Research - Der Pharma Chemica ( 2022) Volume 14, Issue 2
Synthesis and Biological evaluation Of Some New Benzoyl Pyrazole Derivatives bearing a 6,8-Dibromo-2-methylquinazolin-4-one Moiety
Komal Savaliya1* and Pankaj S. Patel22Department of Chemistry, Sheth L.H. Science College, Mansa-382845, (Gujarat) India (Associate professer, Gujarat University, Ahmedabad), India
Komal Savaliya, Centre of Education, Indian Institute of Teacher Education, Gandhinagar, 382016, India, Email: komalsavaliya16@gmail.com
Received: 10-Jan-2022, Manuscript No. dpc-22-51453; Accepted Date: Jan 12, 2022 ; Editor assigned: 12-Jan-2022, Pre QC No. dpc-22-51453; Reviewed: 26-Jan-2022, QC No. dpc-22-51453; Revised: 31-Jan-2022, Manuscript No. dpc-22-51453; Published: 07-Feb-2022
Abstract
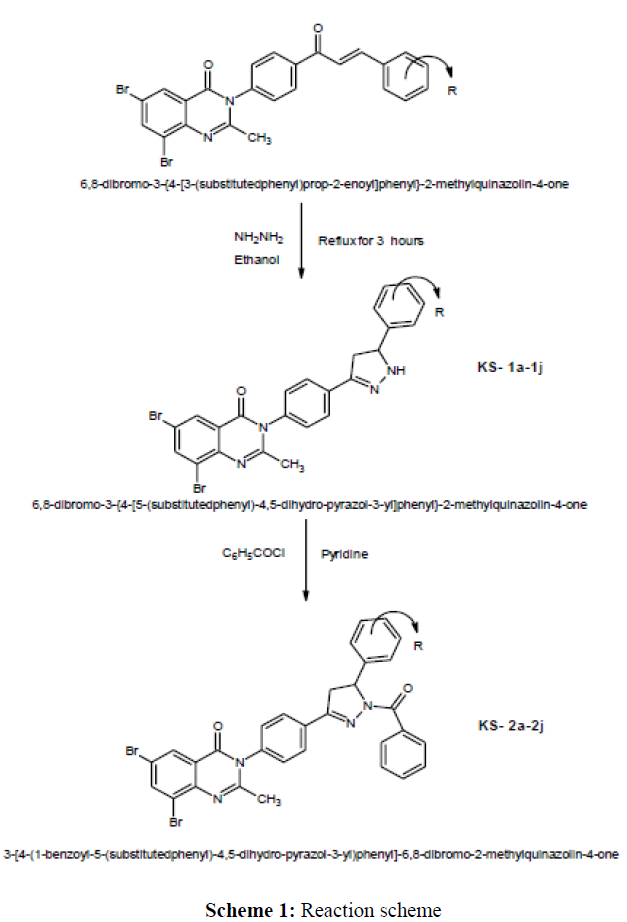
A new series of 3-[4-(1-benzoyl-5-(substitutedphenyl)-4,5-dihydro-pyrazol-3-yl)phenyl]-6,8-dibromo-2-methylquinazolin-4-one have been synthesized by the condensation reaction of 6,8-dibromo-3-{4-[5-(substitutedphenyl)-4,5-dihydro-pyrazol-3-yl]phenyl}-2-methylquinazolin-4-one with benzoyl chloride by using pyridine as a solvent. The intermediate have been synthesized by refluxation of 6,8-dibromo-3-{4-[3-(substitutedphenyl)prop-2-enoyl]phenyl}-2-methylquinazolin-4-one (0.01M) and 99% hydrazine hydrate (0.015M) by using ethanol (50ml) as a solvent with various aldehydes. The chemical structures of synthesized benzoyl pyrazoline derivatives were characterized by their IR, NMR and spectral data. The newly synthesized compounds were screened for their antibacterial and antifungal activities by Agar Cup method.
