Research Article - Der Pharma Chemica ( 2023) Volume 15, Issue 3
STABILITY INDICATING RP- HPLC METHOD FOR SIMULTANEOUS ESTIMATION OF SALICYLIC ACID AND KETOCONAZOLE IN SHAMPOO FORMULATION
Dilip Kowndinya B1*, Basava Rao VV1 and Sailu CH1Dilip Kowndinya B, Department of Pharmacy, University College of Technology, Osmania, Hyderabad-500007, Telangana, India, Email: kowndinyad@gmail.com
Received: 01-May-2023, Manuscript No. dpc-23-101157; Accepted Date: May 29, 2023 ; Editor assigned: 05-May-2023, Pre QC No. dpc-23-101157; Reviewed: 19-May-2023, QC No. dpc-23-101157; Revised: 26-May-2023, Manuscript No. dpc-23-101157; Published: 31-May-2023, DOI: 10.4172/0975-413X.15.3.70-79
Abstract
A reverse phase HPLC method was developed and validated for the simultaneous estimation of Salicylic acid and Ketoconazole in shampoo formulation. Separation of the drugs was done using Kromasil C18 (250×4.6mm, 5μ) column at 322 nm using a mobile phase comprising of Acetonitrile: Buffer (0.01 N Potassium dihydrogen orthophosphate adjusted to a PH of 5.4 using dilute Orthophosphoric acid) in the ratio of 50:50 at a flow rate of 1.0 mL/min. Retention times of Salicylic acid and Ketoconazole were found to be 2.307 min, 3.342 min respectively. Method was found to be linear in the range of 5-30 μg/ml. Developed method was validated and stability studies were performed according to ICH guidelines and applied for the assay of the drugs in the shampoo formulation.
Keywords
Salicylic acid; Ketoconazole; Validation; HPLC; Shampoo
INTRODUCTION
Ketoconazole1-[4-(4-{[(2R, 4S)-2-(2, 4-Dichlorophenyl)-2-(1H-imidazol-1-ylmethyl)-1, 3-dioxolan-4-yl] methoxy} phenyl) piperazin-1-yl] ethan-1-one is antifungal drug used to prevent and treat fungal skin infections (Figure1). Ketoconazole is commercially available in the form of anti- dandruff shampoo, topical creams and lotions. Salicylic acid is a (2-hydroxyl benzoic acid), and naturally occurring in the bark of willow tree (Salix Alba) (Figure 2) The salts and esters of salicylic acid are known as salicylates that are widely used as rubefacient and analgesic in several topical formulations. Salicylic acid alleviates peeling of intercellular cement and binds with scales in the stratum corneum, thereby loosening the keratin. This keratolytic effect also renders an antifungal effect as removal of the stratum corneum suppresses the fungal growth. It is widely used for the treatment of several skin diseases like acne, psoriasis, seborrhoeic dermatitis, calluses, keratosis pilaris, and warts due to its keratolytic, fungicidal, bacteriostatic, and photo protective properties. HPLC methods for estimation of Salicylic acid and Ketoconazole were published individually and in combination with other drugs like pythione. Literature review [1-3] revealed that there are no HPLC methods published till date for the simultaneous estimation Salicylic Acid and Ketoconazole; hence there is a scope for development of a new method for the simultaneous estimation of both the drugs in combined dosage forms. The main objective of present study is to develop a simple, accurate, precise, sensitive, selective, reproducible and rapid analytical technique for simultaneous estimation of Salicylic Acid and Ketoconazole, in bulk and shampoo [4, 5].
Materials Required (2)
Salicylic Acid and Ketoconazole pure drugs (API) received from MSN Pharma Ltd. Combined form of Salicylic Acid and Ketoconazole shampoo purchased from local market (Table 1- 2)
| S. No | Instruments | Make and model |
|---|---|---|
| 1 | HPLC | Waters HPLC system outfitted with 515 dual head reciprocating pump and a 2489 UV Visible detector. The software used was Empower-2. |
| Enable C-18 column (5µm, 250mm×4.6mm i.d) | ||
| 2 | UV-VIS Spectrophotometer | Model-2201, SYSTRONICS. |
| 3 | Digital pH meter | Model-MKVI, SYSTRONICS. |
| 4 | Electronic balance | Model-AY 220, SHIMADZU. |
| 5 | Ultrasonicator | BIOTECHNICS, India. |
| 6 | Vacuum filtration apparatus | Borosil 5340 |
| Vacuum pressure pump | TID-75-S. |
Table 1: Equipment’s and Apparatus.
