Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 8
Synthesis and Biological Activities of [1,3]-Oxazine Derivatives
Chaitra G* and Rohini RM
Department of Pharmaceutical Chemistry, Al-Ameen College of Pharmacy, Bengaluru, India
- *Corresponding Author:
- Chaitra G
Department of Pharmaceutical Chemistry
Al-Ameen College of Pharmacy
Bengaluru, India
Abstract
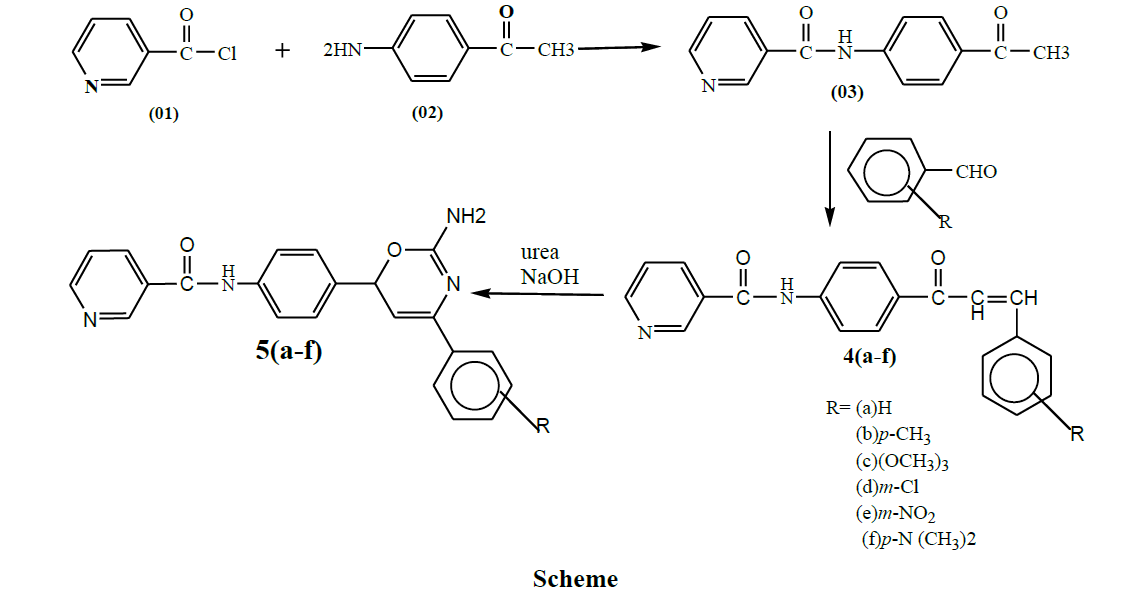
A series of novel [1,3]-oxazine derivative of N-[4-(2-Amino-4-phenyl-6H-[1,3]oxazine-6-yl)-phenyl]-nicotinamide are synthesized due to its wide range of biological activities. To achieve the target compounds the starting material used was nicotinyl chloride. Chalcone derivatives were synthesized by carrying the reaction of benzaldehyde derivatives with p-amino acetophenone. The obtained products were allowed to react with urea to give heterocyclic derivatives of oxazine 5(a-f) respectively. The structures of all synthesized molecules were confirmed by Ultraviolet (UV), Infrared (IR), Proton Nuclear Magnetic Resonance (1H-NMR), Carbon-13 Nuclear Magnetic Resonance (13C-NMR) and Mass spectral data. They were screened for their in-vitro anti-inflammatory activity by bovine serum albumin and protease method and anti-oxidant activity by diphenyl picryl hydrazide and nitric oxide method. N-{4-[2-Amino-4-(3,4,5-trimethoxy-phenyl)-6H-[1,3]oxazine-6-yl]-phenyl}-nicotinamide 5(c) and N-{4-[2-Amino-4-(3-nitro-phenyl)-6H-[1,3]oxazine-6-yl]-phenyl}-nicotinamide methane 5(e) exhibited significant activity when evaluated for BSA and protease methods for anti-inflammatory activity; DPPH and NO methods for anti-oxidant method at 10 μg, 50 μg and 100 μg.
