Short Communication - Der Pharma Chemica ( 2019) Volume 11, Issue 3
Structural Elucidation of a New Novel Pentacyclic Triterpenoid Isolated from Caralluma Attenuata Root
Gurrala Jayalakshmi1, Vejendla Anuradha2*, Setty Ratna Kumari2, Sakamuri Sivarambabu3 and Sk. Rabbani Basha12Department of Basic Sciences and Humanities VNITSW, Guntur, Andhra Pradesh, India
3Department of Chemistry, J.K.C. College, Guntur, Andhra Pradesh, India
Vejendla Anuradha, Department of Basic Sciences and Humanities VNITSW, Guntur, Andhra Pradesh, India, Email: chema2013@gmail.com
Abstract
From the root of Caralluma attenuata belonging to the family Asclepiadaceae, a novel pentacyclic triterpenoid was isolated. Its structure was elucidated on the basis of spectroscopic data. This is the first report of such novel pentacyclic triterpenoid from C. attenuata root.
Keywords
Caralluma attenuata, Asclepiadaceae, Pentacyclic triterpenoid
Abbreviations
TLC: Thin Layer Chromatography, CC: Column Chromatography, MS: Mass Spectrometry, EI: Electron Ionisation, DEPT: Distortionless Enhancement by Polarisation Transfer, MPs: Melting point, NMR: Nuclear Magnetic Resonance
Introduction
Recently, triterpenoids are known to exhibit several pharmacologically activities and extensively discussed in the literature. These compounds can be used as anticancer agents [1-8], anti HIV [9,10], antiinflammatory [11], antiviral [5] and against neurodegenerative disorders [12]. Triterpenoids are the class of compounds which include squalene derivatives, lanostanes, holostanes, cycloartanes cucurbitanes, dammaranes, euphanes, triucallanes, tetranortriterpenoids, quassoids, luphanes, oleananes, friedelanes urasanes, hopanes, serratanes, isomalabaricanes and saponins.
Various medicinal uses of Caralluma species have been reported in Indian traditional medicine system. Most of the Caralluma species are used as anticancer [13], antitubercular and anthelmintic [14], antibacterial [15], healing of ulcers [16], appetite suppressant [17], nootropic [18], nociceptive [19], antioxidant actions [20], ability to lower blood sugar [21]. Species like Caralluma tuberculata, Caralluma Fimbriata, and Caralluma attenuata are the most popular widely utilized species in the genus. Isolation and characterization of oxypregnane glycosides [22,23], pregnane gylcosides [24-31], triterpene saponins [32], flavonoids [33], pregnane esters [34], bisdesmosidic glycosides [35,36], flavone glycosides [37], were earlier reported from the same genus. In the course of our investigation on chemical constituents of Carallumas we have isolated two novel triterpenoids [38,39]. In this paper we report the structural elucidation of another new novel pentacyclic triterpenoid derivative from C. attenuata.
Materials and Methods
Experimental
The plant material of C. attenuata was collected in Tirumala forests during January-2017. MPs uncorrected IR 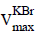 , Proton Nuclear Magnetic
Resonance (1H-NMR) δ ppm, 300 MHz CDCl3, Carbon 13 Nuclear Magnetic Resonance (13C-NMR) 150 MH, distortion less Enhancement by
Polarization Transfer (DEPT) 135, Mass spectrometry (SHIMADZU 2000) Column Chromatography (CC) and Thin Layer Chromatography
(TLC) on silica gel, TLC Chamber (Sigma Aldrich).
, Proton Nuclear Magnetic
Resonance (1H-NMR) δ ppm, 300 MHz CDCl3, Carbon 13 Nuclear Magnetic Resonance (13C-NMR) 150 MH, distortion less Enhancement by
Polarization Transfer (DEPT) 135, Mass spectrometry (SHIMADZU 2000) Column Chromatography (CC) and Thin Layer Chromatography
(TLC) on silica gel, TLC Chamber (Sigma Aldrich).
Preparation of TLC plates
A homogenous suspension of silica gel is prepared by mixing 20 g of 200 mesh silica gel G (Qualigens) in about 45 ml distilled water. This suspension is poured into TLC (UNDPLAN model) spreader, which was adjusted to 0.25 mm thickness. Glass plates (20 cm × 5 cm) are coated with this gel using spreader. These plates are air dried and activated in the oven at 110ºC for 30 min and then kept in a desiccator.
Extraction and isolation
The roots of Carallluma attenuata were air dried, powdered (1.8 kg) and extracted with 3 l of hexane, 3 l of benzene, 3 l of acetone and 3 l of methanol respectively using SOXHLET extractor. n-Hexane extract 50 g was subjected to column chromatography using silica gel 10-40 mesh. It is eluted with various fractions of benzene, acetone and methanol with increasing polarity. The various eluted fractions were observed time to time using TLC plate, benzene fractions (95-100) a yielded a white solid.