| S. No | Chemical/Reagent | Grade | Make |
|---|---|---|---|
| 1 | Methanol | HPLC | Merck |
| 2 | Acetonitrile | HPLC | Merck |
| 3 | Orthophosphoric acid | AR | Merck |
| 4 | Potassium dihydrogen phosphate | AR | Merck |
| 5 | Water | HPLC | In house |
| 6 | Sodium hydroxide | AR | SD fine Chemicals |
| 7 | Hydrochloric acid | AR | SD fine Chemicals |
Table 2: Chemicals and Reagents.
Preparation of Solutions (3)
Preparation of Stock solutions (3.1)
Accurately weighed 100 mg of Ketoconazole and 100 mg of Salicylic Acid and transferred to 100 ml volumetric flask. And 3/4th of diluent was added to these flask and sonicated for 10 minutes. Flasks were made up with diluents and labeled as Standard stock solution. (1000 μg/ml of Ketoconazole and 1000μg/ml of Salicylic Acid)
Preparation of Cc standards (3.2)
Calibration curve standards were prepared by pipetting suitable aliquots of secondary stock solution containing 200μg/ml of Ketoconazole and 200μg/ml of Salicylic Acid into separate 10 ml volumetric flasks and the volume was made up to the mark with diluent to obtain the CC standards in the range of 5 to 30 μg/ ml concentrations for both drugs (Table 3)
| S.No | Pipetted from stock (mL) | Volume of flask (mL) | Concentration in ppm (Sali) | Concentration in ppm (Keto) | %Linearity Level |
|---|---|---|---|---|---|
| 1 | 0.25 | 10 | 5 | 5 | 25 |
| 2 | 0.5 | 10 | 10 | 10 | 50 |
| 3 | 0.75 | 10 | 15 | 15 | 75 |
| 4 | 1 | 10 | 20 | 20 | 100 |
| 5 | 1.25 | 10 | 25 | 25 | 120 |
| 6 | 1.5 | 10 | 30 | 30 | 150 |
Table 3: Preparation of standard Solutions for linearity.
Preparation of diluent (3.2.1)
Acetonitrile and Water taken in the ratio of 50:50 %v/v. It was ultra sonicated for 5min.
Preparation of Cc standards (3.2.2)
Shampoo equivalent to 20mg of Salicylic Acid & 20mg of Ketoconazole was transferred into a 100 ml of volumetric flask, 50ml of diluents was added and sonicated for 25min, and further the volume was made up with diluent and filtered. Resulting solution contain 200μg/ml of Salicylic Acid & 200μg/ml Ketoconazole. 1ml of filtered sample stock solution was transferred in to a 10ml volumetric flask and made up the volume with diluent, resulting solution contain 20μg/ml of Salicylic Acid & 20μg/ml Ketoconazole.
METHODOLOGY
Chromatographic conditions (4)
The method was developed and validated using Kromosil C-18, (250 x 4.5 mm, 5.) column. Mobile phase is prepared by mixing HPLC grade Acetonitrile and 0.01N Potassium phosphate buffer (adjusted to pH 5.4) in the ratio of 50:50 % v/v as mobile phase. Separation was done in isocratic mode at 1.0 mL/min flow rate. Detection wavelength was selected at 322 nm
System suitability (4.1)
The system suitability parameters were determined by using standard solutions of concentration 20 ppm for both Ketoconazole and Salicylic Acid. Six replicate injections of the standards were injected and the parameters like USP peak tailing, resolution and USP plate count were determined.
