Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 2
Spectrophotometric Study Oxidation of Amlodipine Besylate by Chloramine-T in Acidic Buffer pH (4.3) Medium
Fathyah Omar Abdulsalam, Prema Kadappa Reddy and Asha Iyengar T*
Department of Chemistry, Yuvaraja’s College, University of Mysore, Mysuru-570005, Karnataka, India
- *Corresponding Author:
- Asha Iyengar T
Department of Chemistry
Yuvaraja’s College
University of Mysore
Mysuru-570005, Karnataka, India
Abstract
Kinetic oxidation of Amlodipine besylate (AML) with Chloramin-T(CAT) has been studied in acidic buffer medium (4.3). The experimental rate of reaction was found to be -d[CAT]/dt=k [CAT]o[AML]o[OH–]–0.52 at 306 K. The stoichiometry of the reaction was found to be 1:1 and the oxidation products of AML were identified. The effect of added halide ions, reduction product (p-toluene sulphonamide, PTS), ionic strength (NaClO4) and dielectric constant of the medium was studied on the rate of reaction. The activation parameters have been deduced from Arrhenius plots and proper mechanism has been worked out.
Keywords
Chloramine-T, Spectrophotometry, Acidic Buffer, pH
Introduction
Amlodipine is a strong dihydropyridine calcium antagonist [1,2] useful in the management of angina pectoris and hypertension [3]. It belongs to the Dihydropyridine (DHP) family and its derivatives which are photosensitive and are converted to inactive and even toxic products when they are irradiated by ultraviolet visible lights [4-6]. Amlodipine chemically is (2-[(2-amino-ethoxy)-methyl]-4-2-chlorophenyl-1,4-dihydro-6- methyl-3,5-pyridine dicarboxylic acid-3-ethyl-5-methyl ester [7] (Figure 1). It is mainly used for its antianginal, antihypertensive and antiarrhythmic activity. Survey of literature for AML revealed several chromatographic methods for its determination in pharmaceuticals and biological fluids [8-13]. Assay of the drugs has also been studied by different spectroscopic methods [14-21]. Stability of amlodipine in liquid form has been also reported [22,23]. The amlodipine besylate drug in pure form and its figurations are not official in USP pharmacopeia, hence needing more investigations.
According to details of literature survey, no kinetic study of oxidation of amlodipine besylate with chloramine-T has been reported. The chemistry of N-haloamines is of interest due to their diverse behavior [24]. The prominent member of this class of compounds is sodium N-chloro-p-toluenesulfonamide, commonly known as Chloramine-T (CAT). It is a well-known analytical and oxidizing reagent. The kinetic and mechanistic aspects of this reagent have been well documented [25-33]. Our preliminary experimental study of the reaction of AML with CATin alkaline medium revealed it to be very slow to be studied kinetically. However, it was faster in acidic buffer medium. The aim of this investigation is to explain the proper mechanism, to predict experimental rate law and to ascertain the various reactive species of the oxidant to understand the oxidation chemistry of AML with CAT.
Expermintal Section
Materials
The substrate pure Amlodipine Besylate (AML) was purchased from Yarrow Chem Products, Mumbai, India and was used as received. The solubility of AML was found to be sparingly soluble in water, For this reason, the stock solution was prepared by dissolving the drug with 1 cm3 of ethanol and then made up with double distilled water in a volumetric flask (100 ml). It was kept inside a refrigerator to avoid decomposition. The stock solution of the AML was prepared freshly whenever required each time. The buffer solution was purchased from LOBA CHEMIE. An aqueous solution of the oxidant chloramine. CAT from SDFCL, Mumbai, was prepared, standardized periodically by the iodometric method and kept in brown bottles to prevent the photochemical effect. Analar grade chemicals and doubly distilled water were used throughout.
