Research Article - Der Pharma Chemica ( 2017) Volume 9, Issue 3
Synthesis and Anti-Tubercular Study of Some Transition Metal Complexes of Hydrazone Derivatives Derived from Benzofuran-2-Carbohydrazide
Bhushan Nazirkar1*, Udaysingh Patil1, Bapu Thorat1, Mustapha Mandewale1, Hemant Gaokar2, Arjun Pandhare3 and Ramesh Yamgar42IIT-B, Powai, Mumbai-400076, India
3Department of Chemistry, VPM’s B. N. Bandodkar College of Science, Thane, India
4Patkar-Varde College of Arts, Science and Commerce, Mumbai-400062, India
Bhushan Nazirkar, Department of Chemistry, Ismail Yusuf College of Arts, Science and Commerce, Mumbai-400060, India, Email: principaliyc@yahoo.in
Abstract
The benzofuran-2-carbohydrazide based new hydrazone ligands and their transition metal complexes have been prepared. These ligands and metal complexes were characterized by different spectroscopic techniques. The preliminary results of antituberculosis study showed that the hydrazones exhibited very good antituberculosis activity. Among the tested compound 3b was found to be most active with minimum inhibitory concentration of 1.6 μg/mL against Mycobacterium tuberculosis.
Keywords
Benzofuran, Hydrazide, Hydrazones, Tuberculosis, Metal complex
Introduction
Tuberculosis (TB) is caused by an aetiological agent Mycobacterium tuberculosis which is the single largest cause of death globally due to its infections. Existing drug resistant wild strains of TB, increasing incidence of multidrug-resistant (MDRTB) and extensively drug-resistant tuberculosis (XDR-TB) pose dangerous global threat and an urgent need to develop new antimycobacterials [1]. The emergence of strains resistant to the first line drugs causes major concern, as treatment is then deferred to drugs that are less effective, have more toxic side effects, and result in higher death rates, especially among HIV-infected persons [2].
Benzofurans have drawn considerable attention over the last few years due to their profound physiological and chemotherapeutic properties as well as their widespread occurrences in nature [3-5]. In the field of anti-infective agents, benzofurans have been reported to show antifungal, antiprotozoal and antitubercular activity [6-9].
Due to facile synthesis, easily tunable steric and electronic properties, and good solubility in common solvents, hydrazone ligands have been extensively studied in coordination chemistry [10]. Their wide-ranging complexing ability and bewildering versatility make them popular. The significance of acid hydrazides (RCONHNH2) and their corresponding aroylhydrazones (RCONHN=CHR′) and the dependence of theirmode of chelation on transition metal ions present in a living system have been of significant interest [11]. Coordination compounds of aroylhydrazones acting as enzyme inhibitors [12] are useful due to their pharmacological applications [13].
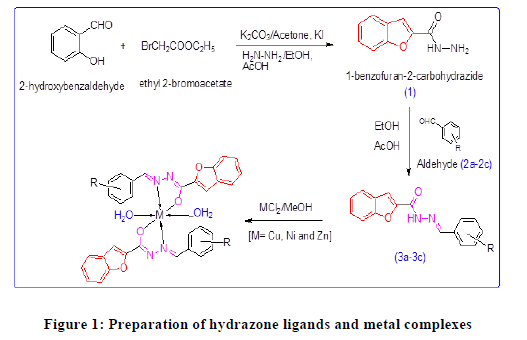
Based on these facts, supported by literature and in continuation of our research for new antituberculosis agents [14-18], we have undertaken research studies on synthesis and biological screening of some new benzofurans-based hydrazone derivatives and their transition metal complexes as shown in Figure 1.
Material and Methods
All reagents used were of Analytical Reagent Grade. Transition metal salts were purchased from E. Merck and used as received. Molar conductivity measurements were carried out with an ELICO-CM-82 conductivity bridge. The 1H and 13C NMR spectra were recorded in deuterated dimethylsulfoxide (DMSO-d6) solvent with a Bruker AV III 500 MHz FT-NMR spectrometer at room temperature using tetramethylsilane as internal reference. The Electron Paramagnetic Resonance (EPR) spectrum of the copper complex was obtained with a Varian EPR model spectrometer. Infrared (IR) spectra were recorded in a KBr matrix using an FTIR- 7600 Lambda Scientific Pvt. Ltd. spectrophotometer in the range 400-4000 cm-1. UV-Visible spectra were obtained on Shimadzu UV-1800 spectrophotometer for scan range 200-800 nm and the path length was 1.0 cm for the measurements. The mass spectrum of the ligand was obtained with a Varian Inc., USA 410 Prostar Binary LC spectrometer. The spectral analyses were done at the University of Mumbai and SAIF, IIT Mumbai, India. The anti-microbial effects synthesized compounds against Mycobacterium tuberculosis (H37 RV strain) ATCC No-27294, were evaluated at the Deparment of Microbiology, Maratha Mandal’s NGH Institute of Dental Sciences and Research Centre, Belgaum-590010, India.