Method validation (4.2)
The method validation was performed in accordance with ICH guidelines
Linearity (4.2.1)
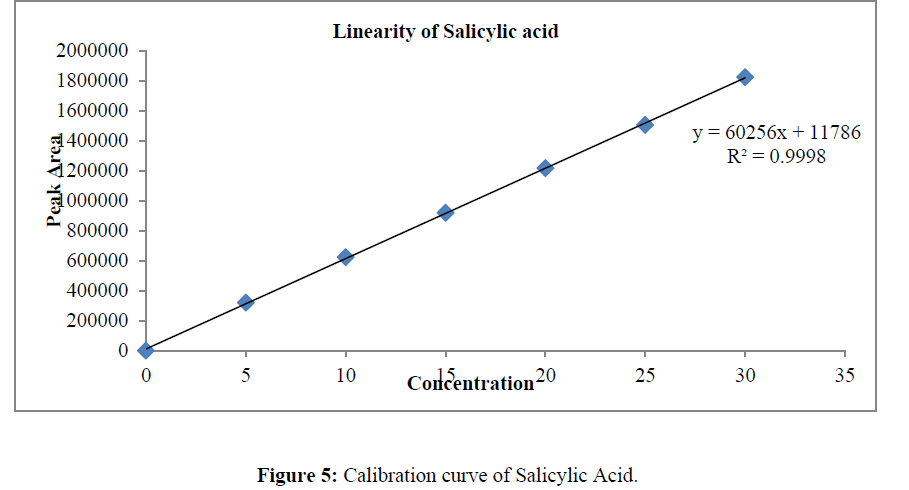
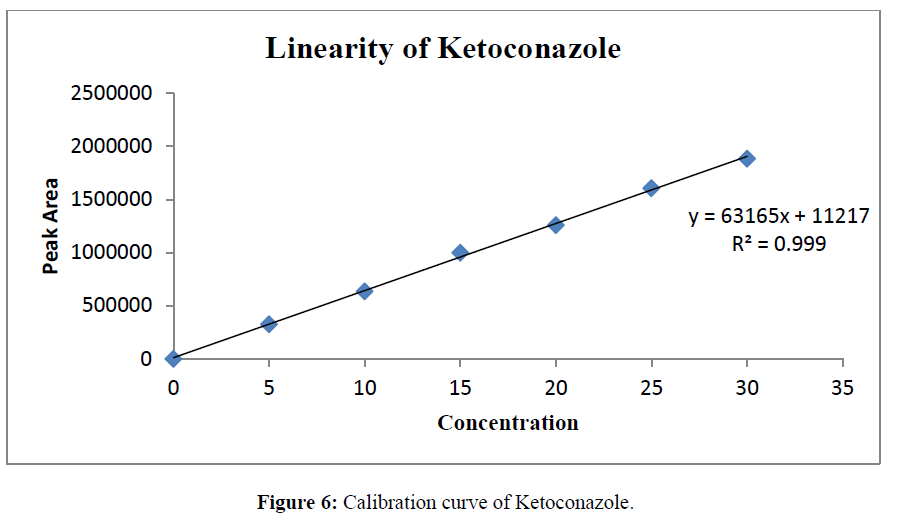
Prepared standard dilutions of Ketoconazole and Salicylic Acid (5-30μg/ml) were injected and chromatograms were recorded. A calibration curve was constructed by taking concentration on X- axis and peak area on Y- axis.
Accuracy (4.2.2)
Accuracy was determined by the recovery studies of the analyte. It is determined by standard addition method, where the test solution of known quantity is spiked with standard solutions at three levels i.e., 50%, 100% & 150% in triplicate. Percentage recoveries at all the levels were calculated.
Precision (4.2.3)
Precision of the method was established at two levels i.e., system precision and method precision. System precision was performed by injecting the working standard solution. Six injections were given from the same standard solution and the peak areas were obtained. Average area, standard deviation and % RSD were calculated for two drugs. Method precision was established by taking multiple sampling from a sample stock solution. Six working sample solutions of same concentrations were prepared, single injection from each working sample solution was given. Average peak area, standard deviation and % RSD were calculated for two drugs.
Robustness (4.2.4)
Small deliberate changes in method like flow rate, mobile phase ratio, and temperature are made. Robustness conditions like Flow minus (0.9ml/min), Flow plus (1.1ml/min), mobile phase minus, mobile phase plus, temperature minus (25°C) and temperature plus (35°C) was maintained and samples were injected in sextet. System suitability parameters are evaluated by making the deliberate changes.
Specificity (4.2.5)
The specificity was established by performing stress degradation studies for the analyte under various conditions like acid degradation, alkali degradation, oxidative degradation, thermal degradation and photo degradation.