Keywords
Oxazine, Chalcone, Antiinflammatory, Antioxidant activity.
Introduction
Heterocyclic derivatives of chalcones that have been synthesized have shown biological and pharmacological activities, resulting good chemotherapeutics compounds [1]. Oxazine derivatives are one among the heterocyclic compounds that shows biological activities [2] like anti-hyperglycemic [3], anti-leishmanial [4], anti- tubercular [5], anti-ulcer [6], anti-cancer [7]. Oxazine are heterocyclic compounds containing one nitrogen and one oxygen with three isomeric forms [8]. Anti-oxidant and anti-inflammatory based drug are used for the prevention and treatment of complex diseases like atherosclerosis, stroke, diabetes, alzheimer’s disease and cancer [9]. The interest on 1,3-oxazine molecules has increased recently because compounds containing dihydro-1,3-oxazine ring system exhibited a wide spectrum of pharmacological activities such as anti-malarial [10], anti-tumor [11], anti-bacterial [12-14], anti-oxidant [15-17], anti-inflammatory activity [18] and their versatility as synthetic intermediates [19]. Particular attention has been paid to these compounds since the discovery of the non-nucleoside reverse transcriptase inhibitor trifluoromethyl-1,3 -oxazine-2-one, which shows high activity against a variety of HIV-1 mutant strains [20]. This has been the prime step for the synthesis of various compounds consolidating the 1,3 -oxazine moiety [21].
Materials and Methods
Melting point of the synthesized compounds was determined in electro thermal apparatus using fused capillary tubes. Monitoring of the reaction and the purity of the compounds was checked by thin-layer chromatography using silica gel G plates of 0.5 mm thickness as stationary phase in combination of n-hexane: ethyl acetate in different ratios as mobile phase. The spots were visualized by using iodine chamber and Ultraviolet (UV) chamber (Scheme 1).
The UV spectra of the synthesized compounds were recorded on Shimadzu UV-1601 and the values of wavelength (λmax) were reported in nm. The Infrared (IR) spectra of the synthesized compounds were recorded on a Fourier Transform IR spectrophotometer (model Shimadzu 8700) in the range of 4000-400 cm-1 using KBr pellets and value of λmax are reported in cm-1 and the spectra were interpreted. Proton Nuclear Magnetic Resonance (1H-NMR) and Carbon-13 Nuclear Magnetic Resonance (13C-NMR) spectra were recorded on Bruker Avance II 400 NMR spectrometer using CDCl3. Chemical shift (δ) are reported in parts per million downfield from internal reference, Tetramethylsilane (TMS) and the spectra were interpreted. Mass spectra were recorded on Mass spectrophotometer (model Shimadzu) by MS-2010A.
Preparation of N-(4-Acetyl-phenyl)-nicotinamide (03)
To an ice cold solution of nicotinyl chloride (0.01 mol) dissolved in methanol, a methanolic solution of p-aminoacetophenone (0.01 mol) was added dropwise with constant stirring. Stirring was continued for 15 min then refluxed for one hour. The reaction mixture was cooled and the obtained precipitate was filtered. Crude product was recrystallized from isopropyl alcohol.
General method for synthesis of substituted 4 (a-f)
Equimolar quantity of N-(4-Acetylphenyl)-nicotinamide (0.01 mol) and substituted benzaldehyde (0.01 mol) were dissolved in absolute alcohol and 40% KOH solution was added slowly with stirring. Continuing the reaction for 9 h and left overnight. The reaction mixture was decomposed in ice-water to obtain product. Crude product was recrystallized from ethanol.
General method for the synthesis of substituted 5 (a-f)
Equimolar quantity of 4 (a-f) and urea were dissolved in ethanolic NaOH and was stirred for 2-3 h at room temperature. Refluxed for 6 h and poured into cold water with continuous stirring for 1 h and was cooled at 0ºC for 42 h. The precipitate obtained was filtered, washed and re-crystalized from ethanol.