Kinetic method
Kinetic measurements were carried out using a UV-Visible spectrophotometer single beam (Digital Spectrophotometer 1200, Labman, India). Kinetic runs were performed under pseudo first-order conditions of large excess of the drug over the oxidant at 306 K Absorbance measurements were made at 390 nm (λmax for AML) for more than two half-lives. The absorbance readings at t=0 and t=t are D0 and Dt. The Plot of log [CAT] vs. time was made to calculate the pseudo-first-order rate constant (k's-1), reproducible within ± 4-5%.
Reaction stoichiometry and product analysis
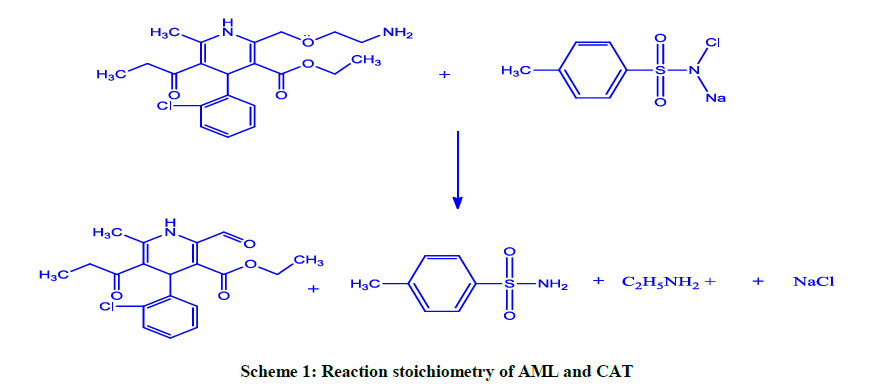
Reaction mixtures containing different ratios of CAT to AML in acidic buffer pH 4.3 were equilibrated at 306 K for 24 h. Determination of the residual oxidant showed that one mole of AML consumed mole of CAT (Scheme 1). The Equation 1 represents the stoichiometric result:

The reaction mixture of AML and CAT in acidic buffer solution was stirred for 48 h at 306 k. After completion of the reaction (Monitored by TLC), the reaction mixture was neutralized with dilute NaOH and the products were extracted with ether acetate.
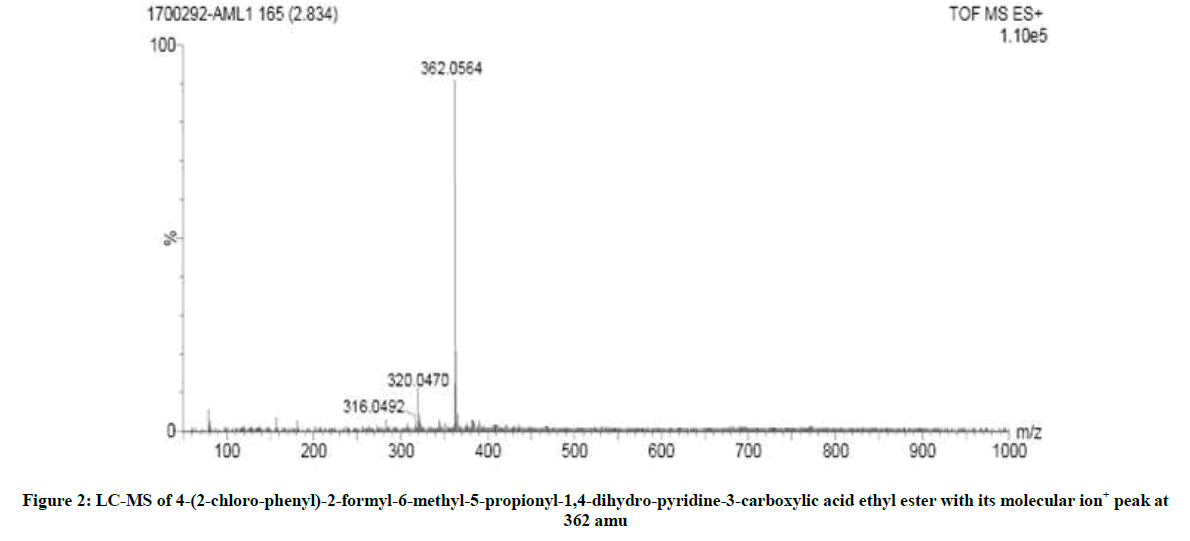