Acid Degradation Studies (4.2.5.1)
To 1 ml of stock solution Salicylic Acid & Ketoconazole 1ml of 2N Hydrochloric acid was added and refluxed for 30mins.The resultant solution was diluted to obtain (20ppm & 20ppm) solution and 10 μl solutions were injected into the system and the chromatograms were recorded to assess the stability of sample
Alkali Degradation Studies (4.2.5.2)
To 1 ml of stock solution Salicylic Acid & Ketoconazole 1 ml of 2N sodium hydroxide was added and refluxed for 30mins. The resultant solution was diluted to obtain (20ppm& 20ppm) solution and 10μl were injected into the system and the chromatograms were recorded to assess the stability of sample.
Thermal Degradation Studies (4.2.5.3)
The standard drug solution was placedinovenat1050C for 6h to study dry heat degradation. For HPLC study, the resultant solution was diluted to (20ppm& 20ppm) solutionand10μl were injected into the system and the chromatograms were recorded to assess the stability of the sample.
Photo Stability studies (4.2.5.4)
The photochemical stability of the drug was also studied by exposing the (200ppm & 200ppm) solution to UV Light by keeping the beaker in UV Chamber for 7days or 200 Watt hours/m2 in photo stability chamber. For HPLC study, the resultant solution was diluted to obtain (20ppm & 20ppm) solutions and 10 μl were injected into the system and the chromatograms were recorded to assess the stability of sample.
Oxidative degradation (4.2.5.5)
To 1 ml of stock solution of Ketoconazole and Salicylic Acid, 1 ml of 20% hydrogen peroxide (H2O2) was added separately. The solutions were kept for 30 min at 60 0C. For HPLC study, the resultant solution was diluted to obtain 60 μg/ ml & 40 μg/ ml solution.10 μl was injected into the system and the chromatograms were recorded to assess the stability of sample.
RESULTS AND DISCUSSION
Assay of formulation (5.1)
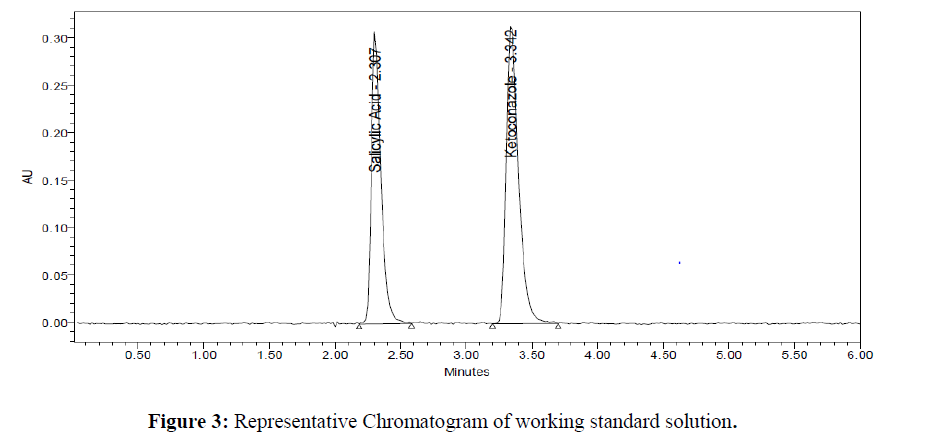
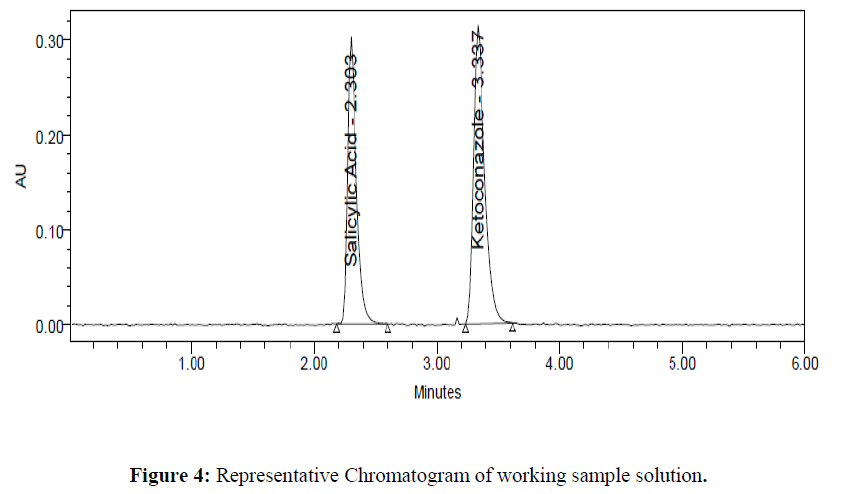
Assay of the formulation is performed in triplicate. The amount of drug present in the formulation was calculated. The % assay of Salicylic Acid and Ketoconazole obtained was 99.02 and 99.51 % respectively. Representative chromatograms for standard and test were given in (Figure 3- 4). Results were given in the below (Table 4)
| S.NO | Peak area of Salicylic Acid | Peak area of Ketoconazole |
|---|---|---|
| 1 | 1205637 | 1248455 |
| 2 | 1201125 | 1245480 |
| 3 | 1211505 | 1242130 |
| 4 | 1206432 | 1245404 |
| 5 | 1214450 | 1249068 |
| 6 | 1213795 | 1242086 |
| Avg | 1208824 | 1245437 |
| Regression equation | y = 60256x + 11786 | y = 6134.x + 432.1 |
| % Assay | 99.02% | 99.51% |
Table 4: Assay of Salicylic Acid and Ketoconazole.