N-(4-Acetyl-phenyl)-nicotinamide (3)
Orange color; M. p. 128ºC; Yield: 64.5%; IR (KBr) cm-1: (C=O) 1736.58 cm-1, (Ar C=C) 1427.07 cm-1, (Ar C-H) 3175.22 cm-1, (C=N) 1677.77 cm-1, (CH3) 2792.42 cm-1, (CONH) 1575 cm-1; 1H-NMR (CDCl3-ppm): δ (ppm)=8.0 (d, 1H, CONH), δ (ppm)=8.84 (m, 4H, Ar), δ (ppm)=7.75 (d, 2H, Ar), δ (ppm)=7.84 (d, 2H, Ar), δ (ppm)=2.55 (s, 3H, CH3).
N-[4-(3-Phenyl-acryloyl)-phenyl]-nicotinamide 4(a)
Pale yellow color; M. p. 121ºC; Yield: 69.28%; IR (KBr) cm-1: (NH) 3359 cm-1, (Ar C-H) 3025.76 and 3083.62 cm-1, (CH3)2917.77 cm-1, (CONH) 1515 cm-1, (C=C) 1643 cm-1, (Ar C=C) 1448.28 cm-1, (C=O) 1604.48 cm-1.
N-[4-(2-Amino-4-phenyl-6H-[1,3]oxazine-6-yl)-phenyl]-nicotinamide 5(a)
Pale yellow color; M. p. 106ºC; Yield: 63.6%; λmax : 379.00 nm; IR (KBr) cm-1: (NH2) 3468 cm-1, (NH) 3355.53 cm-1, (Ar C-H) 3027.69 cm-1, (C-O-C) 1035.59 cm-1, (C=N) 1646 cm-1, (Ar C=C) 1596 and 1492 cm-1, (CONH) 1529 cm-1; 1H-NMR (CDCl3-ppm): δ (ppm)=7.96 (d, 1H, CONH), δ (ppm)=7.57 (d, 2H, ArH), δ (ppm)=7.17 (m, 1H, ArH), δ (ppm)=7.30 (d, 2H, ArH), δ (ppm)=7.21 (s, 2H, ArH), δ (ppm)=2.0 (s, 2H, NH2), δ (ppm)=6.7 (d, 1H, C=C), δ (ppm)=5.19 (d, 1H, CH oxazine), δ (ppm)=5.19 (d, 1H,C-O), δ (ppm)=7.96 (m, 4H, 4-pyridine), δ (ppm)=7.17 (m, 4H, 1-benzene) δ (ppm)=2.0 (d, 2H, NH2).
N-[4-(2-Amino-4-p-tolyl-6H-[1,3]oxazine-6-yl)-nicotinamide 5(b)
Pale yellow color; M. p. 89ºC; Yield: 92.33; λmax: 326.50; IR (KBr) cm-1: (NH2) 3462.56 cm-1, (NH) 3343.96 cm-1, (Ar C-H) 3046.98 cm-1, (C-O- C) 1075 cm-1, (C=N) 1600.63 cm-1, (Ar C=C) 1611 and 1438.64 cm-1, (CONH) 1511.92 cm-1, (CH3) 2918.73 cm-1; 1H-NMR (CDCl3-ppm): δ (ppm)=7.93 (d, 1H, CONH), δ (ppm)=7.51 (d, 2H, ArH), δ (ppm)=7.2 (m, 1H, ArH), δ (ppm)=7.4 (d, 2H,ArH), δ (ppm)=7.29 (s, 2H, ArH), δ (ppm)=2.40 (s, 2H, NH2), δ (ppm)=3.73 (d, 3H, CH3), δ (ppm)=6.69 (d, 1H, C=C), δ (ppm)=5.2 (d, 1H, CH oxazine), δ (ppm)=6.68 (d, 3H, CO).