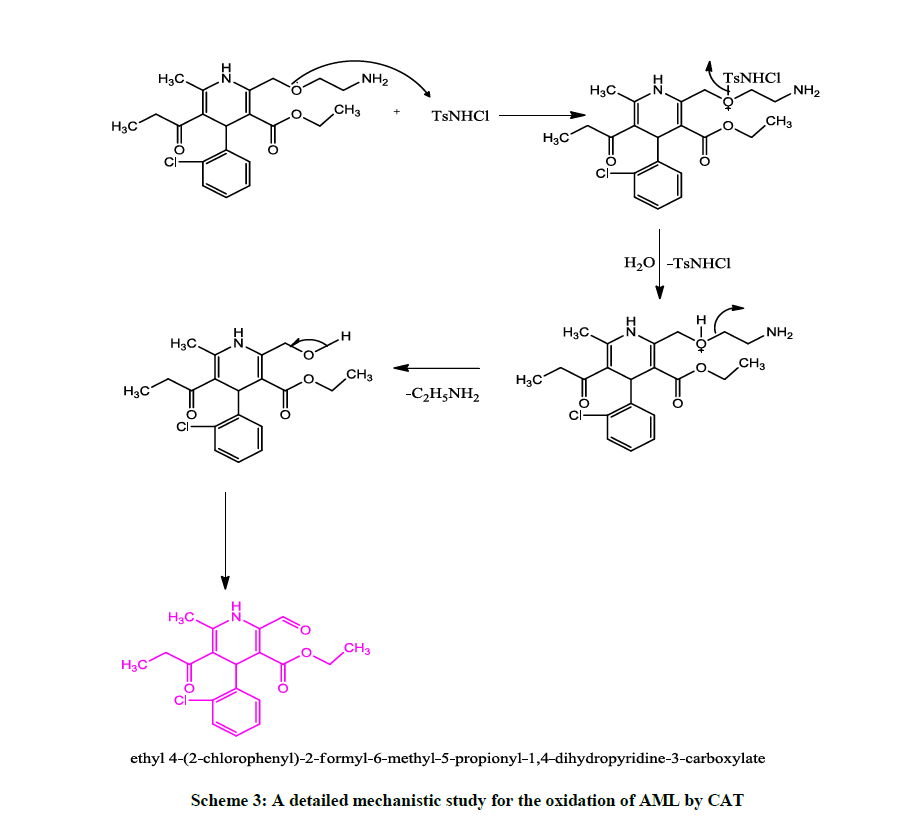
The reduction product of CAT, p-toluene sulfonamide was identified [34] by paper chromatography using benzyl alcohol saturated with H2O as the solvent with 0.5% vanillin in 1% HCl solution in ethanol as the spray reagent (Rf=0.905). Two oxidized product were identified as ethyl 4- (2-chloro-phenyl)-2-formyl-6-methyl-5-propionyl-1,4-dihydro-pyridine-3-carboxylic acid ethyl ester and ethylamine. The first product was confirmed by mass spectral analysis (Figure 2). The mass spectrum showed a molecular ion peak at 362 amu indicates the structure of 4-(2- chloro-phenyl)-2-formyl-6-methyl-5-propionyl-1,4-dihydro-pyridine-3-carboxylic acid ethyl ester. And the second oxidized product ethylamine was confirmed by the carbalamine test [34]. It was noticed that there was no reaction between the products and oxidant under the current set of experimental conditions.
Results
In consider to first experimental data, the oxidation reaction of AML by CAT in acidic buffer medium was good to be measured kinetically. Therefore, the oxidation of AML was studied at several Data initial concentrations of the reactants in acidic buffer media pH=4.3 at 308 k, under similar experirmenal conditions.
