Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 9
Synthesis and Biological Evaluation of 2-Substituted Benzimidazole Derivatives
Nilam B Pawar*, Deepak Yadav and Bhushan R Pawar
Gourishankar Institute of Pharmaceutical Education and Research, Limb, Satar, Survey No. 990, Maharashtra, India
- *Corresponding Author:
- Nilam B Pawar
Gourishankar Institute of Pharmaceutical Education and Research
Limb, Satar, Survey No. 990, Maharashtra, India
Abstract
A novel 2-substituted benzimidazole derivatives were synthesized by the reaction of naproxen with aromatic diamine-o-Phenylenediamine. All the compounds were characterized by InfraRed (IR), Proton Nuclear Magnetic Resonance (1H-NMR). The synthesized derivatives were screened for Anthelmintic and anti-inflammatory activities. Eicosanoids in Helminthes appear to play a dual role, for metabolism or maturation of the organism and communication with the host on a cellular basis. Production of eicosanoids correlates with both penetration and transformation, two activities required for infection. Eicosanoid is involved in control of virulence mechanisms. Therefore in the view of designing novel anthelmintic agent we have synthesized benzimidzole derivative of Naproxen. Naproxens react with free amine group to from benzimidazle derivatives. In this way three derivatives were synthesized and screened for Anthelmintic activities against Indian adult earthworms (Pheretima Posthuma) at various concentrations (5 mg/ml, 10 mg/ml and 20 mg/ml). All compounds showed their significant action for time taken to paralysis and death when compared standard.
Keywords
Nonsteroidal Anti-inflammatory, Albendazole, Anthelmintic, Pheretima posthuma
Introduction
Parasitic diseases are still worldwide problems that have a deep impact on public health [1]. Parasitic infections, such as helminthiosis and protozoosis, are still a major problem in developing countries, affecting mainly the infant population [1].
Intestinal helminthiosis is still a major health problem mainly in developing countries. The economic importance of helmintic infections has long been recognized in the field of animal husbandry. For this reason the most important advances in the chemotherapy of helminthiosis have probably come from the animal health area. The control of helminthiosis relies on the use of anthelmintics. Helminth parasites that have an intra and extra intestinal phase are able to infect humans as well as animals [2]. The treatment for intestinal helminthiosis is usually carried out with Benzimidazole 2-carbamate derivatives, such as albendazole (ABZ) and mebendazole (MBZ) are widely used in the chemotheraphy of many human and animal enteral helminthic infections [3]. The anthelmintic activity of benzimidazole carbamates has been related to their selective antimitotic activity, due to the preferential binding to helmintic tubulin over mammalian tubulin [4]. Albendazole is a potent, broad spectrum Anthelmintic agent used mainly in the treatment of intestinal helminthiosis. Also, it has been demonstrated that Albendazole is effective against some tissue-dwelling infestations, such as trichinellosis, hydatid disease and neurocysticercosis. However, high doses and long treatment are required in the latter cases; due mainly to the poor solubility and absorption of Albendazole [5]. For optimal Anthelmintic activity, BZC must bear an H at the 1-position of the benzimidazole ring [6].
In most rural communities low standard of sanitation and poor socio-economic conditions are obvious predisposing factors to high prevalence of human intestinal helminthiosis [7]. Many parasitic infections, especially those of helminthes origin are asymptomatic, could produce mild or, in a typical cases, confusing symptoms and are often neglected until bizarre, serious or chronic clinical pictures are present. Though the available anthelmintics are effective against these worms but there has been no significant new anthelmintic introduced in clinical trials since ivermectine has entered clinical trials about twenty years ago [8]. Also anthelmintic resistance has been widely reported in livestock’s and it may also be a matter of time before this phenomenon occurs in the parasites of humans. This scenario indicates that there is great need of newer anthelmintics and development of integrated programs to control and combat developing resistance among helminthes [9].
However benzimidazole carbamate is reported for Anthelmintic activity. Eicosanoids in Helminths appear to play a dual role, metabolism or maturation of the organism and communication with the host on a cellular basis. Production of eicosanoids correlates with both penetration and transformation, two activities required for infection. Eicosanoid is involved in control of virulence mechanisms [10-13]. Therefore in the view of designing novel anthelmintic agent we have synthesized 2-substituted benzimidazole and evaluated for Anthelmintic activity against Pheretima posthuma.