Preparation benzofuran-2-carbohydrazide
The benzofuran-2-carboxylate prepared as per procedure reported by Kushwaha [19]. The salicylaldehyde (0.1 mol) was taken in a round bottom flask and 10 cm3 of acetone and K2CO3 (0.25 mol) were added to it, the reaction mixture was stirred for 5 min in icebath. To this reaction mixture ethyl bromo acetate (0.1 mol) was added slowly. Further whole reaction mixture was allowed to stir with catalytic amount of potassium iodide for 20 min. The resulting solution was discharged in crushed ice; the solid obtained was filtered and recrystallized from ethanol.
The solid obtained (ethyl benzofuran-2-carboxylate) (0.1 mol) dissolved in ethanol and 0.12 mol of hydrazine hydrate added with constant stirring. Two drops of acetic acid added to reaction mixture and refluxedfor 3 h. After completion of the reaction it was room temperature. Precipitated solid product filtered and dried (Figure 1).
Preparation of Schiff bases ligands
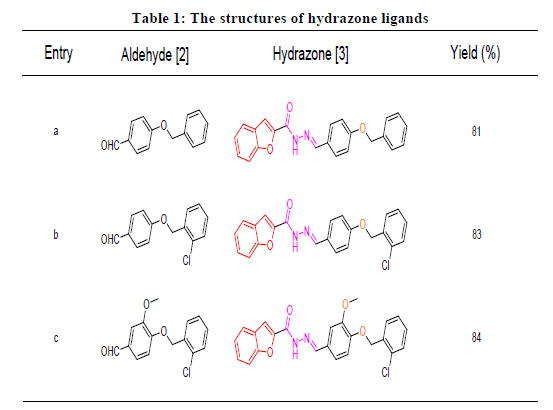
The compound (1) (0.01 mol) and appropriate aldehyde derivatives (2a-2c) (0.01 mol) taken in round bottom flax containing 10 cm3 of ethanol. Reaction mixture was refluxed for 30 min. after addition of catalytic amount of acetic acid. Completion of reaction was checked with TLC. Upon cooling the reaction mixture a solid product (4a-4c) precipitated out, which was filtered, dried and purified by recrystallization from ethanol (Figure 1 and Table 1).
General procedure for the synthesis of metal complexes
A solution of appropriate metal salt (CuCl2, NiCl2 and ZnCl2) in methanol was added gradually to a stirred methanolic solution of the hydrazone ligand (3a-3c) in the molar ratio 1:2. The reaction mixture was further stirred for 2 h at 78ºC. Then it was cooled in ice bath to ensure the complete precipitation of the formed complexes Figure 1 and Table 2. The precipitated solid complex was filtered and washed with water. Finally, the complex was washed with diethyl ether and dried in vacuum desiccators over anhydrous CaCl2.