System suitability (5.2)
System suitability parameters were determined according to ICH guidelines. Plate count should be more than 2000, tailing factor should be less than 2 and resolution must be more than 2. All the system suitable parameters were passed and were within the limits. The results showing system suitability parameters were given in below (Table 5)
| Salicylic Acid | Ketoconazole | ||||||
|---|---|---|---|---|---|---|---|
| Inj | RT(min) | USP Plate Count | Tailing | RT(min) | USP Plate Count | Tailing | Resolution |
| 1 | 2.305 | 5069 | 1.43 | 3.339 | 6721 | 1.43 | 6.8 |
| 2 | 2.307 | 5115 | 1.48 | 3.341 | 6759 | 1.43 | 6.9 |
| 3 | 2.307 | 5169 | 1.47 | 3.341 | 6777 | 1.43 | 6.9 |
| 4 | 2.308 | 5101 | 1.51 | 3.342 | 6631 | 1.42 | 6.9 |
| 5 | 2.309 | 5197 | 1.5 | 3.346 | 6650 | 1.42 | 6.8 |
| 6 | 2.3 | 5235 | 1.49 | 3.346 | 6560 | 1.44 | 6.8 |
Table 5: System suitability parameters for Salicylic Acid and Ketoconazole.
Validation (5.3)
Linearity (5.3.1)
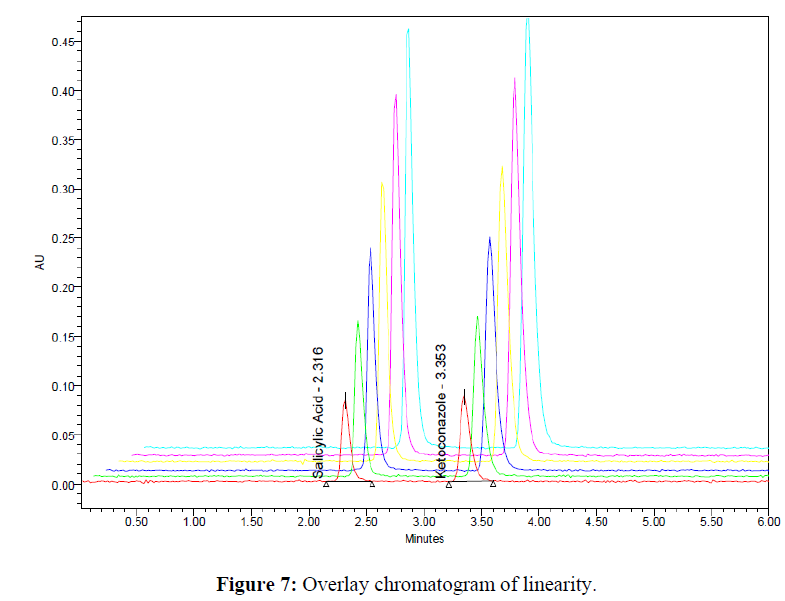
The linearity was determined at concentration range of 5-30μg/ml for Ketoconazole and Salicylic Acid. Graph was plotted using Peak areas against concentration. The calibration curve was shown in (Figure 5-7). The Correlation coefficient (r2) was greater than 0.99 within the concentration range for both the drugs. The results for linearity were given in the below (Table 6)
Accuracy (5.3.2)
Accuracy of the method was established at three levels of concentrations by standard addition method. Triplicate injections were given at each level of accuracy and percentage recoveries were calculated. The mean % Recovery was obtained was 99.09 to 99.46% for Salicylic Acid and 99.14- 99.46 % for Ketoconazole. The results for accuracy was given in the below (Table 7-8)
Precision (5.3.3)
The precision of the method was studied by considering system precision and method precision. System precision was studied by taking six replicate injections from same homogenous standard solution and peak areas were determined. Average area, standard deviation and % RSD were calculated for two drugs. % RSD obtained as 0.9% and 1.0 % respectively for Ketoconazole and Salicylic Acid. The results for system