Effect of reactant concentrations on the rate
The experiments were conducted under pseudo-first order conditions of [AML]0 >> [CAT]0 at constant [AML]0, pH and temperature. The plot of log [CAT]0 vs. time were found to be linear (r=0.996). The rate constant did not change with [CAT]0 confirming the first order dependence of the reaction rate on [CAT]0 (Table 1). Under identical experimental conditions, an increase in the [AML]0 leads to an increase in the k values (Table 1). The plots of log k/vs. log [AML]0 were linear (r=0.998) with unit slopes showing first-order dependence of the rate on the [AML].
| [CAT] 103 (mold/m3) |
[AML] 104 (mold/m3) |
pH | 104 k (s-1) |
|---|---|---|---|
| 1 | 2 | 4.3 | 7.3 |
| 1.5 | 2 | 4.3 | 7.3 |
| 2.5 | 2 | 4.3 | 7.3 |
| 3.5 | 2 | 4.3 | 7.3 |
| 4.5 | 2 | 4.3 | 7.3 |
| 2.5 | 1 | 4.3 | 1.4 |
| 2.5 | 1.5 | 4.3 | 3.2 |
| 2.5 | 2 | 4.3 | 7.3 |
| 2.5 | 2.5 | 4.3 | 9.4 |
| 2.5 | 3 | 4.3 | 13.5 |
| 2.5 | 2 | 4.1 | 13.05 |
| 2.5 | 2 | 4.2 | 9.09 |
| 2.5 | 2 | 4.3 | 7.31 |
| 2.5 | 2 | 4.4 | 3.09 |
| 2.5 | 2 | 4.5 | 2.38 |
Table 1: Effect of variation of [CAT], AML and pH concentrations on the reaction rate at 306 K
Effect of pH and halide ion concentration on the rate
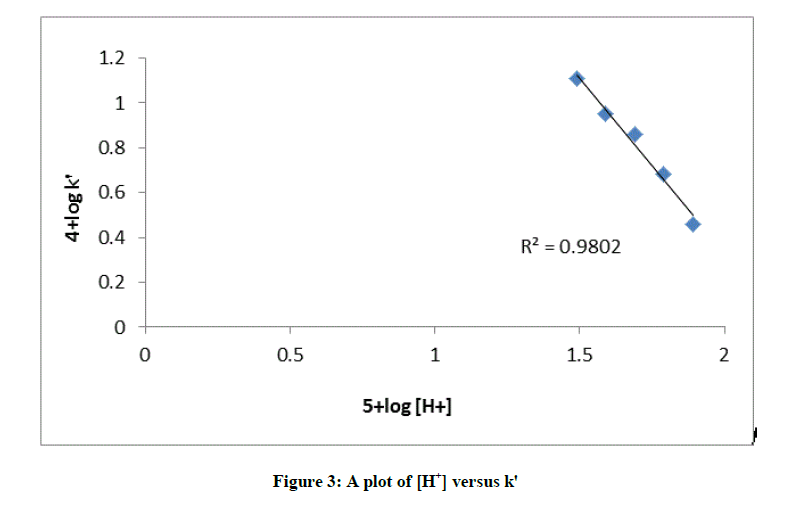
The effect of the acidic buffer [H+] concentration was studied with keeping [AML], [CAT] and temperature at constant values. The rate constants decreased with increase in the [H+]. The plot of log k vs. log [H+] was linear with a negative slope (-0.52) (Table 1 and Figure 3) indicating an inverse fractional-order dependence in [H+]. The addition of Cl- or Br– ions as NaCl or NaBr had no effect on the rate, suggesting no role of halide ions in the reaction sequence.