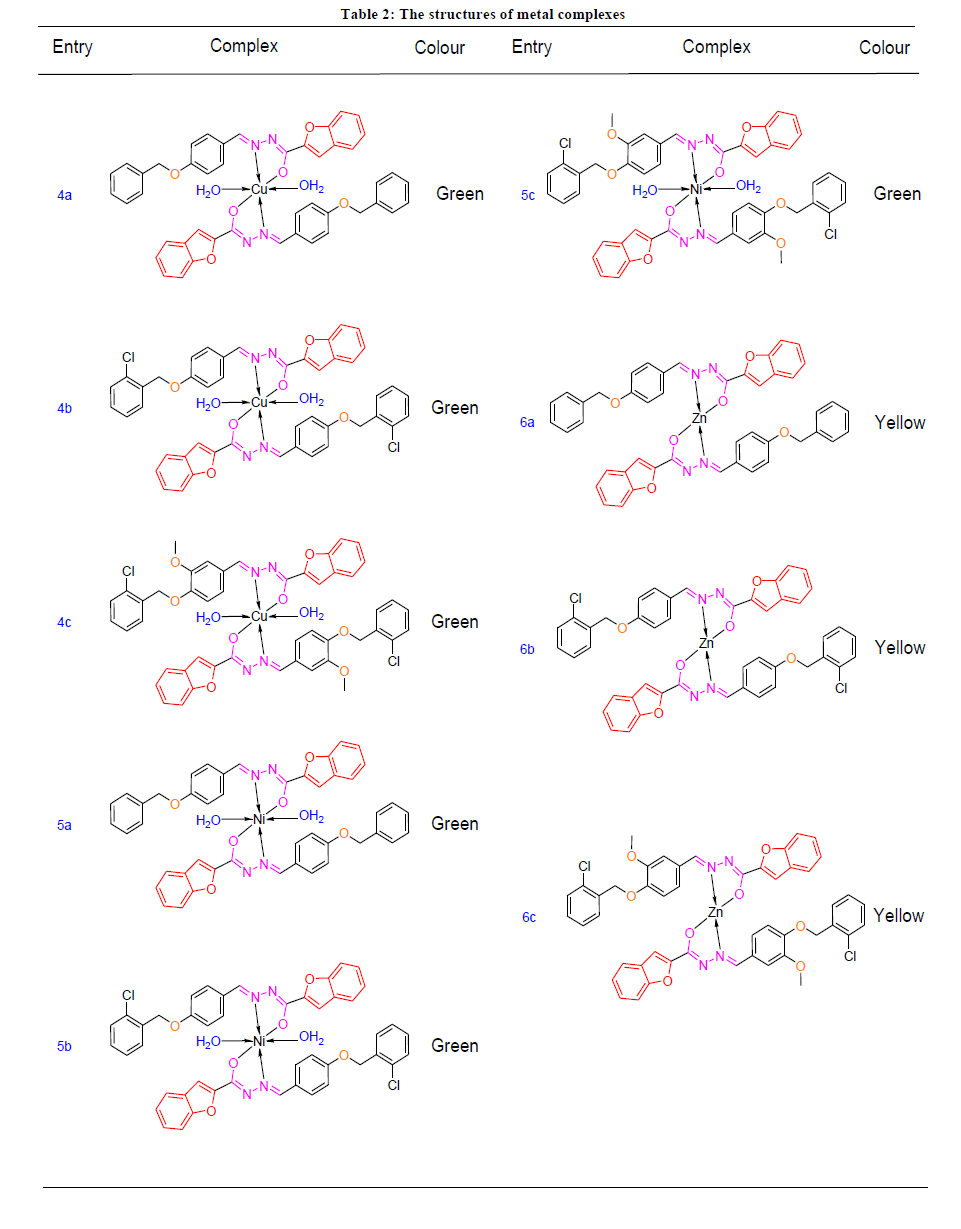
Spectral data
Benzofuran-2-carbohydrazide (1)
White spongy solid (EtOH); mp 174ºC; Yield: 78 %, IR(KBr, υ, cm-1):3324, 3178, 3114, 3019, 1660, 1600, 1546, 1454, 1328, 1187, 1089, 823, 736 cm-1; 1H NMR (DMSO-d6, 300 MHz): δ=10.010 (s, 1H, NH-CO); 7.74 (d, 1H, Ar-H); 7.62 (d, 1H, Ar-H); 7.49 (s, 1H, Furan-H); 7.43 (t, 1H, Ar-H); 7.30 (t, 1H, Ar-H); 4.56 (bs, 2H, NH2); EIMS m/z: 177 [M+H].
N’-{(E)-[4-(benzyloxy)phenyl]methylidene}-1-benzofuran-2-carbohydrazide (3a)
Faint yellow solid (EtOH); mp 208ºC; λmax=325 nm; Yield: 81%, IR(KBr, υ, cm-1): 3195, 3027, 2911, 1639, 1602, 1585, 1540, 1509, 1359, 1294, 12∘51, 1169, 1110, 1062, 1006, 863, 831, 740, 701, 638 cm-1; 1H-NMR (DMSO-d6, 300 MHz): δ=9.810 (s, 1H, -CONH); 8.280 (s, 1H, N=CH); 7.723-7.701 (dd, 2H, Ar-H); 7.617-7.597 (d, 1H, Ar-H); 7.419-7.321 (m, 9H, Ar-H); 6.991–6.968 (dd, 2H, Ar-H); 5.092 (s, 2H, Ar-CH2O);EIMS (C23H18N2O3) m/z: 371.14 [M+H].
N’-[(E)-{4-[(2-chlorobenzyl)oxy]phenyl}methylidene]-1-benzofuran-2-carbohydrazide (3b)
Yellowish brown solid (EtOH); mp 182°C; λmax=324 nm; Yield: 83 %, IR(KBr, υ, cm-1): 3316, 3052, 2894, 1664, 1608, 1535, 1511, 1473, 1450, 1380, 1286, 1257, 1240, 1172, 1033, 983, 827, 809, 742, 559 cm-1; 1H-NMR (DMSO-d6, 300 MHz): δ=9.821 (bs, 1H, -CONH); 8.278 (s, 1H, N=CH); 7.785 (d, 2H, Ar-H); 7.758-7.673 (dd, 1H, Ar-H); 7.624 (s, 1H, Furan-H); 7.555-7.530 (m, 2H, Ar-H); 7.467-7.416 (m, 2H, Ar-H); 7.342-7.300 (m, 3H, Ar-H); 7.021-6.992 (dd, 2H, Ar-H); 5.173 (s, 2H, Ar-CH2O); EIMS (C23H17ClN2O3) m/z:405.9 [M+H].
