to their highly porous structure, notable biocompatibility, ease of preparation, and versatility. These materials are routinely prepared at room temperature and investigated extensively for their promising possibilities in different biomedical applications [1-3]. Silica-based materials are normally amorphous with a high degree of microscopic porosity. The convenient sol-gel process involves the formation of a colloidal suspension of assigned compounds (sol) which is, then, gelified through the hydrolysis followed by polycondensation until a solid network (gel) is formed [1,2]. Bioactive agents can be added during the sol-gel preparation process or introduced into a gel by adsorption onto the surface of the gel [1]. Drug release profile from silica-based xerogels can be affected to some extent by changing the sol-gel preparation parameters, i. e. pH, the water/alkoxide ratio [4], type or concentration of the catalyst (acidic or basic), and temperature [5,6] as well as the drying condition. Drug release, although, is normally known to be diffusion-controlled and relatively fast in the initial (burst) phase in simple Tetraethylorthosilicate (TEOS) -based xerogels without dopants. Several methods have developed to optimize the drug release behaviors of the silica xerogel carriers, including capping of mesoporous silica channels with quantum dots (CdS) [7], functionalization of silica pore with photoactive derivatives [8], and the addition of polymers to create composite drug delivery systems [9]. Polymer/silica xerogel composites are promising in this way because of their unique capability to provide sustained drug release over a long period. The drug release behaviors of a chitosan/silica xerogel composite can be described by both the drug diffusion and biodegradation of the polymer. Chitosan is naturally derived copolymer of N-acetylglucosamine and d-glucosamine, prepared via deacetylated of chitin and has widely used in biomedical applications because of its biodegradability and biosafety profiles [10-12]. Chitosan is metabolized in vivo by the action of human enzymes, especially lysozyme [13-16] and, furthermore, is flexible and formable as compared with inorganic compounds [17]. These properties of chitosan make it a suitable material for coating layer on silica xerogel. In this study, we hybridized a silica xerogel with chitosan, to be used as a coating layer on metallic substrates. The in vitro characteristics of the hybrid composite were evaluated extensively.
Materials and Methods
Materials
Tetraethyl Orthosilicate (TEOS) (>99%, CAS number 78-104), chitosan of molecular weight in the range of 105–3 × 105 g/mol and degree of deacetylation ≥ 75%, ammonium hydroxide, Nitric acid and hydrochloric acid all were purchased locally from Merck (Darmstadt, Germany). Farayand Chimie Hakim (Takestan, Iran) kindly donated Lisinopril. All the materials used without any purification.
Preparation of silica xerogel and silica xerogel nanocomposite
As a routine synthesis procedure, the porous xerogel is prepared by the hydrolysis of Tetraethoxysilane (TEOS) at room temperature. The difference molar ratio of water to TEOS (1 to 10) was tested and it was fixed at eight. TEOS aqueous solution (0.5 %W/V) was added gradually to HNO3 (0.2 N, pH=2.0) while being stirred magnetically at 300 rpm at room temperature. The stirring continued for an additional 1 h at room temperature. After that, the sample was aged for 24 h at room temperature.
The nanocomposite sol of silica xerogel/ chitosan was prepared as follows. Chitosan at a concentration of 0.5% (w/v) was dissolved in acetate buffer (1 M, pH= 4.8) and mixed with TEOS aqueous solution. Silica xerogel and silica xerogel/ chitosan nanocomposite obtained milled into fine powders.
In vitro characterization
FT-IR spectra
FT-IR spectra of nanoparticles taken with a Matson FT-IR spectrophotometer in the range of 400-4000 cm–1 as KBr disks at room temperature.
Scanning electron microscopy
Scanning electron microscopy (SEM), Hitachi f 4160 used to examine the morphology and size distribution of the carriers.
UV–Vis spectroscopy
A UV–Vis spectrometer (Genesys, Madison WI, USA) with 1 cm quartz cells was used for recording and storage of UV–Vis absorbance spectra.
In situ Lisinopril loading during xerogel synthesis
In a typical reaction, lisinopril was loaded into silica xerogel matrix using an in situ method. The best molar ratio of water to TEOS selected. The weight ratio of lisinopril to silica was 0.5%. Briefly, TEOS aqueous solution added drop by drop to HNO3 and then stirred. 5 mg lisinopril powder, then, dissolved in the mixture thus obtained and another stirring step for 1 h at room temperature at the same speed carried out before the gelation process proceeds. The resulting drug-loaded sol, then, allowed be converting slowly to the gel form and, finally, air-drying at 40°C for 2 days. Lisinopril-loaded xerogel obtained, then, milled into fine powders.