Detection by TLC
This white solid is dissolved in chloroform, spotted on TLC plate. The chromatograph was developed using benzene as a mobile phase. The dried plates were then sprayed with methanol-sulphuric acid reagent (98: 2) and heated in an oven for about 45 min, single spot (red colour) with Rf–0.1875 is observed. The solid obtained was recrystallised using benzene and acetone mixture and melting point was determined. MPs 240ºC. This was further analyzed by spectral data.
Spectral Data IR 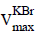
3264.10, 2956.08, 2844.25, 1641.65, 1412.24, 1112.06, 1015.02.
1H-NMR (δ ppm)
1.381, 1.534, 1.436, 1.674, 1.68, 1.18, 1.367, 1.678, 1.42, 1.69, 1.329, 1.13, 1.396, 1.412, 1.712, 1.17, 1.396, 1.495, 1.36, 1.742, 1.14, 1.496, 1.702, 1.372, 1.762, 0.925, 1.432, 0.87, 1.015, 1.315, 0.882, 1.054, 0.79, 5.156, 2.302, 3.379, 1.602, 0.756.
13C-NMR (δ ppm)
35.3611, 36.9137, 79.2, 41.0862, 44.3537, 29.3801, 30.0668, 45.0, 50.5015, 38.0, 21.4534, 26.8097, 47.6693, 42.4, 27.6043, 39.3620, 35.0, 47.8396, 41.391, 42.4, 37.2731, 36.9137, 18.9442, 27.3659, 17.3015, 15.603, 15.8027, 22.1138, 27.4352, 14.9319, 104.9132, 151, 40.187, 22.9581, 14.5294, 14.4079, 65.04.
DEPT
δ 35.3611, 36.9137, 41.0862, 44.3537, 29.3801, 30.0668, 50.5015, 21.4534, 26.8097, 47.66927.6043, 39.3620, 47.8396, 41.319, 37.2731, 18.9442, 27.3659, 17.3015, 15.6035, 15.8027, 22.1138, 27.4352, 14.9319, 104.9132, 40.187, 22.9581, 14.5294, 14.4079, 65.04.
MS EI+
[M+] m/z 538, 523, 537, 523, 510, 509 (100%), 495, 481, 466, 452, 410, 395, 353, 339, 227, 185, 171, 129, 116, 87, 73, 61.
Conclusion
In this paper, we report the isolation, purification and characterization of a new triterpenoid from C. attenuata. The available scientific research on Caralluma signifies its importance as medicinal plant used in a wide range of ethnomedicinal treatment especially for diabetes, obesity, gastro intestinal problems, blood disorders, skin problems and cancer. The medicinal properties of Caralluma are attributed to the presence of chemicals like pregnane glycosides, flavonoids, steroids, steroidal glycosides and terpenoids. Very few species of Caralluma are of these genus have been subjected to stringent scientific evaluation. The scientific evaluation of the triterpenoid isolated may help in exploring the medicinal importance of various Caralluma species. Since no sufficient information is available for the toxicity of the genus or its derivative medicines that need some extensive research. The diverse uses of Caralluma have made it susceptible to over exploitation and it may become threatened due to high trade. However Carallumas is not an invasive species, so modern cultivation techniques should be developed for commercial exploration. Tissue culture techniques must be developed to enhance the production and preserve natural germplasms.
Acknowledgment
I extend my sincere thanks to my research guide, Chairman and Principal of Vignan Degree and P.G. College for providing facilities to carry out my research work. I am also thankful to all my colleagues and family members for their support.
References
- J.M. Li, F. Zheng, L.J. Zhai, Z.H. Xue, Zhongcaoyao., 2014, 45, 2265-2271.
- W. Zhang, X. Men, P. Lei, J. Cancer Res. Ther.,2014, 10, 14-19.
- R. Tundis, F. Menichini, M.R. Loizzo, Stu. Nat. Pro. Chem., 2014, 41, 1-32.
- X.J. Yan, L.H. Gong, F.Y. Zheng, K. Cheng, Z.S.Chen, Z. Shi, Drug Discovery Today., 2014, 19, 482-488.
- R. Paduch, M.K. Szerszer, Mini-Rev. Org. Chem., 2014, 11, 262 -268.
- J.A.R. Salvador, A.S. Leal, D.P.S. Alho, B.M.F. Goncalves, A.S. Valdeira, V.I.S. Mendes, Y. Jing, Stud. Nat. Pro. Chem., 2014, 41, 33-73.
- J.R. Du, F.Y. Long, C. Chen, Enzymes., 2014, 36, 95-130.
- S.M. Kamble, S.N. Goyal, C.R. Patil, RSC Adv., 2014, 4, 33370-33382.
- B. Han, Z. Peng, J. Chem. Pharm. Res.,2014, 6, 438-443.
- R.V. Patel, S.W. Park, Curr. Top. Med. Chem., 2014, 14, 1940-1966.
- G.S. Jeong, J.S. Bae, Mini-Rev. Org.Chem., 2014, 11, 316-329.
- P. Ruszkowski, T.B. Kozlowska, Mini-Rev. Org. Chem., 2014, 11, 307-315.