N’-[(E)-{4-[(2-chlorobenzyl)oxy]-3-methoxyphenyl}methylidene]-1-benzofuran-2-carbo hydrazide (3c)
Faint yellow solid(EtOH); mp 171°C; λmax=337 nm; Yield: 84%, FTIR(KBr, υ, cm-1): 3305, 3205, 2996, 1644, 1600, 1554, 1509, 1465, 1450, 1419, 1373, 1301, 1267, 1230, 1180, 1139, 1029, 863, 750, 644, 613 cm-1; 1H-NMR (DMSO-d6, 300 MHz): δ=9.812 (bs, 1H, -CONH); 8.226 (s, 1H, N=CH); 7.711-7.646 (m, 3H, Ar-H); 7.540-7.272 (m, 8H, Ar-H); 6.856-6.830 (dd, 2H, Ar-H); 5.269 (s, 2H, Ar-CH2O); 3.998 (s, 3H, OCH3); EIMS (C24H19ClN2O4) m/z: 435.82 [M+H].
Cu (II) complex of N’-{(E)-[4-(benzyloxy)phenyl]methylidene}-1-benzofuran-2-carbohydrazide (4a)
Green solid; mp: above 300°C; λmax=325 nm; FTIR (KBr, υ, cm-1): 3423, 1605, 1574, 1447, 1422, 524, 453.
Cu (II) complex N’-[(E)-{4-[(2-chlorobenzyl)oxy]phenyl}methylidene]-1-benzofuran-2-carbohydrazide(4b)
Green solid; m.p: above 300°C; λmax=326 nm, FTIR (KBr, υ, cm-1): 3442, 3361, 1635, 1602, 1557, 1450, 1420, 534, 453.
Cu (II) complex N’-[(E)-{4-[(2-chlorobenzyl)oxy]-3-methoxyphenyl}methylidene]-1-benzofuran-2-carb ohydrazide (4c)
Green solid; m.p.: above 300°C; λmax=330 nm; FTIR (KBr, υ, cm-1): 3423, 1657, 1640, 1577, 1448, 1422, 532, 468, 453.
Ni (II) complex of N’-{(E)-[4-(benzyloxy)phenyl]methylidene}-1-benzofuran-2-carbohy drazide (5a)
Green solid; m.p.: above 320°C; λmax=334 nm; FTIR (KBr, υ, cm-1): 3445, 1601, 1423, 523, 482, 457.
Ni (II) complex of complex N’-[(E)-{4-[(2-chlorobenzyl)oxy]phenyl}methylidene]-1-benzofuran-2-carbohydrazide (5b)
Green solid; m.p.: above 320°C; λmax=326 nm; FTIR (KBr, υ, cm-1): 3421, 1655, 1603, 1450, 1421, 634, 523, 461, 438.
Ni (II) complex of N’-[(E)-{4-[(2-chlorobenzyl)oxy]-3-methoxyphenyl}methylidene]-1-benzofuran-2-carb ohydrazide (5c)
Green solid; m.p.: above 320°C; λmax=325 nm; FTIR (KBr, υ, cm-1): 3422, 1604, 1450, 1421, 531, 484, 445.
Zn (II) complex of N’-{(E)-[4-(benzyloxy)phenyl]methylidene}-1-benzofuran-2-carbohy drazide (6a)
Off white; mp: above 300°C; λmax=327 nm; FTIR (KBr, υ, cm-1): 3423, 3197, 3027, 2910, 1639, 1604, 1585, 1509, 1361, 831, 642, 604.
Zn (II) complex of complex N’-[(E)-{4-[(2-chlorobenzyl)oxy]phenyl}methylidene]-1-benzofuran-2-carbohydrazide (6b)
Off white; mp: above 300°C; λmax=324 nm; FTIR (KBr, υ, cm-1): 3471, 3064, 1604, 1579, 1509, 1475, 1340, 1160, 821, 626, 490.
Zn (II) complex of N’-[(E)-{4-[(2-chlorobenzyl)oxy]-3-methoxyphenyl}methylidene]-1-benzofuran-2-carb ohydrazide (6c)
White solid; mp: above 330°C; λmax=333 nm; FTIR (KBr, υ, cm-1): 3438, 3307, 3016, 2910, 1621, 1598, 1525, 1515, 1471, 1338, 1140, 835, 622, 607.