Keywords
Condensation; Benzoyl pyrazolines; Chalcone; antibacterial activity; Agar Cup method
Introduction
The heterocyclic compounds have a great importance in medicinal chemistry and play an important role in regulating biological process. One of the most important heterocycles in medicinal chemistry are quinazolines possessing wide spectrum of biological properties like Quinazolin-4(3H)-one are a class of fused hetero-cycles that are of considerable interest on account of the diverse range of their biological properties. Much attention has been paid to the synthesis of heterocyclic compounds bearing nitrogen containing ring system like pyrazoline mainly due to their higher pharmacological activity. Pyrazole is an unsaturated five-membered ring containing two adjacent nitrogen atoms as represented by the molecular formula C3H4N2. The noun pyrazole was given for the first time by Ludwig Knorr in 1883 [1]. The first natural pyrazole, 1-pyrazolyl-alanine was isolated from seeds of watermelons [2] (Figure 1).
Pyrazole and its derivatives have great interest in agrochemical, pharmaceutical, and chemical industries [3,4]. Pyrazolines are known to have anticancer [5], cytotoxic [6], antifungal [7], antitubercular [8], antioxidant [9]. Some pyrazolines are also reported to have anti-inflammatory, antidiabetic, anesthetic and analgesic properties [10-12]. The main skeletal of pyrazole and its related structures can be reported as important building blocks in organic synthetic for the design of a variety of biologically active compounds [13,14].
Materials and Methods
All reagents were of analytical reagent grade and were used without further purification. All the product were synthesized and characterized by their spectral analysis. All Melting points were determined by open capillary tube and are uncorrected. The IR spectra were recorded on Bruker Model; Alpha, Laser Class1, made in Germany and Brooker instrument was used for NMR Spectroscopy and (CH3)4Si (tetramethyl silane) used as internal standard and DMSO was used as a solvent. Purity of the compounds was checked by TLC on silica-G plates. Antimicrobial activities were tested by Agar Cup method. Standard drugs like Streptomycin and Fluconazole were used for the comparison purpose.
Result and Discussions
Preparation of 6, 8-dibromo-3-{4-[5-(substitutedphenyl)-4,5-dihydro-pyrazol-3-yl]phenyl}-2-methyl quinazolin-4-one (KS-1a-1j)
A mixture of 6,8-dibromo-3-{4-[3-(substitutedphenyl)prop-2-enoyl]phenyl}-2-methylquinazolin-4-one (0.01M) and 99% hydrazine hydrate (0.015M) in ethanol (50ml) refluxed gently for 3 hours. Then the mixture was concentrated and allowed to cool. The resulting solid was filtered, washed with ethanol and recrystallized from ethanol. 1HNMR (DMSO); (KS-1a): δ ppm 2.507, Singlet (3H) (-CH3), 3.368, Doublet(2H) (-CH2), 3.942 Triplet (1H) (-CH<), 7.377, Singlet (1H) (-NH), 7.277-8.340, Multiplet (10H) (Ar-H). 1HNMR (DMSO); (KS-1g): δ ppm 2.505, Singlet (3H) (-CH3), 3.355, Doublet(2H) (-CH2), 3.959 Triplet (1H) (-CH<), 7.379, Singlet (1H) (-NH), 7.379-8.411, Multiplet (10H) (Ar-H), 9.659, Singlet(1H) (-OH). IR(KBr); KS-1f (cm-1): 3379 (>NH-), 3269 (-OH), 3029 ( =C-H ), 2965 (-C-H Stretching), 1671 (>C=O Stretching), 1587 (>C=N stretching), 1503 (>C=C< Aromatic), 1442 (-CH2 bending) , 1402 (-CH3), 1304 (C-N), 1264 (N-N), 1169 (C-O-C) , 535 (C-Br). IR(KBr); KS-1i (cm-1): 3357 (>NH-), 3087 (=C-H ), 2906 (-C-H Stretching), 1662 (>C=O Stretching), 1587 (>C=N stretching), 1507 (>C=C< Aromatic), 1443 (-CH2 bending), 1420 (-CH3), 1294 (C-N), 1249 (N-N), 1168 (C-O-C), 548 (C-Br) (Scheme 1) (Tables 1and 2).