- M.V. Jyothi, E. Bhargav, C. NareshBabu, K. Somasekhar Reddy, B. Pradeep Kumar, R. Prasad Rao, J. Pharm. Bioallied Sci.,2019, 11, 38-42.
- P. Mounika, M. Vijayajyothi, P. Ramalingam, J. Vinush aReddy, K. Anusha, Int. J. Pharmaceut. Sci. Res., 2016, 4561-4564.
- M. Sireesha, R. Venkatnadh, K. Suresh Babu, Pullaiah, Med. Chem., 2017, 1374821.
- G. Sunil, S. Srivastava, K. singh, A. Sharma, G. Kavitha, Ancient Sci. of life., 2016, 35, 222-226.
- R. Kuriyan, T. Raj, S.K. Srinivas, M. Vaz, R. Rajendran, A.V. Kupad, Apetite., 2007, 338-344.
- M.M. Keshtkar, A.G. Khani, Renewable Energy Focus., 2018, 27, 1-13.
- R. Rajendra, D.B. Ambikar, R.A. Khandare, V.D. Sannapuri, N.S. Vyawahare, P. Clayton, Food Nutri. Sci., 2014, 5, 147-152.
- S. Ray, N. Rahman, A. Basu, S.G. Ray, J. Pharm Biomed. Sci., 2012, 18, 2230.
- V. Mahesh, D.T. Priya Darshini, J.M. Sasikumar, J. Food Sci. Technol., 2014, 51, 2415-2424.
- S. Latha, K. Rajaram, P.S. Kumar, Int. J. Pharm. Sci.,2014, 6, 975- 991.
- E.A. Sattar, M.A.A. Al-Yahya, N. Nakamur, M. Hattori, Phytochem., 2001, 57, 1213-1217.
- E.A. Sattar, R.M. Meselhy, M.A.A. Al-Yahyam, Planta Med., 2002, 68, 430-434.
- V.U. Ahmad, U. Khan, G.H. Rizwani, J. Nat. Prod.,1988, 51(6), 1092-1097.
- M.A.A. Al-Yalhya, E.A. Sattar, E. Guittet, J. Nat. Pro.,2000, 63, 1451-1453.
- A. Braca, A. Badar, I. Morelli, R. Scarpato, G. Turchi, C. Pizza, N.D. Tammasi, Tetrahedron., 2002, 58, 5837-5842.
- A.F. Halim, A.T. Khalil, Phytochem.,1996, 42(4), 1135-1139.
- K. Hayashi, L. Lida, Y. Nakao, K. Kaneko. Phytochem., 1998, 27(12), 3919-3924.
- L.J. Lin, G.L.R.R. Lin, L.G.A. Cordell, M. Ramesh, B. Srilatha, B.M. Reddy, A.V.N. Appa Rao, Phytochem., 1994, 35(6), 1549-1553.
- T. Tanaka, S. Tsukamoto, K. Hayashi, Phytochem.,1990, 29(1), 229-237.
- J.W. Jhoo, K.S. Sang, X. Cheng, N. Ruth, E. Stark, Q. Yizheng, R.T. Rosen, C.T. Ho, J. Agri. Food Chem., 2001, 40, 5969-5974.
- M. Kamil, A.F. Jayaraj, F. Ahmad, C. Gunasekhar, S. Samuel, K. Chan, M. Habibullan, J. Pharm. Pharmacol., 1999, 51, 225.
- A.T. Khalil, Fitoterapia., 1995, 66(3), 261-264.
- S.X. Qiu, L. Lin, G.A. Cordell, M. Ramesh, B. Ravi kumar, M. Radhakrishna, G. Krishnamohan, B.M. Reddy, N.Y. Rao, B. Srinivas, N.S. Thomas, A.V.N. Appa Rao, Phytochem.,1997, 46(2), 333-340.
- S.X. Qiu, G.A. Cordell, M. Ramesh, B. Ravi kumar, M. Radhkrishna, G.K. Mohan, B.M. Reddy, Y.N. Rao, B. Srinivas, N.S. Thomas, A.V.N. Appa Rao, Phytochem., 1999, 50, 485-491.
- M. Ramesh, Y. Nageswara Rao, M. Ramakumar Kumar, G. Krishna Mohan, Ravi kumar, B. Madhakumar, A.V.N Appa Rao, M. Radhakrishna, B. Madhava Reddy, Biochem. Systematics Ecol., 1999, 27: 85-86
- G. Jayalakshmi, V. Anuradha, K. Kalyani, S. Sivaram Babu, Eur. J. Pharmaceut. Med. Research, 2016; 3(6), 342-344.
- G. Jayalakshmi, K. Kavitha, S. Ratnakumari, V. Anuradha, S.Sivaram Babu, Eur. J. Pharmaceut. Med. Res.,2017, 4(6), 710-712.
- J.W. Jhoo, K.H.S. Sang, X. Chennm, N. Zhu, R.E. Stark, Q.Y. Zheng, R.T. Rosen, C.T. Ho., J. Agri. Fable ood Chem., 2001, 49, 12.