precision were given in (Table 6). Method precision was studied by preparing six test solutions of same concentration and injected once from each solution and peak areas were determined. Average area, standard deviation and % RSD were calculated for two drugs and obtained as 0.4 % and 0.2 % respectively for Ketoconazole and Salicylic Acid. The results for method precision were given in the (Table 9-10)
| Salicylic Acid | Ketoconazole | ||
|---|---|---|---|
| Conc (μg/mL) | Peak area | Conc (μg/mL) | Peak area |
| 5 | 320230 | 5 | 325672 |
| 10 | 623245 | 10 | 635101 |
| 15 | 919360 | 15 | 998492 |
| 20 | 1217201 | 20 | 1259266 |
| 25 | 1505773 | 25 | 1606603 |
| 30 | 1823620 | 30 | 1885676 |
Table 6: Linearity data of Salicylic Acid and Ketoconazole.
| %Level(N=3) | Spiked Concentration(μg/mL) | Standard concentration (μg/mL) | Amount recovered(μg/mL) | % Recovery | Mean % Recovery |
| 50% | 10 | 20 | 9.960559 | 99.61 | 99.48. |
| 10 | 20 | 10.02872 | 100.29 | ||
| 10 | 20 | 9.85448 | 98.54 | ||
| 100% | 20 | 20 | 19.62445 | 98.12 | 99.09 |
| 20 | 20 | 19.87763 | 99.39 | ||
| 20 | 20 | 19.95562 | 99.78 | ||
| 150% | 30 | 20 | 30.14734 | 100.49 | 99.96 |
| 30 | 20 | 29.91877 | 99.73 | ||
| 30 | 20 | 29.8999 | 99.67 |
Table 7: Accuracy results of Salicylic Acid.
| % Level (N=3) | Spiked Concentration (μg/mL) | Standard concentration (μg/mL) | Amount recovered(μg/mL) | % Recovery | Mean % Recovery |
|---|---|---|---|---|---|
| 50% | 10 | 20 | 9.939957 | 99.4 | 99.44 |
| 10 | 20 | 9.842787 | 98.43 | ||
| 10 | 20 | 9.920437 | 99.2 | ||
| 100% | 20 | 20 | 19.8967 | 99.48 | 99.44 |
| 20 | 20 | 19.85804 | 99.29 | ||
| 20 | 20 | 19.91413 | 99.57 | ||
| 150% | 30 | 20 | 29.75129 | 99.17 | 99.16 |
| 30 | 20 | 29.68003 | 98.93 | ||
| 30 | 20 | 29.81963 | 99.4 |
Table 8: Accuracy results of Ketoconazole.
| System precision | ||
|---|---|---|
| S. No | Area of | Area of |
| Salicylic Acid | Ketoconazole | |
| 1 | 1065046 | 1223116 |
| 2 | 1076051 | 1220824 |
| 3 | 1084787 | 1218110 |
| 4 | 1078299 | 1227569 |
| 5 | 1058304 | 1247270 |
| 6 | 1074445 | 1213756 |
| Mean | 1072822 | 1225108 |
| S.D | 9565.1 | 11809.9 |
| %RSD | 0.9 | 1 |
Table 9: System Precision data of Salicylic Acid and Ketoconazole.