| % carbon | % Nitrogen | % Hydrogen | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sr No | Sub. No. | R | M.F. | Mol.Wt (g/m) |
Yield % |
M.P.C | Found | Calcd | Found | Calcd | Found | Calcd |
| 1 | KS-2a | -2-Cl | C31H21Br2ClN4O2 | 676.78 | 65 | 170 | 55.00 | 55.01 | 8.27 | 8.28 | 3.12 | 3.13 |
| 2 | KS-2b | -4-Cl | C31H21Br2ClN4O2 | 676.78 | 78 | 190 | 55.01 | 55.01 | 8.24 | 8.28 | 3.12 | 3.13 |
| 3 | KS-2c | -3,4-(OCH3)2 | C33H26Br2N4O4 | 702.39 | 58 | 136 | 56.41 | 56.43 | 7.97 | 7.98 | 3.72 | 3.73 |
| 4 | KS-2d | -H | C31H22Br2N4O2 | 642.33 | 69 | 158 | 57.95 | 57.96 | 8.72 | 8.72 | 3.43 | 3.45 |
| 5 | KS-2e | -2-OH | C31H22Br2N4O3 | 658.33 | 73 | 165 | 56.53 | 56.56 | 8.50 | 8.51 | 3.32 | 3.37 |
| 6 | KS-2f | -4-OH-3- OCH3 | C32H24Br2N4O4 | 688.36 | 78 | 190 | 55.81 | 55.83 | 8.14 | 8.14 | 3.51 | 3.51 |
| 7 | KS-2g | -4-OH | C31H22Br2N4O3 | 658.33 | 77 | 143 | 56.52 | 56.56 | 8.50 | 8.51 | 3.36 | 3.37 |
| 8 | KS-2h | -4-N(CH3)2 | C33H27Br2N5O2 | 685.40 | 72 | 205 | 57.83 | 57.83 | 10.21 | 10.22 | 3.97 | 3.97 |
| 9 | KS-2i | -4-OCH3 | C32H24Br2N4O3 | 672.36 | 68 | 165 | 57.12 | 57.16 | 8.32 | 8.33 | 3.6 | 3.6 |
| 10 | KS-2j | -3-NO2 | C31H21Br2N5O4 | 687.33 | 70 | 150 | 54.16 | 54.17 | 10.16 | 10.19 | 3.05 | 3.08 |
| SR No | COMP NO | R | Zone of inhibition in mm | |||
|---|---|---|---|---|---|---|
| Antibactrerial Activity | Antifungal Activity |
|||||
| S. aureus | E.coli | Aspergillue niger | Saccharomyces | |||
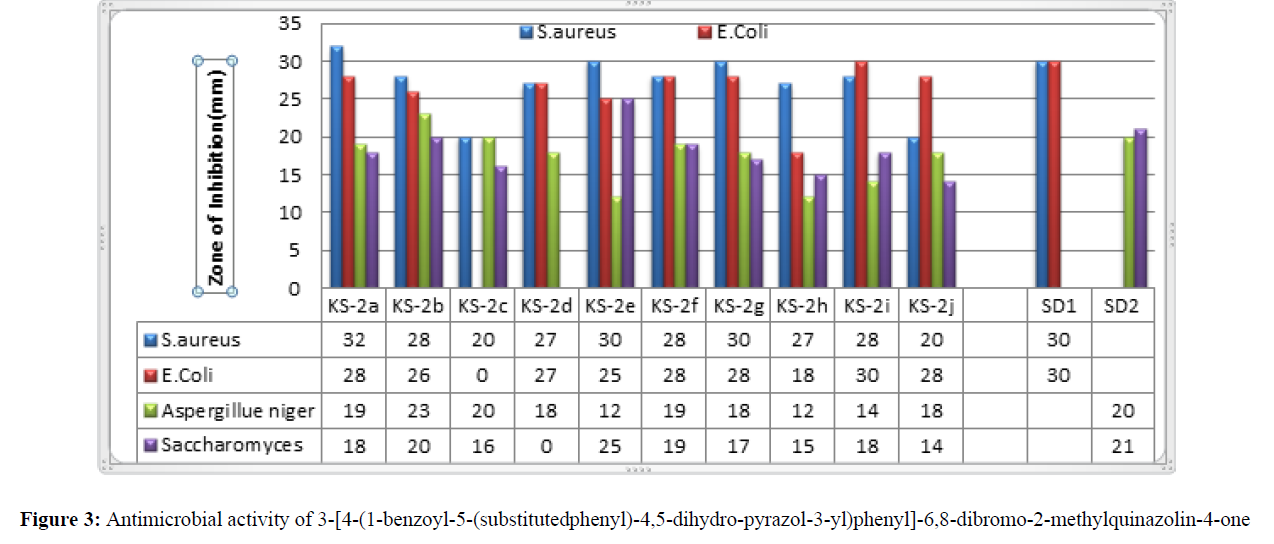
| 1 | KS-2a | 2-Cl | 32 | 28 | 19 | 18 |
| 2 | KS-2b | 4-Cl | 28 | 26 | 23 | 20 |
| 3 | KS-2c | -3,4-(OCH3)2 | 20 | NA | 20 | 16 |