N-{4-[2-Amino-4-(3,4,5-trimethoxy-phenyl)-6H-[1,3]oxazin-6-yl]-phenyl}-nicotinamide 5(c)
Yellow color; M. p. 156ºC; Yield: 98.2; λmax : 325.60 nm; IR (KBr) cm-1: (NH2) 3457.74 cm-1, (NH) 3365.17 cm-1, (Ar C-H) 3232.1 cm-1, (C-O-C) 1126.22 cm-1, (C=N) 1628.59 cm-1, (Ar C=C) 1611 and 1438 cm-1, CONH 1593.88 cm-1, OCH3 2997.8, 2938.98, 2832.92 cm-1; 1H-NMR (CDCl3-ppm): δ (ppm)=7.9 [1H, (d, Ar H)], δ (ppm)=7.6 [1H, (s, Ar H)], δ 7.7 [1H (d, Ar H)], δ (ppm)=7.7 [1H, (d, Ar H)], δ (ppm)=7.4 [ 2H, (s, -C=C)], δ (ppm)=7.2 [2H, (s, -C=C)], δ (ppm)=6.1 [1H, (s, -C-H)], δ (ppm)=6.7 [1H, (d, -C=C)], δ (ppm)=2.41 [2H, (s, -NH2)], δ (ppm)=6.4 [2H, (d, Ar H)], δ (ppm)=3.7 [9H, (m, -OCH3)]; 13C-NMR (CDCl3-ppm): (2C, aromatic)-152.97, (4C, oxazine, C=N) δ (ppm)=151.61, 151.54, (1C, oxazine,C=O) δ (ppm)=197.17, (1C, oxazine, C-NH2) δ (ppm)=188.16, (1C,C=C) δ (ppm)=121.34, (1C,C-C) δ (ppm)=128.04, (1C, ArH) δ (ppm)=137.05, (2C, ArH) δ (ppm)=127.41 and 127.18, (2C, ArH) δ (ppm)=121.34, (1C, C-N) δ (ppm)=137.05, (1C, C-O) δ (ppm)=64.36, (1C,C-N) δ (ppm)=130.85, (2C, ArH) δ (ppm)=105.44, (3C, OCH3) δ (ppm)=56.18, 56.09 and 56.01; m/e: 476.0 (molecular ion).
N-{4-[2-Amino-4-(3-chloro-phenyl)-6H-[1,3]oxazin-6-yl]-phenyl}-nicotinamide 5(d)
Yellowish; M. p.179ºC; Yield: 70.0; λmax: 370.40 nm; IR (KBr) cm-1: (NH2) 3419 cm-1, (NH) 3332 cm-1, (Ar C-H) 3052 cm-1, (C-O-C) 1126.29 cm-1, (C=N) 1634 cm-1, (Ar C=C) 1442.49 cm-1, (CONH) 1518.3 cm-1; 1H-NMR (CDCl3-ppm): δ (ppm)=7.81 (d, 1H, CONH), δ (ppm)=7.52 (d, 2H, ArH), δ (ppm)=7.2 (m, 1H, ArH), δ (ppm)=7.4 (d, 2H, ArH), δ (ppm)=7.3 (s, 2H, ArH), δ (ppm)=2.0 (s, 2H, NH2), δ (ppm)=6.69 (d, 1H, C=C), δ (ppm)=5.1 (d, 1H, CH oxazine), δ (ppm)=6.68 (d, 3H, C-O).
N-{4-[2-Amino-4-(3-nitro-phenyl)-6H-[1,3]oxazin-6-yl]-phenyl}-nicotinamide methane 5(e)
Dark yellow; M. p. 159ºC; yield: 72.4; λmax: 374.60 nm; IR (KBr)cm-1: (NH2) 3425.92 cm-1, (NH) 3338.18 cm-1, (Ar C-H) 3089 cm-1, (C-O-C) 1176.36 cm-1, (C=N) 1634.38 cm-1, (Ar C=C) 1444.42 cm-1, (CONH) 1532.17 cm-1; 1H-NMR (CDCl3-ppm): δ (ppm)=7.83 (d, 1H, CONH), δ (ppm)=7.59 (d, 2H, ArH), δ (ppm)=7.31 (m, 1H, ArH), δ (ppm)=7.54 (d, 2H, ArH), δ (ppm)=7.29 (s, 2H, ArH), δ (ppm)=2.20 (s, 2H, NH2), δ (ppm)=6.69 (d, 1H, C=C), δ (ppm)=5.2 (d, 1H, CH oxazine), δ (ppm)=6.68 (d, 3H, C-O).