Materials and Methods
Experimental (Tables 1-3) [9-22]
| Comp. No. | Mol. Form. (Mr)a | M. p. (ºC) | % Yield | Rf valueb |
|---|---|---|---|---|
| DY1 | 230 | 106-108 | 57.52 | 0.67 |
| DY2 | 353 | 132-134 | 51.78 | 0.74 |
| DY3 | 244 | 142-146 | 61.45 | 0.56 |
aSolvent for crystallization: Ethanol; bSolvent systems: Ethyl acetate: Ethanol (1: 1), Ethanol:n-hexane(1: 1), Methanol: benzene(4: 1)
Table 1: Physicochemical data of Benzimidazole derivatives
| S. No. | Parameters | Conc. | DY1 | DY2 | DY3 | STD (Albendazole) |
|---|---|---|---|---|---|---|
| 1 | Time taken for paralysis in minutes | 1% | 14.35 ± 0.15 | 11.47 ± 0.10 | 17.45 ± 0.10 | 7.30 ± 0.2 |
| 2% | 12.17 ± 0.15 | 9.36 ± 0.10 | 13.15 ± 0.10 | 6.34 ± 0.2 | ||
| 5% | 6.30 ± 0.15 | 8.40 ± 0.1 | 9.25 ± 0.10 | 5.45 ± 0.2 | ||
| 2 | Time taken for death in minutes | 1% | 27.32 ± 0.15 | 21.39 ± 0.15 | 29.50 ± 0.15 | 10.5 ± 0.2 |
| 2% | 18.52 ± 0.15 | 16.52 ± 0.1 | 19.32 ± 0.15 | 8.45 ± 0.2 | ||
| 5% | 10.12 ± 0.15 | 11.07 ± 0.15 | 15.12 ± 0.15 | 7.37 ± 0.2 |
Table 2: Anthelmintic activity of test comp. on Pheretima posthuma
| Name of Microorganism | Zone of Inhibition in mm | |||
|---|---|---|---|---|
| Standard | DY1 | DY2 | DY3 | |
| Pseudomonas aeruginosa | 3.9 | 3.8 | 2.2 | 1.9 |
| Bacillus subtilis | 3.4 | 3.1 | 1.8 | 1.6 |
| Staphylococcus aureus | 3.5 | 2.5 | 1.8 | 1.9 |
| Escherichia coli | 3.2 | 2.8 | 1.4 | 1.1 |
Table 3: Antimicrobial activity
All chemicals and solvents used for the experimental work are AR grade and LR grade, purchased from PALLAV, Research Lab. The reactions were carried out by conventional method. Melting points were determined by open capillary method. All chromatographic purifications were done on silica gel. The IR spectra were recorded on JASCO FTIR -410 using KBr pressed pellet technique, proton magnetic resonance spectra (1H-NMR) were recorded on a Bruker 300 MHz instrument (Bruker, Germany) in dimethyl sulfoxide-d6/CDCl3 using tetramethylsilane as internal standard. Chemical shifts are reported in ppm (δ) relative to the solvent peak.
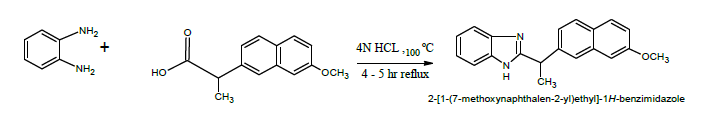
Synthesis of 2-[1-(7-methoxynaphthalen-2-yl) ethyl]-1H-benzimidazole (DY1)
A mixture of 1.08 g 0.0l mol of 0-Phenylenediamine, 2.30 g (0.01 mol) of Naproxen, 5 ml of dilute hydrochloric acid were taken into a 250 ml round bottomed flask and the mixture was heated on a water bath at 100°C for 4-5 h. The reaction mixture was cooled and 10% sodium hydroxide solution was added slowly with constant stirring of the flask, until the mixture was just alkaline to litmus paper. The brown coloured solid obtained was filtered on a Buchner funnel.
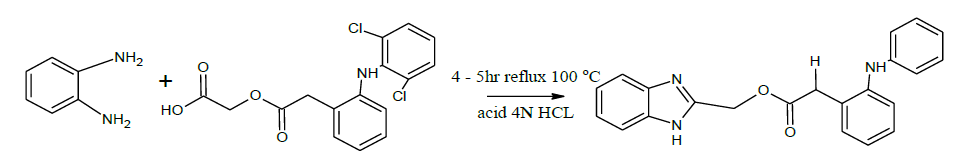
Synthesis of (2-[(2-chlorophenyl) amino] benzyl 1H-benzimidazol-2-ylacetate) (DY2)
A mixture of 1.08 g 0.0l mol of 0-Phenylenediamine 3.54 g (0.01 mol) of aceclofenac, 5 ml of dilute hydrochloric acid were taken into a 250 ml round bottomed flask and the mixture was heated on a water bath at 100°C for 4 h. The reaction mixture was cooled and 10% sodium hydroxide solution was added slowly with constant stirring of the flask, until the mixture was just alkaline to litmus paper. The brown coloured solid obtained was filtered on a Buchner funnel.