Drug release study
The in vitro drug release profile evaluated for both the silica xerogel and silica xerogel/chitosan nanocomposite. Briefly, for each variant, 100 mg of the dried nanocarrier dispersed in 10 ml of phosphate-buffered saline (PBS, pH 7.4) at 37°C under mild stirring (100 rpm). In order to determine the drug amount released out of the carrier, at times 1 h, 2 h, etc. 1.0 ml of solution taken out and replaced with 1.0 ml of fresh PBS maintained at 37ºC. The collected samples were, then, filtered (syringe filter, 220 nm) and the lisinopril content in the filtrates was determined spectrophotometrically at 230 nm. Finally, the percent ratio of the released drug, calculated based on an equal-concentration free drug solution kept in the same condition, plotted against the corresponding times to construct the drug release profile.
Results and Discussion
The lisinopril-loaded silica xerogels were prepared by dissolving lisinopril into TEOS sol and followed by gelling and drying. As a result, the majority of the lisinopril physically entrapped in the porous silica xerogel, though a small amount of lisinopril can form hydrogen bonds with the silica xerogel. Since lisinopril is highly soluble in water, the physically entrapped lisinopril molecules were diffused into an aqueous medium quickly., the lisinopril- loaded xerogels released about 30% of lisinopril in one day, displaying an initial burst. This is unfavorable for our aim to provide for a long-term sustained delivery. To optimize the drug release behavior, the lisinopril-loaded silica xerogel nanocomposites, then, coated with chitosan. During the production process of the nanocomposites, an interaction between amino groups of chitosan and carboxylic groups of lisinopril might occur, which results in long-term sustained delivery. In our present study, we have demonstrated the possibility of using silica xerogel and silica xerogel/chitosan nanocomposites prepared at room temperature by the sol-gel process as lisinopril delivery system.
FT-IR analysis
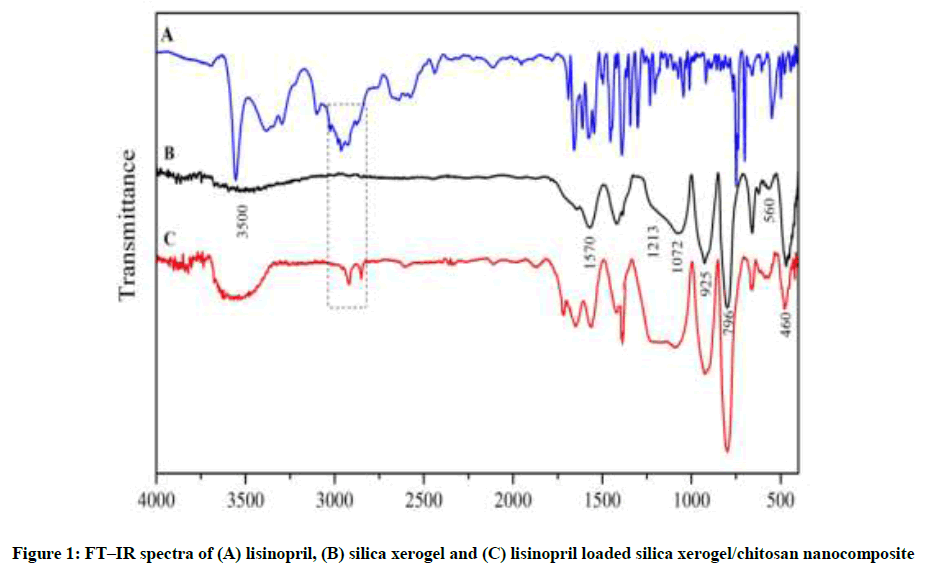
The addition of lisinopril to the xerogel did not change the physical characteristics of the nanocarriers. This indicates that the drug homogeneously distributed throughout the xerogel without any macroscopic phase separation. To avoid any damage or loss of activity of the drug, room temperature and low acidity parameters used. The chemical structure and functional groups of silica xerogel were investigated by FT-IR (Figure 1). The pure silica xerogel showed characteristic bands at 3500 (–OH stretching mode), 1570 (molecular H2O bending mode), 1213-1072 (Si–O–Si asymmetric stretching mode), 925 (Si–OH stretching mode), 796 (Si–O–Si symmetric stretching mode) and 560-460 cm-1 (Si–O–Si mode). A comparison of the spectra shown in Figure 1 indicates the absence of any chemical interactions between the drug and the nanocarrier.