N-{4-[2-Amino-4-(3-dimethylamino-phenyl)-6H-[1,3]oxazin-6-yl]-phenyl}-nicotinamide methane 5(f)
Dark yellow; M. p. 190ºC; yield: 72.5; λmax: 412.60 nm; IR (KBr) cm-1: (NH2) 3400.85 cm-1, (NH) 3334.32 cm-1, C-H (Ar) 3046.98 cm-1, C-O-C 1028.84 cm-1, C=N 1598.7 cm-1, C=C (Ar) 1432.85 cm-1, (CONH) 1524.45 cm-1, [(CH3)3] 2807.85, 2911.99 cm-1; 1H-NMR (CDCl3-ppm): δ (ppm)=7.90 (d, 1H, CONH), δ (ppm)=7.50 (d, 2H, ArH), δ (ppm)=7.29 (m, 1H, ArH), δ (ppm)=7.4 (d, 2H, ArH), δ (ppm)=7.32 (s, 2H, ArH), δ (ppm)=2.0 (s, 2H, NH2), δ (ppm)=6.69 (d, 1H, C=C), δ (ppm)=5.19 (d, 1H, CH oxazine), δ (ppm)=6.68 (d, 3H, C-O), δ (ppm)=2.85 [m, 6H, N(CH3)2].
In vitro anti-inflammatory activity
Bovine serum albumin (BSA) method [22]
A solution of 0.2% w/v of Bovine serum albumin BSA was prepared in Tris buffer saline. Test sample stock solutions of 1000 μg/ml was prepared. From stock solutions two different concentrations of 100 μg/ml and 200 μg/ml were prepared by using methanol as a solvent. To 0.1 ml of each test sample 5 ml of 0.2% BSA was added. The blank consisted 5 ml 0.2% w/v BSA solution and 0.1 ml methanol. The positive control consist 0.1 ml (100 μg/ml) of Indomethacin in methanol and 5 ml 0.2% w/v BSA solution. The test samples were heated at 72˚C for five minutes and then cooled for 10 min. The absorbance of these solutions was determined by using spectrophotometer at a wavelength of 660 nm. The denaturation of the protein was determined on a percentage basis relative to the blank control using the following formula:-

Protease inhibition assay method [23]
The reaction mixture containing 0.5 ml of protease solution (0.06 mg of trypsin in 20 mM of Tries HCl buffer), 0.5 ml of Tris HCl buffer (20 mM) and 1 ml of test sample of different concentration was prepared. The mixture was incubated at 37ºC for 15 min and then 1 ml of 0.8% w/v casein was added. The mixture was incubated for an additional 20 min. Then 2 ml of 70% perchloric acid was added to terminate the reaction. Cloudy suspension was centrifuged and the absorbance of the supernatant was read at 210 nm against buffer as blank. The % inhibition was calculated using following formula:-

In vitro antioxidant activity
Diphenyl picryl hydrazide (DPPH) method [24]
A stock solution of DPPH (10 mg) was prepared by dissolving in 10 ml of methanol. From this stock solution, different dilutions were made to obtain concentrations of 10 to 100 μg/ml. The absorbance was recorded at 517 nm.
Standard solution ascorbic acid (10 mg) was dissolved in 10 ml of methanol. From this stock solution dilutions 10 to 100 μg/ml were measured. To ml of different concentration of ascorbic acid 1 ml of DPPH solution of 30 μg/ml concentration was added and volume was made up to 10 ml with methanol. The absorbance was recorded for these dilutions at 517 nm after incubation for 30 min.
The effect of ascorbic acid (vitamin C) on DPPH was assessed for comparison with that of synthesized compounds. The test samples were prepared by initial preparation of stock solution of (1000 μg/ml) in methanol and different concentrations were prepared (100, 50 and 10 μg/ml). To all these dilutions, 1 ml of DPPH solution (30 μg/ml) was added and absorbance were recorded at 517 nm after duration of 30 min. Percentage inhibition of free radical activity was calculated using the following formula.

Nitric oxide radical scavenging method [25]
To 0.5 ml of 10 mM sodium nitroprusside (10 mM) in phosphate buffer saline was added to 1 ml of test sample of different concentration, incubated at 25ºC for 180 min then added 1 ml of griess reagent [Solution A: 1% sulphanilamide in 2.5% phosphoric acid; Solution B: 0.1% napthyl ethylene diamine in 2.5% phosphoric acid].
Solution A and solution B were mixed in equal volume and were used before 12 h. The positive control ascorbic acid solution was prepared similarly and was incubated. A blank solution was prepared. The absorbance of chromophore formed was measured at 546 nm on UV-Visible spectroscopy.
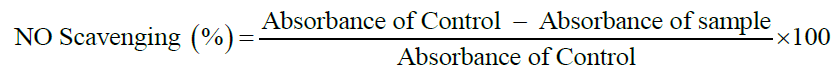
Results and Discussion
Anti-inflammatory activity
Bovine serum albumin (BSA) method
Among the screened compounds N-{4-[2-Amino-4-(3,4,5-trimethoxy-phenyl)-6H-[1,3]oxazine-6-yl]-phenyl}-nicotinamide 5(c) and N-{4-[2- Amino-4-(3-nitro-phenyl)-6H-[1,3]oxazine-6-yl]-phenyl}-nicotinamide methane 5(e) showed good activity compared to positive control of indomethacin. Other compounds showed dose dependent good in vitro anti-inflammatory activity as compared to standard indomethacin at 200 μg/ml. the results have been depicted in Table 1.
| Compound code | Percentage Inhibition (%) | |
|---|---|---|
| Dose (100 μg/ml) | Dose (200 μg/ml) | |
| Indomethacin | 78.01 | 94.8 |
| 5a | 53.3 | 73 |
| 5b | 53.3 | 66.6 |
| 5c | 66.6 | 86.6 |
| 5d | 46.6 | 86.6 |
| 5e | 73.3 | 86.6 |
| 5f | 66.6 | 80.6 |
Table 1: Anti-inflammatory activity by BSA method
Proteases method
All the synthesized compounds were screened for in-vitro anti-inflammatory activity by proteases method. It was evident by the observation that the compound 5(c) and 5(e) exhibited excellent anti-inflammatory activity. The results have shown in following Table 2.
| Compound code | Percentage Inhibition (%) | ||
|---|---|---|---|
| Dose (100 μg/ml) | Dose (50 μg/ml) | Dose (10 μg/ml) | |
| Indomethacin | 89.32 | 69.31 | 30.15 |
| 5a | 77.6 | 70.4 | 52.22 |
| 5b | 77.88 | 57.64 | 50.55 |
| 5c | 77.74 | 71.43 | 67.64 |
| 5d | 77.6 | 66.33 | 64.89 |
| 5e | 86.34 | 68.91 | 68.77 |
| 5f | 54.74 | 71.01 | 61.66 |
Table 2: Anti-inflammatory activity by Protease method
Anti-oxidant activity
Diphenyl picryl hydrazide (DPPH) method
The hydrogen atom donating ability of the synthesized was determined by the decolorization of methanol solution of 2,2-diphenyl-1- picrylhydrazyl (DPPH). DPPH produced violet color in methanol and faded to yellow color in the presence of antioxidants. Compared to standard ascorbic acid 5(c) and 5(e) compounds exhibited good activity. The results have shown in below Table 3.
| Compound code | Percentage Inhibition (%) | ||
|---|---|---|---|
| Dose (100 μg/ml) | Dose (50 μg/ml) | Dose (10 μg/ml) | |
| Ascorbic acid | 99.25 | 95.41 | 90.5 |
| 5a | 94.1 | 90.6 | 89.4 |
| 5b | 98.6 | 86 | 85.9 |
| 5c | 94.7 | 94.4 | 94 |
| 5d | 87 | 82.6 | 80 |
| 5e | 90 | 85 | 84.6 |
| 5f | 76.6 | 62.2 | 61.5 |
Table 3: Anti-oxidant activity by DPPH method
Nitric oxide (NO) method
The compounds have been tested in 10, 50 and 100 μg/ml which have shown good activity among them 5(c) and 5(e) exhibited highest activity as compared to standard ascorbic acid. The results have depicted in Table 4.
| Compound code | Percentage Inhibition (%) | ||
|---|---|---|---|
| Dose (100 μg/ml) | Dose (50 μg/ml) | Dose (10 μg/ml) | |
| Ascorbic acid | 81.112 | 80.4 | 78.81 |
| 5a | 81.11 | 81.11 | 81.73 |
| 5b | 80.48 | 81.11 | 81.11 |
| 5c | 81.11 | 81.73 | 81.11 |
| 5d | 89.11 | 85.11 | 81.11 |
| 5e | 81.11 | 81.11 | 81.11 |
| 5f | 81.11 | 83.46 | 81.11 |
Table 4: Anti-oxidant activity by NO method
Conclusion
From our present study, it can be concluded that six derivatives of substituted 1,3-oxazine were obtained from the intermediate chalcone on treatment with urea in presence of ethanolic NaOH. The compounds were synthesized in good percentage of yield their physical and analytical determination was done by using melting point apparatus, purification of compounds by TLC. The structural assignments of these compounds were identified and characterized by using IR, 1H-NMR, 13C-NMR and Mass techniques. It is evident from the research work that the series of synthesized and screened compounds can further be explored for in vivo studies.
References
- P. Anusha, P. Mani Chandrika, S. Shruthi, S.U. Nishat, S. Sultana, World. J. Pharm. Pharm. Sci., 2015, 4(11), 885-895.
- G.R. Kamala, L. Patnaik, M. Annapurna, S. Sasanka, M.S.K. Naidu, J. Med. Chem. Drug. Discovery., 2016, 8, 2332.
- H. Jamal, W.H. Ansari, S.J. Rizvi, Biol. Med., 2009, 1, 107-115.
- Y. Zuo, Euro. J. Med. Chem., 2012, 50, 393-404.
- V. Kotra, India. J. chem., 2010, 49, 1109-1116.
- N.S. Pamar, India. J. Phy. Pharma. Col., 1998, 42, 343-351.
- H.P. Singh, Sch. Res. Lib., 2010, 2, 460-472.
- F.V. Lauro, D.C. Franciso, L.R. Marcela, G.C. Elodia, P.G. Eduardo, LR. Maria, J. Chem., 2014, 1-9.
- K. Elumalai, M.A. Ali, M. Elumalai, K. Eluri, S. Srinivasan, J. Acute. Disease., 2013, 316-321.
- N.A. Khalaf, K. Ashok, Shakya, A. Al-Othman, Z. El-agbar, H. Farad, Turk. J. Biol., 2008, 32, 51-55.
- Duffin, W.M. Rollo, I.M. Br, J. Pharm. Col., 1957, 12, 171.
- M.E. Kuehne, E.A. Konopke, J. Med. Chem., 1962, 5, 257.
- J.B. Chylinska, T. Urbanski, J. Med. Chem., 1963, 6, 484.
- L.Y. Hsu, C.H. Lin, Heterocycles., 1996, 43, 2687.
- O.S. Pedersen, E.B. Pedersen, Synthesis., 2000, 2000 (4), 479-495.
- A.J. Cocuzza, D.R. Chidester, B.C. Cordova, S. Jeffrey, R.L. Parsons, Bacheler, Bioorg. Med. Chem. Lett., 2001, 11, 1177.
- T.N. Doan, D.T. Tran., Pharmacol. Pharm., 2011, 2, 282-288.
- C.M. Bhalgat, M.I. Ali, B. Ramesh, G. Ramu, Ara, J. Chem., 2011, 1-8.
- O.V. Singh, H. Han, Tetrahedron Lett., 2007, 48, 2345.
- N. Zonatta, Squizani, A.M.C, Fantinel, N. Bonacorso, J. Braz. Chem. Soc., 2005, 16, 1255-1261.
- Shingare, A.H. Kategaonkar, S.S. Sonar, U. Rajkumar, A.H. Kategaonkar, B. Bapurao, Bull. Korean. Chem. Soc., 2010, 31, 6, 1657.
- Z. Nowakowska, Eur. J. Chem., 2007, 42, 125-137.
- Oyedapo, J. famurewaa, Int. J. P’çog., 1995, 33, 65-69.
- M. Philip, J. Songklanakarin, Scitechnol., 2004, 26, 2, 211-219.
- B.A. Allne, M.M. Michel, A.L. Margareth, Med. Chem., 2014, 4-7.