Effect of dielectric constant on the rate
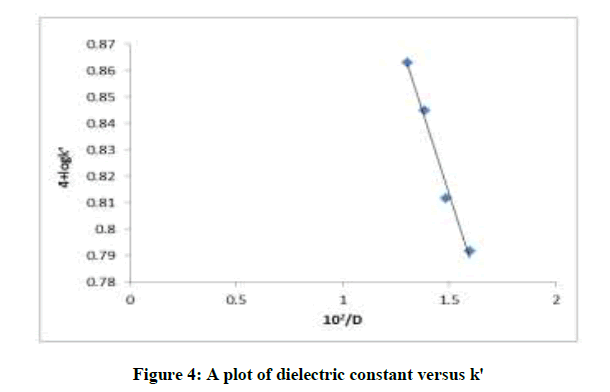
The dielectric constant (D) of the medium was investigated by the addition of methanol to the reaction medium. The addition of methanol showed a decrease in reaction rate supporting the rate limiting step with no charge dispersal (Table 2). The plot of dielectric constant [D] vs. log k/is shown in (Figure 4) was linear with a negative slope of -0.44. It was noticed that no reaction of dielectric with CAT under the experimental conditions used.
| Temperature (K) | 104 k (s-1) | Activation parameters |
|---|---|---|
| 296 | 2.02 | Ea=79.5 kJ/mol-1 |
| 301 | 4.65 | ∆H≠=75.2 kJ/mol-1 |
| 306 | 7.31 | ∆S≠= -77.7 Jk-1/mol-1 |
| 311 | 9.93 | ∆G≠= 99.5 kJ/mol-1 |
| 316 | 16.88 | Log A=11.3 |
Table 2: Effect of varying temperature and activation parameters for oxidation of AML by CAT
Effect of P-toluene sulfonamide and ionic strength on the rate
The addition of p-Toluene Sulfonamide (PTS), the reduction product of CAT to the reaction mixture did not affect the rate significantly, This indicates that PTS is not involved in the rate determining step in the scheme proposed. Similarly, The effect of ionic strength of the medium on the rate of reaction was carried out using NaClO4 solution with all other experimental conditions held constant. It was found that ionic strength had negligible effect on the reaction rate signifying no need to keep the ionic strength of the reaction mixture constant in the kinetic runs.
Effect of temperature on the rate
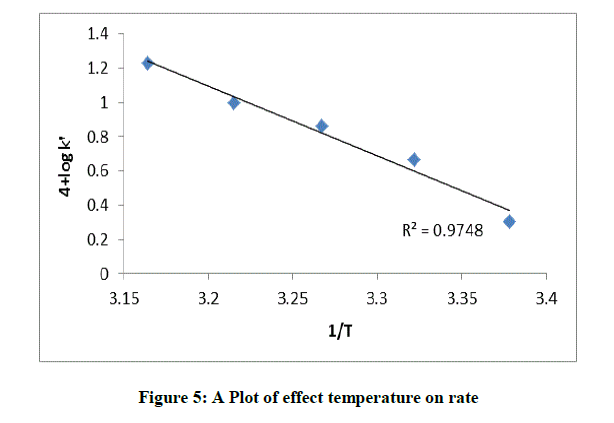
The rate constant for the oxidation of AML with CAT in the temperature range (296-311 K) was measured by keeping the other experimental conditions constant. It was noticed that the rate constant of the reaction increases with increase in temperature for given concentration of AML (Table 2 and Figure 5). From the Arrhenius plot of log k/vs. 1/T activation parameters were computed (Table 2).
Test for free radical
The addition of the monomer of aqueous acrylamide solution to the reaction mixture showed the absence of free radical generation.
Discussion
Chloramine-T acts as a moderate oxidant in both acid and alkali solutions with a two electron change to give reduction products (PTS, NaCl). Different ionic species of acidic chloramie-T the conjugate free acid (TsNHCl), dichloramine-T (TsNHCl2), hypochlorous acid (HOCl) and possibly H2OCl+ had reported early by Cambelland and Johnston [26], Bishop E and Jennings [27], Morris et al., [28].