| 4 | KS-2d | -H | 27 | 27 | 18 | NA |
| 5 | KS-2e | -2-OH | 30 | 25 | 12 | 25 |
| 6 | KS-2f | -4-OH-3-OCH3 | 28 | 28 | 19 | 19 |
| 7 | KS-2g | -4-OH | 30 | 28 | 18 | 17 |
| 8 | KS-2h | -4-N(CH3)2 | 27 | 18 | 12 | 15 |
| 9 | KS-2i | -4-OCH3 | 28 | 30 | 14 | 18 |
| 10 | KS-2j | -3-NO2 | 20 | 28 | 18 | 14 |
Preparation of 3-[4-(1-benzoyl-5-(substitutedphenyl)-4,5-dihydro-pyrazol-3-yl)phenyl]-6,8-dibromo-2-methylquinazolin-4-one (2a-2j)
A mixture of 6,8-dibromo-3-{4-[5-(2-substitutedphenyl)-4,5-dihydro-pyrazol-3-yl]phenyl}-2-methylquinazolin-4-one (0.001M) and benzoyl chloride (0.0011M) dissolved in dry pyridine (25ml) and stirred at room temperature for 1 hours, after which the reaction mixture treated with cold dilute HCl (2N). The resulting solid was filtered and washed successively with water, cold NaOH (2%) and water, and recrystallized from glacial acetic acid.
1HNMR (DMSO); (KS-2a): δ ppm 2.515, Singlet (3H) (-CH3), 3.388, Doublet(2H) (-CH2), 3.961, Triplet (1H) (-CH<), 7.492-7.971, Multiplet (15H) (Ar-H).
1HNMR (DMSO); (KS-2i): δ ppm 2.513, Singlet (3H) (-CH3), 3.352, Doublet(2H) (-CH2), 3.744, Singlet (3H) (-OCH3), 3.973, Triplet (1H) (-CH<), 7.491-7.969, Multiplet (15H).
IR(KBr); KS-2a (cm-1): 3044 (=C-H), 2969 (-C-H stretching), 1681 (>C=O stretching), 1580 (>C=N stretching), 1494 (>C=C< Aromatic), 1451 (-CH2 bending), 1419 (-CH3), 1323 (C-N), 1288 (N-N), 704 (C-Cl), 534 (C-Br). IR(KBr); KS-2c (cm-1): 3051 (=C-H), 2938 (-C-H stretching), 1666 (>C=O stretching), 1584 (>C=N stretching), 1510 (>C=C< Aromatic), 1445 (-CH2 bending), 1415 (-CH3), 1312 (C-N), 1257 (N-N), 1174(C-O-C), 545(C-Br) (Figure 2).