Results and Discussions
All the hydrazone ligands (3a-3c) have been synthesized by condensation of benzofuran-2-carbohydrazide with various 4-substituted benzaldehydes (2a-2c). Each hydrazone ligands (3a-3c) finally refluxed separately with nickel chloride, copper chloride and zinc chloride in ethanol with molar ratio 2:1 to yield nickel complexes (4a-4c), copper complexes (5a-5c) and zinc complexes (6a-6c).
All complexes are stable at room temperature and are non-hygroscopic in nature. These metal complexes are insoluble in water but are soluble in DMF and DMSO. These ligands and metal complexes were characterized by different spectroscopic techniques (FT-IR, 1H and 13C NMR, MS, UV–Visible and Fluorescence, TGA and XRD).
The 1H-NMR spectrum revealed, in addition to expected aromatic signals, a singlet at 9.810-9.821 ppm which is assigned to hydrazone N-H proton and singlet at 8.226-8.280 ppm attributed to azomethine proton. The aromatic protons resonate in the region 7.19–7.92 ppm.
The IR spectra of the metal complexes were compared with that of the free hydrazone ligand in the region 400-4000 cm-1. The IR spectrum of free ligand shows a broad band at 3316 cm-1, assigned to hydrazone υ(NH) group. The peaks at 1664 and 1608 cm-1 are due to carbonyl υ(C=O) andazomethine υ(C=N), respectively. The peaks attributed to carbonyl υ(C=O) and azomethine υ(C=N) are shifted to lower frequencies in the spectra of all the complexes due to the participation of carbonyloxygen and azomethine nitrogen in coordination. Taken collectively, these spectral data indicate that the hydrazone ligand is behaving as a monobasic bidentate (NO) chelating ligand in each of the complexes. The Copper and Nickel metal complexes shows broad peak in the region of 3445-3421 cm-1 corresponding to co-ordinated water molecules. The low frequency region of the spectra indicated two new medium intensity bands at about 440-470 cm-1 due to ν(M-O) vibrations. Also IR spectra shows bands in the range 480-540 cm-1 due to ν(M-N) stretching.
The formation of hydrazone is also confirmed by the presence of intense molecular ion peak in the mass spectra of hydrazone derivatives (3a-3c). Spectral evaluation predicts the molecular weights of the desired hydrazone compounds.
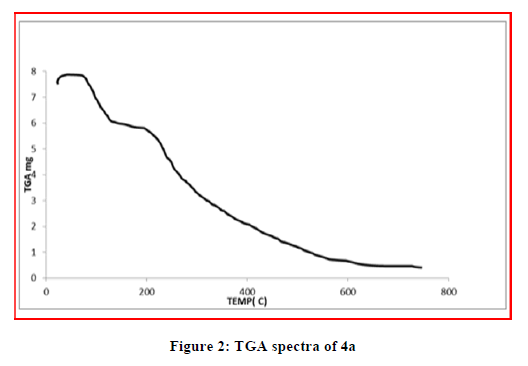
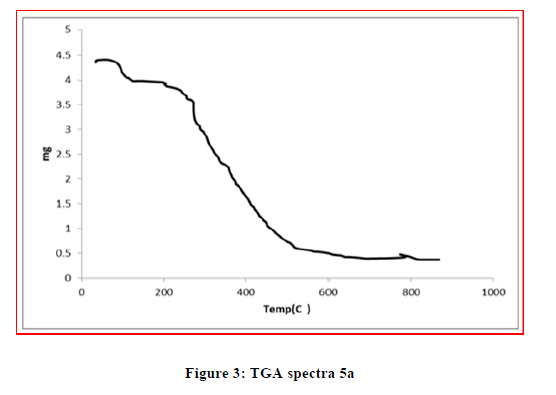
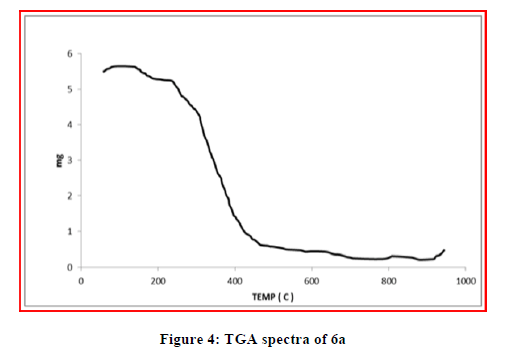
The TGA analysis was carried out to explain the thermal stability of the complexes. The thermal behavior of the metal complexes was studied in temperature range of 25°-1000°C. The TGA studies of Cu (II) and Ni (II) complexes showed that the decomposition occurs in three steps. The first stage involves weight loss below 100ºC due to the removal of the lattice cell water in the complexes. Second step involves weight loss in the temperature range 110°C–160°C is due to elimination of coordinated water. A plateau was observed above 600ºC corresponds to the formation of corresponding metal oxide. While TGA study of Zn (II) do not show any weight loss upto 200°C, indicates absence of coordinated water molecules. The considerable weight loss from 250°C to 450°C indicates loss due to volatile matter. Above 450ºC there is a plateau seen indicates formation of stable zinc oxide (Figures 2-4).