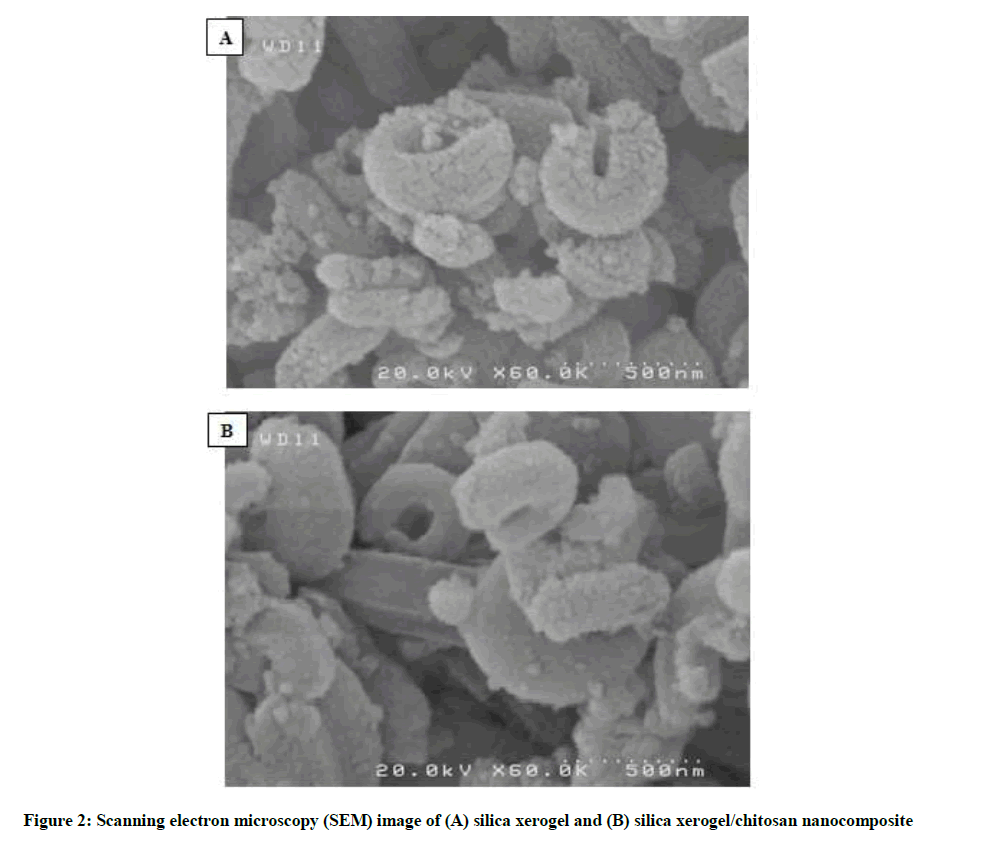
SEM images employed to examine the detailed structure and morphology of as-synthesized silica xerogel nanocomposites. Figure 2A and B shows a typical SEM image of silica xerogel and silica xerogel/chitosan nanocomposites, respectively. After coated with chitosan, the small cracks on the surface of silica xerogel are disappeared (Figure 2B).
Drug release
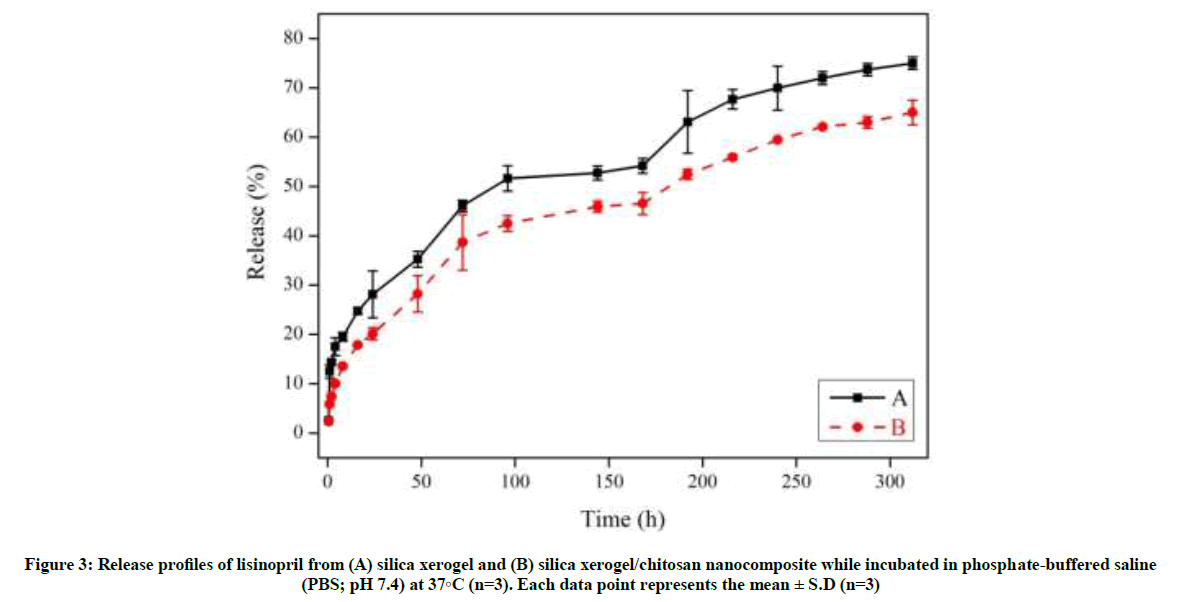
Figure 3 shows the cumulative release profiles of lisinopril from the silica xerogel and silica xerogel/chitosan nanocomposite with three distinct stages of lisinopril release, including initial burst stage (stage 1) and two sustained release stages (stage 2 and stage 3), observed for both variants. At the initial burst stage, both types of nanocarriers released about 30 % of lisinopril in the first 1 day time period. The second release stage of the nanocarriers involved a further 30% of the drug during the time period between day 1 to 7. Finally, at the end of the third release stage, about 75% of the loaded lisinopril was released after 13 days. In comparison, the cumulative release of the drug was about 10 percent lower in the case of nanocomposites than the bare xerogels. This difference is significant with the possibility to be even better with more investigations.