If TsNHCl2 was the reactive species, then the rate law would predict a second order dependence of rate on [CAT], which is contrary to experimental observation. if HOCl is primary oxidizing species, a first order retardation of the rate by the added TsNH2 (PTS) is expected, Narayana and Rao [29] have reported that monohaloamines can be protonated in acidic medium at pH ˃ 4 as shown in equation (1) for chloramine-T.
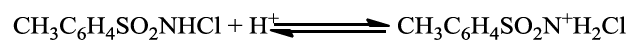
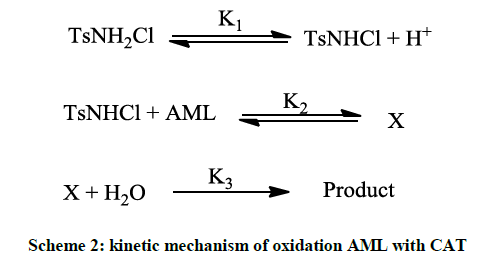
The second protonation constant for CAT is 102 at 25°C, deprotonation of TsN+H2Cl results in the generation of TsNHCl in case of inverse fractional order in [H+] as involved in present study. In accordance to with experimental data, the active oxidizing species is TsNHCl and obtained Scheme 2 is proposed to explain the reaction mechanism for the oxidation of AML by CAT (Scheme 3).
Reactive specie
 (1)
(1)
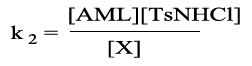 (2)
(2)
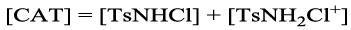 (3)
(3)
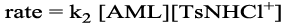 (4)
(4)
From eq (1) substituting in eq (3) got
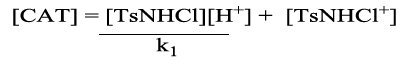 (5)
(5)
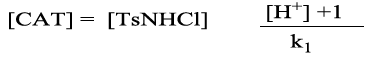 (6)
(6)
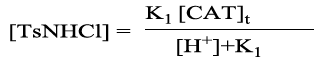 (7)
(7)
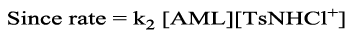
Then the rate law will be
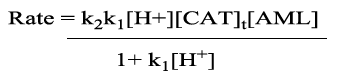 (8)
(8)
Rate law in Equation 8 is in good agreement with experimental results with a first order dependence of rate on both [AML]0 and [CAT]0, and an inverse-fractional order dependence on [H+], the proposed mechanism is proved by the observed data. Methanol in different proportions (0-30%, v/v) is added to alter dielectric constant of the medium. The negative dielectric effect as obtained from the plot of log k' versus 1/D reveals the presence of dipole-dipole interaction in the reaction 21, 23. Addition of reduction product PTS was unable to change the rate indicating its non-involvement in pre-equilibrium. The rate remains un-effected on varying ionic strength of the medium indicating the involvement of non-ionic species in the rate limiting step. Halide ions have no significant effect on the reaction rate. Further the computed thermodynamic parameters support the proposed mechanism. A large negative value of entropy of activation and moderate positive values of free energy of activation and enthalpy of activation indicate the formation of properly ordered compact transition state complex having less degrees of freedom.
Conclusion
The kinetics of oxidation of AML drug by CAT has been investigated spectrophotometrically at 306 K. The oxidation reaction pursues and obeys the rate law: rate [CAT]o, [AML]o equal to unity. The rate of [H+] was inverse fractional order (-0.51). Activation parameters were calculated and the suitable mechanism and rate law are done according to experimental results obtained.
Acknowledgments
One of Authors (Fathyah Omar) thankfully acknowledges the financial support of embassy of Libya in New Delhi, India.
References
- D.R. Abernethy, Am. Heart J., 1989, 118(5 Pt 2), 1100-1103.
- G. Kungys, H. Naujoks, C. Wanner, Eur. J. Clin. Pharmacol., 2003, 59(4), 291-595.
- J. Reid, P. Meredith, H. Elliot, J. Cardiovasc. Pharmacol., 1988, 12- 22.