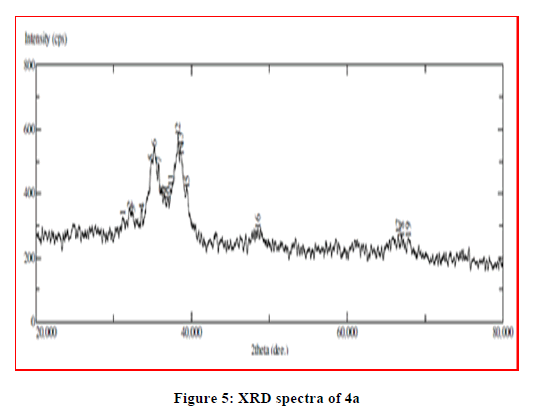
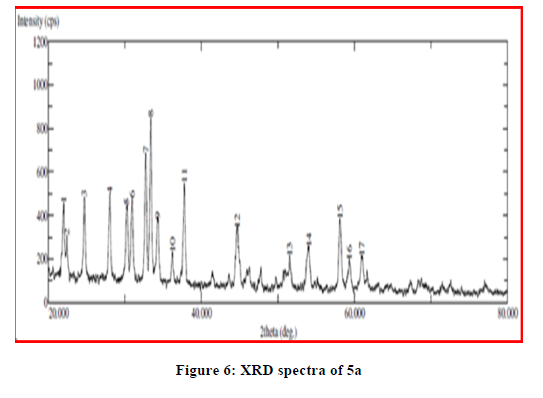
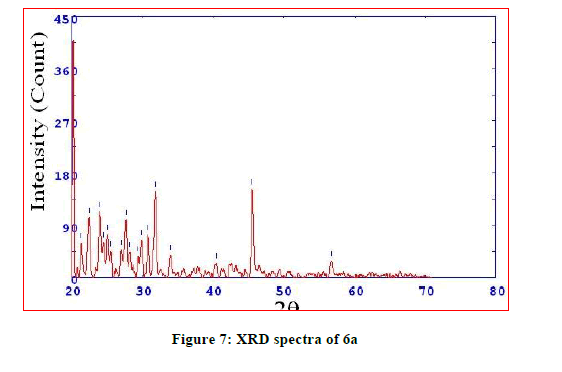
The X-ray powder diffraction data of most of the complexes provide vital structural information of materials which do not yield single crystals of good quality. Single crystals of the complexes could not be prepared thus powder diffraction data were acquired structural description. The X-ray diffractogram of the complexes was measured in the range of 20°-80°, 2θ values. XRD pattern indicates complexes have well defined crystalline patterns, with various degrees of crystallinity. All this supports the formation of complexes (Figures 5-7).
From the mathematical relation Λm=K/C the molar conductance of the metal complexes (Λm) can be calculated by dissolving it in appropriate solvent where C is the molar concentration of the metal complex solutions [20]. The metal complexes were dissolved in DMF to prepare 10−3 M of their solutions. The molar conductivities were measured at 25 ± 2ºC. The study show negligible molar conductance values for metal complexes (2.60-14.80Ω−1 mol−1 cm2), indicating that the complexes are non-electrolytes. The ESR analysis is carried out on VARIAN ESR model. The ESR spectra of powdered Cu(II) complex samples (5a-5c) were recorded at room temperature. The spectra have asymmetric bands with g11 (2.42)>g⊥ (2.23)>TCNE (2.00277), indicating the unpaired electron lie predominately in the dx2-y2 orbital with possibly mixing of dz2 because of the low symmetry [1,2]. The exchange interaction parameter ‘G’ is calculated using formula
G=(g11-2)/(g⊥-2). The value of ‘G’ is less than 4 indicate exchange interaction between the metal ions.