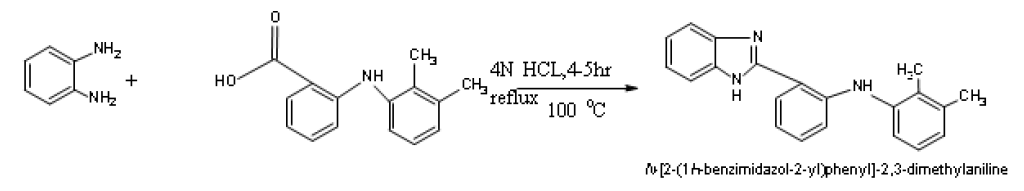
Synthesis of N-[2-(1H-benzimidazol-2-yl)phenyl]-2,3-dimethylaniline) (DY3)
A mixture of 1.08 g 0.0l mol of O-Phenylenediamine, 2.44 g (0.01 mol) of Mefenamic acid 5 ml of dilute hydrochloric acid were taken into a 250 ml round bottomed flask and the mixture was heated on a water bath at 100°C for 4-5 h. The reaction mixture was cooled and 10% sodium hydroxide solution was added slowly with constant stirring of the flask, until the mixture was just alkaline to litmus paper. The brown coloured solid obtained was filtered on a Buchner funnel.
Spectral data of synthesized derivatives (DY1-DY3)
2-[1-(7-methoxynaphthalen-2-yl) ethyl]-1H-benzimidazole (DY1)
IR (KBr, cm-1): 3331.07 (N-H str), 1502.55 (c=c), 3051.39 (Ar C-H), 742.59 (=C-H out of plane), 1604.77(N-H bend).
(2-[(2-chlorophenyl) amino] benzyl 1H-benzimidazol-2-ylacetate) (DY2)
IR (KBr, cm-1): 3371.32 (N-H), 1583.56 (N-H) bend, 742.56 (C-Cl), 1259.89 (C-O), 1629.34 (C=O), 3037.8 (ArCH), 3174.45 (Al CH). 1H-NMR ( ppm): 13.980 (s, 1H NH), 7.096, 7.231, 7.354, 7.518 (m, Ar-H), 6.414 (d, NH), 3.805 (s, CH2).
N-[2-(1H-benzimidazol-2-yl) phenyl]-2, 3-dimethylaniline) (DY3)
IR (KBr, cm-1): 1496.76 (C=C), 3371.32 (N-H Str), 3037.89 (Ar C-H), 3174.89 (al C-H), 1259 (C-F) 1685.67 (N-H Bend).
Pharmacology
In vitro-anthelmintic activity [2,5,10]
The newly synthesized compounds were tested for anthelmintic activity. P. posthuma (earthworm obtained from Satara region) of nearly equal size (10 cm ± 1) were used for the in vitro Anthelmintic bio assay of all newly synthesized prototypes. The worms were acclimatized to the laboratory condition before experimentation. The worms were divided into the respective groups containing 4 earth worms in each group. All the synthesized products (DY1-DY3) dissolved in respective solvents and diluted with normal saline for making the concentration of 1%, 2%and 5%. All the prototypes and the standard drug solution were freshly prepared before commencement of the experiments. All the earthworms were washed in normal saline solution before they were released into 20 ml of respective formulation as follows, The anthelmintic activity was determined in four observations. Four worms of about the same size per Petri dish were used. They were observed for their spontaneous motility and evoked responses. Observations were made for the time taken to paralysis and death of individual worms. Paralysis was said to occur when the worms do not revive even in normal saline. Death was concluded when the worms lost their motility followed with fading away of their body colour.
Antimicrobial activity [10,13,15,16]
The compounds were tested for their in vitro growth inhibitory activity against different bacteria. The various organisms like Staphylococcus aureus, Bacillus subtilis, (all Gram positive) and Pseudomonas aeruginosam, E. coli (all Gram negative) were procured from Microbes Specialty Lab, The inhibition zones of synthesized compounds were determined using cup plate method. The sterilized medium (autoclaved at 121°C for 20 min) was inoculated using 18 h slant cultures of the test organisms and transferred into sterile petri dishes and allowed to solidify the media. Cups of 8 mm diameters were made on solidified media. Solutions of the synthesized compounds at a concentration of 50 μg/ml and 500 μg/ml were prepared in DMSO. Each solution was placed in cups by means of sterile pipette. The plates thus prepared were left for 90 min in a refrigerator for diffusion. The plates were incubated at 37°C for 24 h and examined for inhibition zones. The experiment was performed in duplicate and the average diameter of the zones of inhibition was recorded. Ciprofloxacin (50 μg/ml) was used as standard.
Results and Discussion
Benzimidazole derivatives were synthesized by proposed route of reaction. Various NSIADs were used. Reactions were monitored by Thin Layer Chromatography (TLC) and used to assess the purity of intermediates and the final compounds, by using different solvent system. The physicochemical properties of anticipated compounds showed desirable solubility and acceptable melting point range. The structural elucidation of the synthesized compounds was carried out with the help of IR spectroscopy and 1H-NMR spectroscopy.
The IR spectrum of synthesized derivatives exhibits various bands at frequencies 3439 cm-1 (N–H str), 3000-3100 cm-1 (Aryl C–H str), 2900-3000 cm-1, (Alkyl C–H str), 1730-1700 cm-1 (C=O str), 1550-1640 cm-1 (Amide N–H str).
The NMR spectrum of synthesized derivatives showed characteristic peaks at δ values at 3.8 (CH3), 6.4-7 (Aryl C–H), 13(imino N-H). All the synthesized compounds were screened for antimicrobial, anthelmintic activities. Various strains used for activity are B. Subtilis, Staphylococcus aureus (Gram +ve), Escherichia coli, and P. aeruginosa (Gram -ve). Ciprofloxacin was used as standard drug for the comparing the antibacterial activity of synthesized benzimidazole derivatives.
In case of B. subtilis Compounds 2-[1-(7-methoxynaphthalen-2-yl) ethyl]-1H-benzimidazole (DY1) showed equipotent antibacterial activity. Compounds DY2 showed moderate activity compared with standard. (DY3), showed slight activity.
In case of S. aureus Compounds 2-[1-(7-methoxynaphthalen-2-yl) ethyl]-1H-benzimidazole (DY1) showed high antibacterial activity. Compounds DY2 showed moderate activity compared with standard. (DY3), showed slight activity.
In case of P. aeruginosa Compound 2-[1-(7-methoxynaphthalen-2-yl) ethyl]-1H-benzimidazole (DY1) showed high antibacterial activity. Compounds DY2 showed moderate activity compared with standard. (DY3), showed slight activity.
In case of E. coli Compounds 2-[1-(7-methoxynaphthalen-2-yl) ethyl]-1H-benzimidazole (DY1) showed high antibacterial activity. Compounds DY2 showed moderate activity compared with standard. (DY3), showed slight activity.
All the synthesized compounds were subjected to Anthelmintic activity studies against species of earthworm P. posthuma. Distilled water was used as control whereas Albendazole used as a Standard compounds. The paralyzing and death times were noted and their mean was calculated. The death time was ascertained by placing the earthworms in warm water (50°C) which stimulated the movement, if the worm was alive. Almost, all the newly synthesized compounds were found to exhibit moderate to good Anthelmintic activity against earthworm species. Comparison of Anthelmintic activity data revealed that Compounds (2-[(2-chlorophenyl) amino] benzyl 1H-benzimidazol-2-ylacetate) (DY2) exhibit higher bioactivity against earthworm.species. Compounds 2-[1-(7-methoxynaphthalen-2-yl) ethyl]-1H-benzimidazole (DY1) showed moderate Anthelmintic activity. Compound N-[2-(1H-benzimidazol-2-yl) phenyl]-2,3-dimethylaniline) (DY3), showed slight activity in comparison to standard drugs (Albendazole).
Conclusion
The synthesis of different Benzimidazole derivatives was carried out by using novel synthetic scheme using O-Phenylene diamine as starting material. This can be reacted with NSAIDs to from benzimidazole derivatives. The reaction afforded desired derivatives in yield ranging from 50% to 60%. All synthesized compounds were meeting the expected spectral data.
All the confirmed compounds were screened for anti-microbial, antiinflammmatory and anthelmintic activity. Results suggested that compound DY3 showed high antibacterial activity against all bacteria when compared with standard drug Ciprofloxacin While compound DY2 exhibited higher anthelmintic against earthworm species P. posthuma.