Mechanistically, since the trends of drug release from both types of nanocarriers, i. e., bare xerogel and chitosan-coated nanocomposite, are the same, it seems that the chitosan coat has no mechanistically significant effect on drug release, but a retarding effect. The first, second, and third phases of drug release should be related to the drug loaded on the surface, middle, and deep parts of the xerogel pores, respectively.
Conclusion
This work verified the drug release behaviors of silica xerogel and silica xerogel/chitosan nanocomposites. Three obvious drug release stages, a sharp initial burst lasting for 1 day, a slowly sustained release stage lasting for 13 days, were observed for the silica xerogel nanocomposites. The sharp initial release burst of the silica xerogel is mainly due to the precipitation of the lisinopril on the surfaces of the nanocomposites. The sustained release behavior in the silica xerogel is controlled by chitosan encapsulations. The double-layered nanocomposite exhibited a reduced initial burst. The silica xerogel/chitosan nanocomposites can provide additional advantages in the localized drug delivery applications, as compared to the silica xerogel.
Acknowledgement
We are most grateful for the continuing financial support of this research project by Zanjan University of Medical Sciences.
References
- C.J. Brinker, G.W. Scherer GW, Sol–gel science, the physics and chemistry of sol–gel processing, Boston, 1990.
- R.K. Iler, The chemistry of silica. Solubility, polymerization, colloid and surface properties, and biochemistry, John Wiley and Sons, Chichester, 1979.
- N. Roveri, M. Morpurgo, B. Palazzo, B. Parma, L. Vivi, Anal. Bioanal. Chem., 2005, 381(3), 601-606.
- P. Kortesuo, M. Ahola, M. Kangas, A. Yli-Urpo, J. Kiesvaara, M. Marvola, Int. J. Pharm., 2001, 221(1), 107-114.
- J.C. Ro, J.C. In, J. Non-Cryst. Solids., 1991, 130(1), 8-17.
- M.D. Curran, A. Stiegman, J. Non-Cryst. Solids., 1999, 249(1), 62-68.
- C.Y. Lai, B.G. Trewyn, D.M. Jeftinija, K. Jeftinija, S. Xu, S. Jeftinija, V.S. Lin, J. Am. Chem. Soc., 2003, 125(15), 4451-4459.
- N.K. Mal, M. Fujiwara, Y. Tanaka, Nature., 2003, 421(6921), 350-353.
- D. Arcos, J. Peña, M. Vallet-Regí, Chem. Mater., 2003, 15(21), 4132-4138.
- S. Sadighian, K. Rostamizadeh, H. Hosseini-Monfared, M. Hamidi, Colloids. Surf. B., 2014, 117, 406-413.
- F. Hosseini, S. Sadighian, H. Hosseini-Monfared, N.M. Mahmoodi, Desalin. Water. Treat., 2016, 57(51), 24378-24386.
- S. Sadighian, K. Rostamizadeh, M. J. Hosseini, M. Hamidi, H. Hosseini-Monfared, Toxicol. Lett., 2017, 278, 18-29.
- X. Lu, Y. Wang, Y. Liu, J. Wang, SQu, Feng B, J. Weng, Mater. Lett., 2007, 61(18), 3970-3973.
- S.H. Teng, E.J. Lee, B.H. Yoon, D.S. Shin, H.E. Kim, J.S. Oh, J. Biomed. Mater. Res. A., 2009, 88(3), 569-580.
- K. Sukhbir, V. Chawla, R.K. Narang, G. Aggarwal, Asian J. Pharm. Clin. Res., 2017, 10(6), 125-130.
- G. Hariharan, P. Sinha, Asian. J. Pharm. Clin. Res., 2017, 10(10), 136-141.
- S. Sadighian, H. Hosseini-Monfared, K. Rostamizadeh, M. Hamidi, Adv. Pharm. Bull., 2015, 5(1), 115.