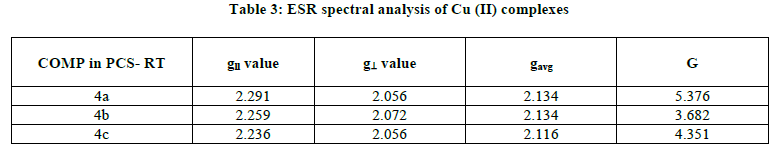
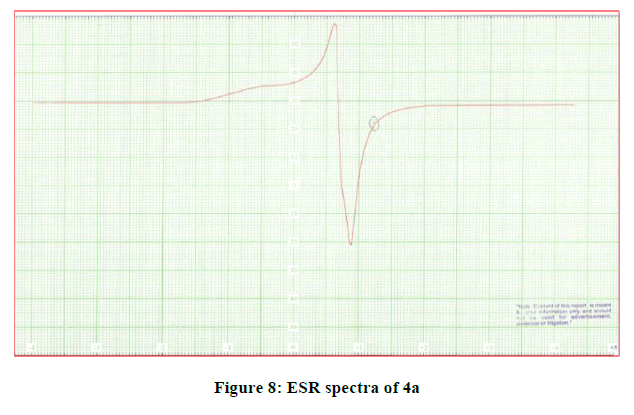
The ESR spectrum in a polycrystalline state of the Cu (II) complex was recorded at room temperature. The gll and g⊥ values for 4a-4c are reported in the Table 3. The gavg value is calculated and reported in the same table. The spectrum showed asymmetric bands with two g values. The values are almost same for all complexes indicating [21,22] that the type of bonding is the same in all complexes and that the Cu-O bond lengths do not vary much from complex to complex (Figure 8).
The trend gll> g⊥> 2.00277, indicating that the unpaired electron lay predominately in the dx2-y2 orbital with possibly mixing of dz2 orbital because of the low symmetry.
The axial symmetry parameter ‘G’ is determined as:
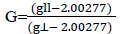
G values found to be more than 4 suggesting very weak or no interaction in the solid state.
Biologicalevaluation (Anti-tuberculosis Activity)
The anti-microbial effects of the new hydrazones and their Zinc complexes against Mycobacterium tuberculosis (H37 RV strain) ATCC No-27294, were evaluated at the Department of Microbiology, Maratha Mandala’s NGH Institute of Dental Sciences and Research Centre, Belgaum-590010, India. The method applied is similar to that reported by Maria and Lourenco [23]. Ciprofloxacin (MIC 3.12 g/mL), Pyrazinamide (MIC 3.12 g/mL) and Streptomycin (MIC 6.25g/mL) were used as references to evaluate the potency of the synthesized compounds.
All the studied samples are showing different effectiveness due to the active barrier of an outer cell wall membrane of Mycobacterium tuberculosis for entry of outside substances like test compounds under this study. However, hydrazone ligands showed better activity than their metal complexes. As shown in Table 4, hydrazone 3b has unpredictable high anti-tuberculosis activity against Mycobacterium tuberculosis as their MIC value is 1.6 μg/mL.
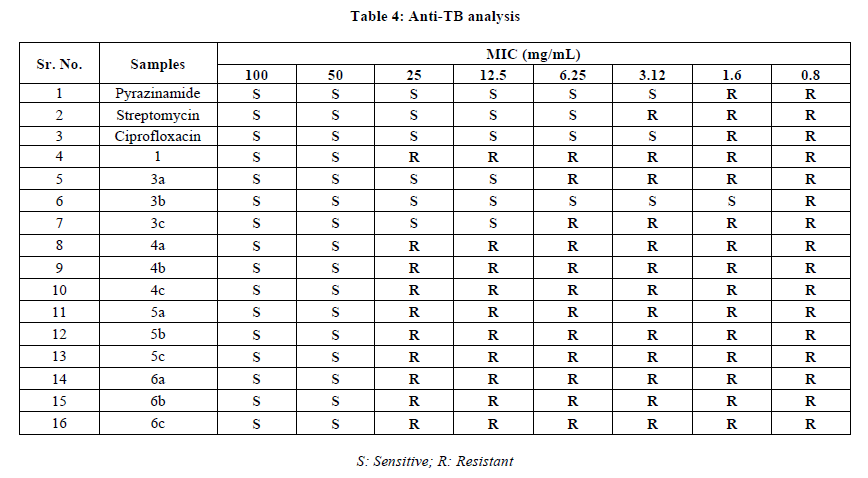
This could be as a result of the hydrazone, which bear polar and nonpolar properties together; this makes them suitable for permeation to the bacterial cell. This finding indicates that complex formation altered the solubility; hence the penetration of complex into the cell wall of the Mycobacterium tuberculosis is restricted. This results in decrease in the anti-tubercular activity of hydrazones upon complexation.
The observed results of the test compounds indicates the future potential for the development of hydrazones to solve the boundaries due to presently existing anti-tuberculosis agents to treat multiple drug resistant Tuberculosis.