Heterocyclic and fused heterocyclic substituents were exhibited and might be responsible for maximum anthelmintic activity also they may be responsible anti-inflammatory activity.
In future, further studies using various in vivo models, broad therapeutic spectrum of the compound can be established. Thus the compounds may be further explored for their pharmacophoric model using QSAR studies to develop compounds for enhanced activities.
Acknowledgement
The authors wish to thank Sophisticated Analytical Instrument Facility Division, Kolhapur University, Kolhapur; for providing 1H-NMR spectral data. Authors also wish to extend their gratitude to Dr. A.V. Yadav sir, and Principal of Gouri shankar Institute of pharmaceutical education and research limb, Satara for providing necessary facilities and Microbiology Department.
References
- H. Francisco, H. Alicia, C. Rafael, N. Gabriel, S. Olivia, H. Manuel, Y. Lillian, Eur. J. Med. Chem., 2010, 45, 3135-3141.
- J. Rivera, R. Yepez-Mulia, A. Hernandez-Campos, R. Moreno-Esparza, R. Castillo, G. Gabriel Navarrete-Vazquez, I. Fuentes- Noriega, H. Jung-Cook, Int. J. Pharmaceut., 2007,344, 159-165.
- R. Yarely, Y. Lillian, C. Ivan, L. Fabian, S. Olivia, H. Alicia, C. Rafael, H. Francisco, Bioorg. Med. Chem., 2011, 19, 789-797.
- G. Navarrete-Vazquez, R. Cedillo, R. Hernandez-Campos, R. Yepez, F. Hernandez-Luis, J. Valdez, R. Morales, R. Corte, M. Hernandezc, R. Castilloa. Bioorg. Med. Chem. Lett., 2001, 11, 187-190.
- F. Hernandez-Luis, A. Hernandez-Campos, L. Yepez-Mulia, R. Cedillo, R. Castilloa. Bioorg. Med. Chem. Lett., 2001, 11, 1359-1362.
- J. Valdez, R. Cedillo, A. Hernandez-Campos, R. Yepez, F. Hernandez-Luis, G. Navarrete-Vazquez, A. Tapia, Corte R, M. Hernandez, R. Castillo, Bioorg. Med. Chem. Lett., 2002, 12, 2221-2224.
- M. Gundiri and O. B. Akogun, The Nigerian J Parasitol., 2000,21, 117-124.
- R.K. Griffith, Burger’s medicinal Chemistry and drug discovery, 6th (Edn.), A John Wiley and Sons Inc. Publication., New York 2003, pp. 1090-1093.
- T. Eugale, G. Tilahum, A. Debella, A. Feleke, E. Makonen, 2007, 110, 428-433.
- W. Ralph. Howard and W. David, Stanley. Annals of the Entomological Society of America.92 (1999) 880-890.
- A. Belley, K. Chandee, Parasitology today., 1995, 11, 327-334.
- C.M. Noverr, R. John, E. Downward, G. Huffnagle, Clin. Microbiol. Rev., 2003, 16, 517-533.
- http://www.chemguide.co.uk/physical/catalysis/hydrolyse.html (April 22, 2012)
- http://en.wikipedia.org/wiki/schotten–baumann reaction, 2012.
- http://en.wikipedia.org/wiki/Triethylamine, 2012.
- P. Wentworth, A. Datta, S. Smith, A. Marshall, L. Partridge, G. Blackburn. J. Am. Chem. Soci., 1997, 119, 2315-2316.
- http://en.wikipedia.org/wiki/acyl chloride (April 12 2012)
- R.P. Schwarzenbach, Philips M, Gschwend, Dieter M, Imboden, Environmental organic chemistry. J.F. Rossignol, R.Cavier.US, 1976, 3, 950351, 13.
- P.L. Kriz, Introduction to Spectroscopy, 3rd (Edn.) Harcourt College Publishers, 2007, 13-352.
- B. Lakshmanan, P. Mazumder, D. Sasmal, S. Ganguly, S. Jena,Acta. Parasitologica Globalis.,2011, 1 01-05.
- S. Pawar, P. Gorde, R. Kakde, Scholars Research Library., 2010, 2, 80-85.
- M. Shahare, V. Kadam, D. Jagdale, P. Gandhi, P. Gaikwad. Int. J. Res. pharmacy Chem., 2012, 2, 132-136.