- D. Jang, E. Jeong, H. Lee, B. Kim, S. Lim, C. Kim, Eur. J. Pharm. Sci., 2006, 28, 405.
- G. Ragno, E. Cione, A. Garofalo, G. Genchi, G. Ioele, A. Risoli, A. Spagnoletta, Int. J. Pharm., 2003, 26, 125.
- G. Ragno, A. Garofalo C. Vetuschi, J. Pharm. Biomed. Anal., 2002, 27, 9.
- J.E.F. Reynolds, Royal Pharmaceutical Society of Great Britain, London, 1996.
- A. Zarghi, S.M. Foroutan, A. Shafaati, A. Khoddam, IL Farmaco., 2005, 60, 789.
- S. Tatar, S. Atmaca, J. Chromatogr. B., 2001, 758, 305.
- G. Bahrami, S. Mirzaeei, J. Pharm. Biomed. Anal., 2004, 36, 163.
- M. Josefsson, A. Zackrisson B. Norlander, J. Chromatogr. B., 1995, 672, 310.
- S.C. Monkman, J.S. Ellis, S. Cholerton, J.M. Thomason, R.A. Seymour, J.R. Idle, J. Chromatogr. B., 1996, 678, 360.
- A.P. Beresford, P.V. Marcae, D.A. Stopher, B.A. Wood, J. Chromatogr. B., 1987, 64, 178.
- J.H. Kumar, R.K. Agramal, Indian Drugs., 37, 2000, 196.
- C.V.N. Prasad, C. Parikar, T.R. Chowdhary, S. Purohit, P. Parinroo, Pharm. Pharmacol. Commun., 1998, 4, 325.
- P. Anu, G.K. Suvarna, D. Sathyanarayana, East. Pharm., 2000, 43, 111.
- N. Rahman S.N.H. Azmi, IL Farmaco., 2001, 56, 731.
- T.K. Murthy, M.N. Reddy, M.D. Reddy, D.G. Sankar, Asian J. Chem., 2001, 13, 771.
- N. Rahman, M.N. Hoda, J. Pharm. Biomed. Anal., 2003, 31, 381.
- N. Rahman, M. Singh, M.N. Hoda, IL Farmaco., 2004, 59, 913.
- K. Basavaiah, U. Chandrashekar, H.C. Prameela, IL Farmaco., 2003, 58, 141.
- N. Rahman, S.N.H. Azmi, Acta Biochim. Polon., 2005, 52, 915.
- N. Rahman, S.N.H. Azmi, Sci. Asia., 2006, 32, 429.
- X. Li, A. Bjorkman, T.B. Andersson, M. Ridderstrom, C.M. Masimirembwa, J. Pharma. Exp. Therapeut., 2002, 300, 399.
- M.M. Campbell, G. Johnson, Chem. Rev.,1978, 78, 65.
- E. Bishop, V. Jennings, J. Talanta., 1958, 1, 197.
- J.C. Morris, J. A Salazar, J. Winemann, J. Am. Chem. Soc.,1948, 70, 2035.
- K.K. Banerji, B. Jayaram, D.S. Mahadevappa, J. Sci. Ind. Res., 1987, 4665.
- A. Geethanjali, Synlett., 2005, 18, 2857.
- E. Kolvari, A. Ghorbani-Choghamarani, P. Salehi, F. Shirini, M.A. Zolfigol, J. Iran Chem. Soc., 2007, 4126.
- Puttaswamy, R.V Jagadeesh, Appl. Catal. A: Gen.,2005, 2923, 259.
- K.N. Vinod, Puttaswamy, K.N. Ninge Gowda, Ind. Eng. Chem. Res.,2010, 49, 3137.
- Puttaswamy, A. Sukhdev, J.P Shubha, J. Mol. Catal A: Chem.,2009, 310, 24.
- Puttaswamy, R.V. Jagadeesh, V. Nirmala, A. Radhakrishna, J. Mol. Catal. A: Chem.,2005, 229, 211.