Conclusion
New heterocyclic hydrazone ligands and their transition metal complexes have been synthesized and their in vitro antituberculosis potential was evaluated. These synthesized compounds were characterized using physicochemical techniques such as elemental analysis and NMR, UV–visible, IR, TGA, ESR, XRD and mass spectroscopies. Molar conductivity measurements show that all the complexes are non-electrolytic in nature. Metal-to-ligand stoichiometry is found to be 1:2 as evident all the spectral characterization. Antimicrobial results showed that the hydrazone ligands are more potent than the metal complexes. Antituberculosis activity of the hydrazone 3b revealed an MIC value of 1.6 μg/mL.
Acknowledgment
The authors thank the Principal, Government of Maharashtra, Ismail Yusuf College, Jogeshwari east, Mumbai-60 for providing facilities for research work. The authors thank Dr. Kishore Bhat, Deparment of Microbiology, Maratha Mandal’s NGH Institute of Dental Sciences and Research Centre, Belgaum-590010, India, for antituberculosis study.
References
[1] T. Aboul-Fadl, H.A. Abdel-Aziz, M. K.Abdel-Hamid, T. Elsaman, J. Thanassi, M.J. Pucci, Molecules., 2011, 16, 7864.
[2] H. Lang, G. Quaglio, O. Olesen, Tuberculosis., 2010, 90, 1.
[3] M.W. Khan, M.J. Alam, M.A. Rashid, R. Chowdhury, Bioorg. Med. Chem., 2005, 13, 4796.
[4] J.H. Jang, K. Kanoh, K. Adachi, Y. Shizuri, J. Antibiot., 2006, 59, 428.
[5] S.M. Rida, S.A. El-Hawashi, H.T. Fahmy, A.A. Hazza, M.M. El-Meligy, Arch. Pharm. Res., 2006, 29, 16.
[6] C. Ryu, A. Song, J.Y. Lee, J.Hong, J.H. Yoon, A. Kim, Bioorg. Med. Chem. Lett., 2010, 20, 6777.
[7] B.F. Abdel-Wahab, H.A. Abdel-Aziz, E.M. Ahmed, Eur. J. Med. Chem., 2009, 44, 2632.
[8] K. Manna, Y.K. Agrawal, Eur. J. Med. Chem., 2010, 45, 3831.
[9] M. Brandvang, V. Bakken, L. Gundersen, Bioorg. Med. Chem. Lett., 2009, 17, 6512.
[10] Q. Shi, L. Xu, J. Ji, Y. Li, R. Wang, Z. Zhou, R. Cao, M. Hong, A.S.C. Chan, Inorg. Chem. Commun., 2004, 7, 1254.
[11] S. Gemma, L. Savini, M. Altarelli, P. Tripaldi, L. Chiasserini, S.S. Coccone, V. Kumar, C. Camodeca, G. Campiani, E. Novellino, S. Clarizio, G. Delogu, S. Butini, Bioorg. Med. Chem., 2009, 17, 6063.
[12] J.R. Dilworth, Coord. Chem. Rev., 1976, 21, 29.
[13] J.R. Merchant, D.S. Clothia, J. Med. Chem., 1970, 13, 335.
[14] M.C. Mandewale, B.R. Thorat, Y. Nivid, R. Jadhav, A.S. Nagarsekar, R.S. Yamgar, J. Saudi. Chem. Soc., 2016.
[15] M.C. Mandewale, S. Kokate, B.R. Thorat, S.S. Sawant, R.S. Yamgar, Arabian. J. Chem., 2016.
[16] M.C. Mandewale, B. Thorat, D. Shelke, R. Yamgar, Bioinorg. Chem. Appl., 2015, 14.
[17] M.C. Mandewale, B. Thorat, R. Yamgar, Der Pharma Chemica., 2015, 7, 207-215,
[18] R. Yamgar, Y. Nivid, S. Nalawade, Mustapha Mandewale, R. Atram, S. Sawant, Bioinorg. Chem. Appl., 2014, 2014, 10.
[19] D.S. Kushwaha, Y.K. Mishra, M. Ajay M. Chaturvedi, Int. J. Chem. Stud., 2014, 2, 25.
[20] G.G. Mohamed, M.M. Omar, A.M. Hindy, Turk. J. Chem., 2006, 30, 361.
[21] I.S. Ahuja, R. Singh, J. Indian. Chem. Soc., 2001, 78, 39.
[22] U. Sakaguchi, D.N. Anderson, J. Chem. Soc. Dalton. Trans., 1979, 600.
[23] M.S. Lourenco, M.N. DeSouza, A.C. Pinheiro, M.L. Ferreira, R.B. Goncalves, T.M. Nogneira, M.A. Peralta, ARKIVOC, 2007, 15, 181.