| Salicylic Acid | Ketoconazole | |||||
|---|---|---|---|---|---|---|
| S. No | Area of Standard solution | Area of test solution | % Assay | Area of Standard solution | Area of test solution | % Assay |
| 1 | 1193010 | 1205637 | 98.75 | 1252212 | 1248455 | 99.39 |
| 2 | 1225777 | 1201125 | 98.39 | 1242571 | 1245480 | 99.16 |
| 3 | 1215780 | 1211505 | 99.24 | 1258468 | 1242130 | 98.89 |
| 4 | 1206484 | 1206432 | 98.82 | 1250160 | 1245404 | 99.15 |
| 5 | 1225638 | 1214450 | 99.48 | 1256925 | 1249068 | 99.44 |
| 6 | 1212195 | 1213795 | 99.42 | 1253390 | 1242086 | 98.89 |
| Mean | 1217175 | 1208824 | 99.02 | 1252288 | 1245437 | 99.15 |
| S.D | 12436 | 5266.9 | 0.431 | 5654.4 | 2982 | 0.24 |
| %RSD | 1 | 0.4 | 0.4 | 0.5 | 0.2 | 0.2 |
Table 10: Method Precision results of Salicylic Acid and Ketoconazole.
Robustness (5.3.4)
Robustness of the method was studied by making deliberate changes in flow rate, mobile phase ratio and column oven temperature. After making each change in the conditions, chromatograms were recorded by injecting the standard solutions in six replicates. System suitability parameters were checked at each level. System suitability parameters were not much affected and all the parameters were passed. %RSD was within the limit. Results were given in the below (Table 11).
| S. No | Robustness Condition | Salicylic Acid %RSD (n= 6) | Ketoconazole %RSD (n= 6) |
| 1 | Flow rate (-) 0.9ml/min | 1 | 0.7 |
| 2 | Flow rate (+) 1.1ml/min | 0.9 | 1 |
| 3 | Mobile phase (-) 60B:40A | 0.9 | 0.8 |
| 4 | Mobile phase (+) 50B:50A | 1 | 0.7 |
| 5 | Temperature (-) 25°C | 0.6 | 0.6 |
| 6 | Temperature (+) 35°C | 0.5 | 0.8 |
Table 11: Robustness data for Ketoconazole and Salicylic Acid.
Specificity (5.3.5)
Specificity of the method was established by performing the degradation studies under the stress conditions like acid, alkali, oxidation, photo and thermal degradation. The study was conducted as mentioned for the period of 30 minutes. The chromatograms were recorded after subjecting the drugs under stress and the percentage recoveries were calculated. The drugs were found stable under these conditions. The results were given in the below (Table 12).
| Type of degradation | Ketoconazole | Salicylic Acid | ||||
|---|---|---|---|---|---|---|
| Area | %Recovered | % Degraded | Area | %Recovered | % Degraded | |
| Acid | 827320 | 95.62 | 4.38 | 236197 | 94.64 | 5.36 |
| Base | 803986 | 92.92 | 7.08 | 207754 | 83.24 | 16.76 |
| Peroxide | 814804 | 94.17 | 5.83 | 228696 | 91.63 | 8.37 |
| Thermal | 830881 | 96.03 | 3.97 | 242450 | 97.14 | 2.86 |
| Uv | 847962 | 98.01 | 1.99 | 246679 | 98.84 | 1.16 |
| Water | 860915 | 98.01 | 1.99 | 247465 | 99.15 | 0.85 |
Table 12: Degradation data.
CONCLUSION
A simple, Accurate, precise method was developed for the simultaneous estimation of the Salicylic Acid and Ketoconazole in shampoo formulation. Retention time of Salicylic Acid and Ketoconazole were found to be 2.307 min and 3.342 min. %RSD of the Salicylic Acid and Ketoconazole were and found to be 0.4 and 0.2 respectively. %Recovery was obtained as 99.14 % and 99.46 % for Salicylic Acid and Ketoconazole respectively. The method was found to be specific for the drugs without having interference from the degradants. Method developed was simple and economical that can be adopted in regular Quality control laboratories.
REFERENCES
- Yang YW, Zhu Y, Su XQ. J. Hyg. 2005, 34(5): p. 626-628.
- Chao L. Int J Cosmet Sci. 2001, 23(3): p.183-188.
- Fraihat S, Bahgat K. J. Pharm. Res.2014, 9: p. 1511-1514.
- ICH, Q2A Text on validation of analytical procedures, Oct, 1994. ICH, Q3B Validation of analytical procedures: methodology, Nov, 1996.
- Asian Guidelines for Validation of Analytical Procedures adopted from ICH guidelines. ICH Q2A-27th Oct. 1994 ICH Q2B-6th Nov. 1996.
Indexed at, Google Scholar, Crossref