Anti bacterialactivity
A short review of results of anti-bacterial screening of the compounds of this section is mention here.
Against Staphylococcus aureus
Over all analysis of the screening result suggest KS-2a showed good anti-bacterial activity than the standard test-drug like Streptomycin also. Hence these compounds should be further tested under various conditions for their pharmaceutical applications. Minimum antibacterial activity was shown by the compounds 2c and 2j against S. aureus.
Against Escherichia Coli
From screening results, compound 2a, 2f, 2g and 2j were found to possess maximum antibacterial activity against Escherichia Coli. The minimum antifungal activity was shown by the compound KS-2h for E. Coli. 2c was found to be inactive against E. Coli.
Anti fungalactivity
A short review of results of anti-fungal screening of the compounds of this section is mention here.
Against Aspergillus Niger
Over all analysis of the screening result suggest that KS-2b showed good anti-fungal activity than the standard test-drug like Fluconazole also. Hence these compounds should be further tested under various conditions for their pharmaceutical applications. The minimum antifungal activity was shown by the compound KS-2e and 2h for Aspergillus Niger.
Against Saccharomyces
Over all analysis of the screening result suggest that KS-2e showed good anti-fungal activity than the standard test-drugs used for bio-assay. Hence these compounds should be further tested under various conditions for their pharmaceutical applications. 2d was found to be inactive against Saccharomyces. The minimum antifungal activity was shown by the compound KS-2j for Saccharomyces.
Conclusion
The Main objective of present research work was to synthesize, characterize and evaluate antimicrobial activities of the newly synthesized compounds with the help of analytical data such as 1H-NMR and IR. In conclusion, in present we prepared a series of 3-[4-(1-benzoyl-5-(substitutedphenyl)-4,5-dihydro-pyrazol-3-yl)phenyl]-6,8-dibromo-2-methylquinazolin-4-one. It is been observed that from the compounds tested, most of all were found to show good to moderate antibacterial and antifungal activity as compared to the standard drugs like Streptomycin and Fluconazole respectively.
Acknowledgements
We are grateful to the Principal Dr. Janakkumar R. Shukla and Management of Sheth L.H. Science College, Mansa for providing facilities for carrying out research work.
References
- Evelyn CS. Fec Publishing. 2012.
- [2] Eicher T and Hauptmann T. Wiley-VCH, Weinheim. 2003, p.7206.
- Keter KF and Darkwa. J Biometals. 2012, 25: p. 9-21.
- Fustero S, Sanchez-Rosello M, Barrio P et al., Chem Rev. 2011, 111: p. 6984-7034.
- Banjo Semire and Abel Kolawole Oyebamiji. Bulletin of Pharmaceutical Research. 2017, 7(3): p.1-9.
- Esvet Akbas, Fatih Caglar Celikezen, Hasan Turkez et al., Cogent Chem. 2017, 134411: p.1-10.
- Liu JJ, Zhao MY, Zhang X et al., Mini Rev Med Chem. 2013, 13: p.1957.
- Alagarsamy V, Solomon VR, Krishnamoorthy G et al., J Serb Chem Soc. 2015, 12: p.1471.
- Sharath V, Kumar HV and Naik N. J Pharm Res. 2013, 6: p.785-790
- Regaila HA, El-Bayanki AK and Hammad M. Egypt J Chem. 1979, p. 20.
- Krishna R B, Ranade R. Bhaithwal S P. and Parmar S, Eur. Med. J. Chem. Chimther; 1980, p.15567.
- Husain MI and Shukla S. Indian J. Chem. 1986, 25B: p. 983.
- Parmar NJ, Pansuriya BR, Labana BM et al., Eur J Org Chem. 2012, p. 5953-5964.
- Ramiz MM, Hafiz IS, Reheim MA et al., J Chin Chem Soc. 2012, 59: p.72-80.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref