Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 4
Synthesis and Biological Evaluation of Sulphonyl Derivatives of Naturally Occurring Chromone Alkaloid of Rohitukine as Anticancer Agents
Sunil K Mishra1,2, Pallavi Srivastava3, Rath SK3, Mahdi AA4, Agarwal SK4 and Lakshmi V1,4*
1Medicinal and Process Chemistry Division, Central Drug Research Institute, Lucknow-226031, India
2Toxicology Division, Central Drug Research Institute, Lucknow-226031, India
3Department of Bioengineering Faculty of Engineering Integral University Lucknow-226026, India
4Department of Biochemistry, King George’s Medical University, Lucknow-226003, India
- *Corresponding Author:
- Lakshmi V Medicinal and Process Chemistry Division
Central Drug Research Institute
Lucknow-226031, India
Abstract
Aim: The present study deals with isolation of active constituent rohitukine from stem bark of Dysoxylum binectariferum, and synthesis of a series of its new sulphonyl derivatives aiming to enhance its therapeutic efficacy.
Experimental: Rohitukine has been isolated to >95% purity and ten new semisynthetic analogs have been prepared using different sulphonyl chlorides. These derivatives were evaluated for anticancer activity against Breast Cancer Cell Line (MCF-7) (ER +ve) and MDA-MB-231 (ER -ve) breast cancer cell lines.
Major findings: Compounds K6, K8 and K10 showed significant activity against breast cancer cell lines at a concentration of 17.5 μM, 17 μM and 19 μM in MCF-7 and 20 μM, 19.8 μM and 28.5 μM in MDA-MB-231 respectively.
Conclusion: These compounds induced significant apoptosis in MCF-cell line. Further work may enhance the anticancer activity.
Keywords
Natural product, Anticancer activity, Dysoxylum binectariferum
Introduction
Cancer is a multifactorial disease with excessive and robust biological networks and is a major public health burden of present era. In addition to developed countries and developing countries are also being increasingly afflicted with cancer, due to increased life expectancy and advanced pattern of socio-cultural life dominated by western medicines which may lead to increased cancer risk [1]. It is thought to be caused by the interaction between genetic susceptibility and environmental factors. The characteristic feature of cancer cells include genetic instability, deregulation of multiple cellular signaling pathways, uncontrolled replication, avoidance of apoptosis, persistent angiogenesis, invasion of neighboring tissues and metastasis. Furthermore intratumoral heterogeneity caused due to genetic instability results in adaptive resistance, therefore the survival rate of cancer patients remains in many cases very limited [2]. Cancer can be treated to some extent using chemotherapeutic agents with specific targets for example, the cell cycle. This has led to a search for materials that have specific targets in cancerous cells that control the cell cycle. In fact compounds that target multiple intracellular components and distinct molecular mechanisms may be preferable and considered more promising.
Most of the anticancer drugs act through inducing apoptosis during mitotic arrest, otherwise after mitotic slippage following intrinsic pathway new which need stimulation of Mitochondrial Outer Membrane Permeabilization (MOMP) [3,4]. In this regard, there is considerable interest in the design and development of molecules with higher efficacy, selective anticancer activity and human body tolerability. Accumulating evidence has shown that natural products and their semi-synthetic derivatives remain one of the major sources of potential anti-cancer agents with nearly 50% of the new chemical entities launched in the market [5,6]. These are known to exert site-specific action on multiple cellular signaling pathways without causing undesired toxicity in normal cells. These non-toxic natural agents could be useful in combination with conventional chemotherapeutic agents for the treatment of human malignancies with lower toxicity and higher effectiveness. Nevertheless, proper characterization of naturally derived compounds particularly in view of cancer specific action without cytotoxic effects on normal cells is essential. Breast cancer is the most prevalent form of cancer and the second leading cause of deaths in women caused by cancer all over the world [7,8]. Tamoxifen an ER agonist acting through ER receptor is the most effective antibreast cancer molecule which act trough inhibiting estrogen action in ER +ve breast cancer [9,10]. But it is not effective against ER negative tumors. Therefore, there is an urgent need to develop novel agents with activity against estrogen dependent as well as estrogen independent breast cancer.
As part of our drug development programme from natural resources we aimed to explore and to develop new antibreast cancer molecules from indigenous medicinal plant Dysoxylum binectariferum Hook. f (Meliaceae). The stem bark of D. binectariferum is used as folk medicine for treatment of several diseases. Rohitukine a benzopyranones motif containing chromone alkaloid is the major active constituent of this plant, showing significant biological activities [11,12] benzopyranones are key structural motifs found in a large number of biologically active molecules from natural products [13,14] that form a family of active compounds with a wide range of biological activities including, antidiabetic, anti-inflammatory, antioesteogenic, antimicrobial, anti-allergic, antioxidant, antitumor and cytotoxic activities [15,16]. Rohitukine a benzopyranone ring containing chromone alkaloid is major active constituent of D. binectariferum. It has received significant attention for their medicinal activities; consequently efforts have been made to synthesize its semi-synthetic analogs. In present study we have investigated anticancer activity of new sulphonyl derivatives of rohitukine against MCF-7 (ER +ve) and MDA-MB-231 (ER -ve) breast cancer cell lines.
Results and Discussion
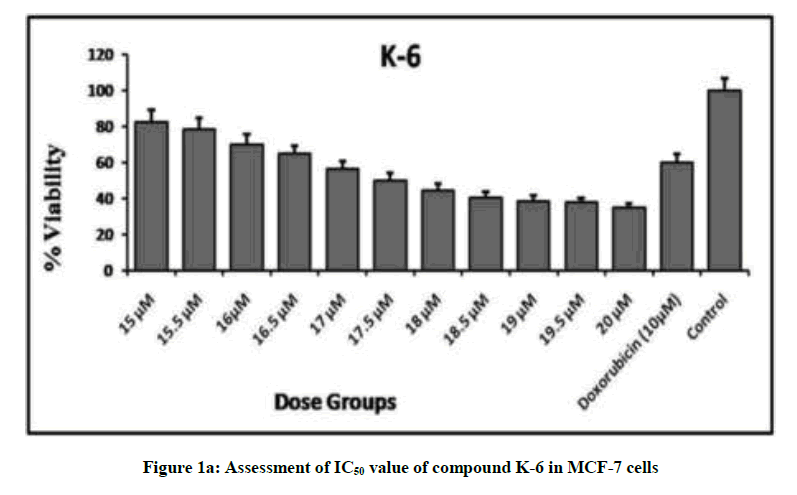
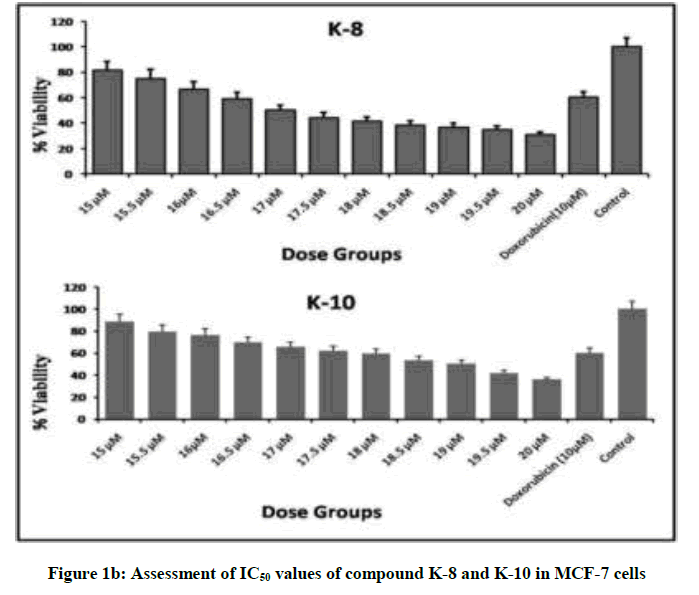
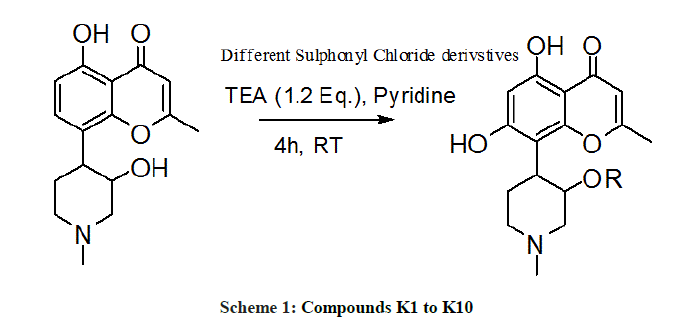
We embark to synthesize new semi synthetic derivatives of the lead molecule rohitukine in order to explore the anticancer effect of different substituted sulphonyl chlorides. Compounds K1-K10 were synthesized in moderate to good yield (Table 1) and evaluated for antiproliferative activity against MCF-7 and MDA-MB-231 cell lines alongside doxorubicin as standard drug. Compounds K-6, K-8 and K-10 showed promising antiproliferative activity showing minimum IC50 values against MCF-7. IC50 of K-6, K-8 and K-10 are 17.5, 17.0 and 19.0 μM respectively, these compounds also showed antiproliferative potential with IC50 values 20 μM, 19.8 μM and 28.5 μM respectively against MDA-MB-231. The cytotoxic activities for compounds K-6, K-8 and K-10 against MCF-7 have been presented in Figure 1a and 1b.
| Compound | R | Molecular formula | Molecular weight | Yield% |
|---|---|---|---|---|
| K-1 | 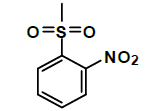 |
C22H22N2O9S | 490 | 81% |
| K-2 | 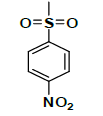 |
C22H22N2O9S | 490 | 72% |
| K-3 | 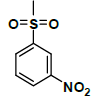 |
C22H22N2O9S | 490 | 76% |
| K-4 | 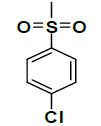 |
C22H22ClNO7S | 479 | 68% |
| K-5 | 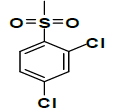 |
C22H21Cl2NO7S | 513 | 75% |
| K-6 | 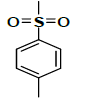 |
C23H25NO7S | 459 | 70% |
| K-7 | 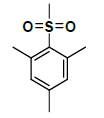 |
C25H29NO7S | 487 | 68% |
| K-8 | 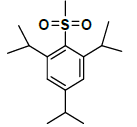 |
C31H41NO7S | 571 | 77% |
| K-9 | 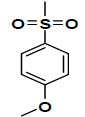 |
C23H25NO8S | 475 | 74% |
| K-10 | 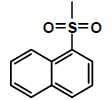 |
C26H25NO7S | 495 | 80% |
Table 1: Percentage yield of different sulphonyl derivatives of rohitukine
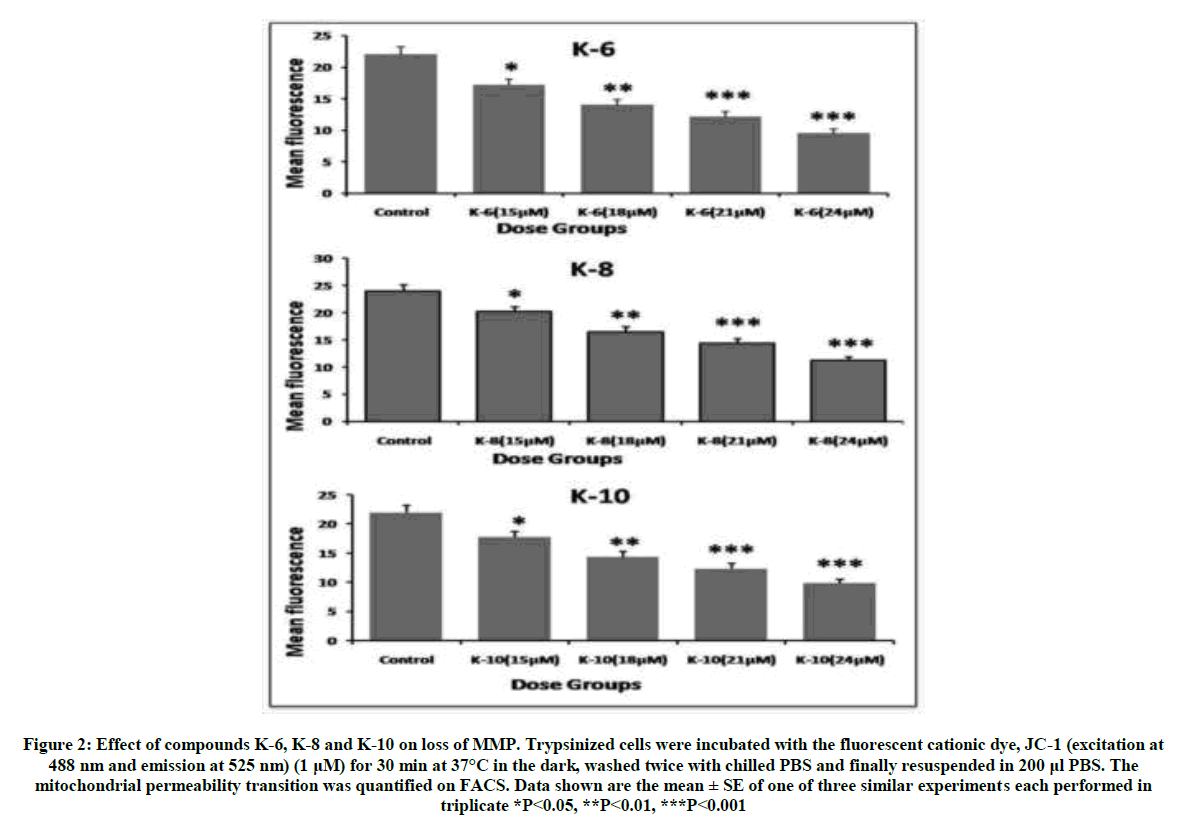
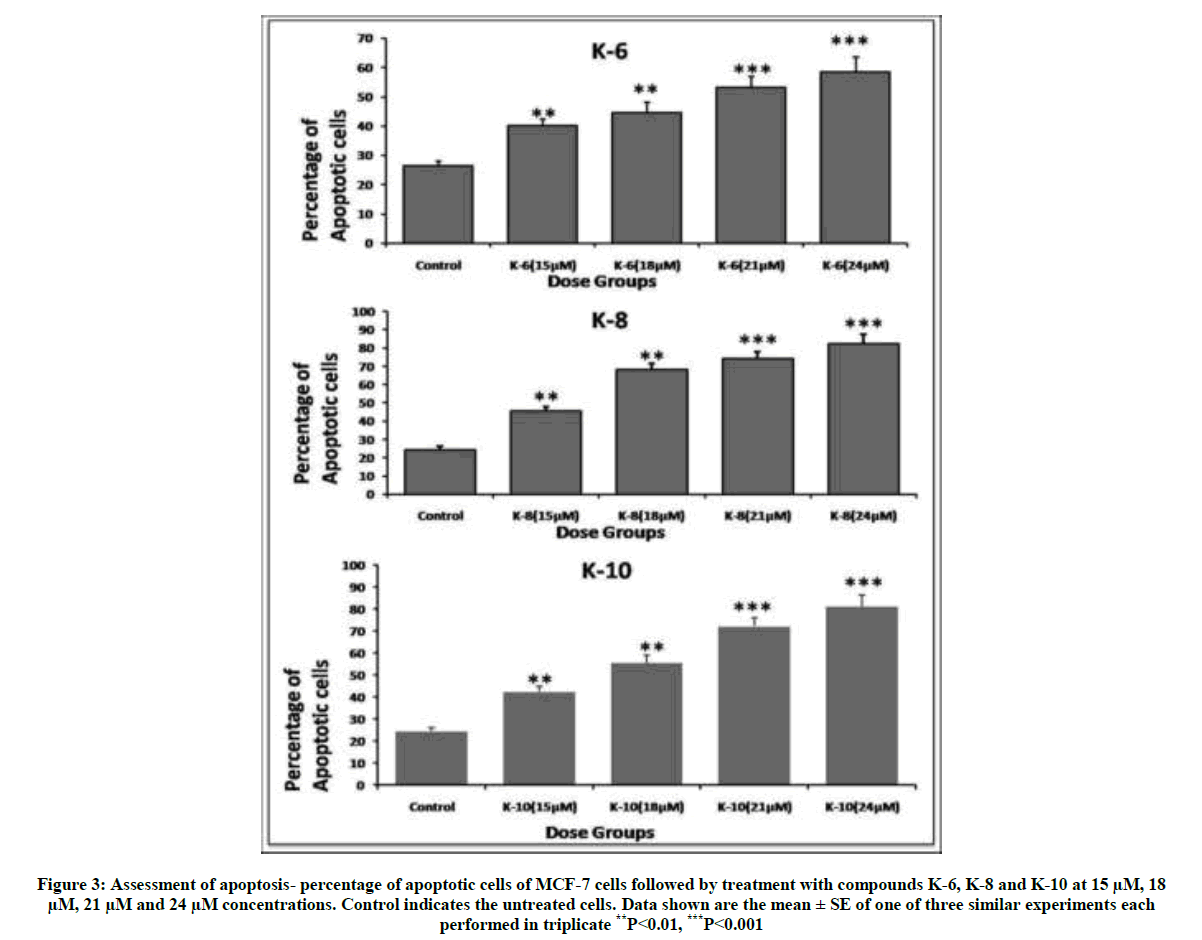
Thus these semi-synthetic analogs of Rohitukine have been found to be effective against both breast cancer cell lines and this is the rationale to further evaluate various parameters to determine the mechanism of action of these derivatives. The compounds K-6, K-8 and K-10 showed significant decrease in mitochondrial membrane potential in a dose dependent manner which is reflected by decrease in mean fluorescence with respect to control (Figure 2). Percentage of cells undergoing apoptosis was determined using flow cytometry. Treatment of MCF-7 cells with compounds K-6, K-8 and K-10 showed induction of apoptosis in a dose dependent manner (Figure 3). D. binectariferum stem bark as well as its major active constituent rohitukine possesses diverse biological activities. However, for the first time its Sulphonyl derivatives have been evaluated for anticancer activity, compounds K-6, K-8 and K-10 have shown significant results. It is shown to impart its anticancer property by induction of apoptosis.
Figure 2: Effect of compounds K-6, K-8 and K-10 on loss of MMP. Trypsinized cells were incubated with the fluorescent cationic dye, JC-1 (excitation at 488 nm and emission at 525 nm) (1 μM) for 30 min at 37°C in the dark, washed twice with chilled PBS and finally resuspended in 200 μl PBS. The mitochondrial permeability transition was quantified on FACS. Data shown are the mean ± SE of one of three similar experiments each performed in triplicate *P<0.05, **P<0.01, ***P<0.001
Figure 3: Assessment of apoptosis- percentage of apoptotic cells of MCF-7 cells followed by treatment with compounds K-6, K-8 and K-10 at 15 μM, 18 μM, 21 μM and 24 μM concentrations. Control indicates the untreated cells. Data shown are the mean ± SE of one of three similar experiments each performed in triplicate **P<0.01, ***P<0.001
Experimental Section
Plant material
Stem bark of D. binectariferum, was collected from Sindhuburg, Maharashtra, India, and identified by the Botany Division of Central Drug Research Institute, Lucknow, India. A voucher specimen number 4032 has been kept in the Herbarium of the Institute.
Extraction/Fractionation/Isolation procedure
Air dried ground stem bark (2.0 kg) were extracted with 95% aqueous ethanol (4 × 5.0 lit.) and the combined extracts were filtered and concentrated under reduced pressure below 50°C to minimum volume of 1.0 lit. It was further dried in hot air vacuum oven at 45°C to brown powder (Crude extract, yield 44.2 g). The brown powder (32 g) was fractionated in to chloroform soluble (10.2 g) and chloroform insoluble fractions by maceration with chloroform. The chloroform fraction on repeated column chromatography over silica gel and final purification by High Performance Liquid Chromatography (HPLC) on reverse phase C18 R.P columns using acetonitrile-water 55:45, v/v, flowrate-1.0 ml/min using UV detector (l 230 nm) yielded Rohitukine (1.2%) as major constituent, which was characterized using NMR, mass, derivatization and comparing the data with those reported in literature [12].
General procedure for the synthesis of compound K1-K10
Rohitukine was stirred in the pyridine triethylamine (6:2) solution at 40°C for 1 h. Substituted sulfonyl chlorides were added to this stirring solution and reaction was continued for 4 h. The reaction mixture was neutralized with 1 N HCl and extracted with DCM. The reaction mixture was washed with water (20 ml × 2), brine solution (20 ml × 2) and dried anhydrous Na2SO4. The reaction mixtures were evaporated in vacuo and residue were purified with column chromatography on SiO2 (100-200 mesh), affording compound K1 to K10 (Scheme 1). These sulfonyl derivatives were synthesized in moderate to good yield (Table 1).
K-1 [4-(5,7-dihydroxy-2-methyl-4-oxo-4H-chromen-8-yl)-1-methylpiperidin-3-yl-2-nitrobenzenesulfonate]: Compound K-1 was prepared from rohitukine and 2-nitrobenzene-1-sulfonylchloride using representative procedure to yield pure compound through elution with 80% chloroform in hexane. Yield: 81%; 1H-NMR: (300 MHz, CDCl3) δ: 12.95 (s, 1H), 8.0-7.50 (m, 4H), 6.50 (s, 1H), 6.28 (s, 1H), 4.96-4.90 (m, 1H), 3.06-2.99 (m, 1H), 2.89-2.81 (m, 3H), 2.55-251 (m, 1H), 2.31 (s, 3H), 2.25 (s, 3H), 2.00-192 (m, 1H), 1.47-1.43 (m, 1H); 13C-NMR (200 MHz, CDCl3) δ: 183.81, 166.26, 162.26, 161.86, 161.04, 151.14, 138.38, 137.15, 132.16, 130.60, 128.12, 111.32, 109.82, 106.76, 100.04, 68.02, 56.94, 55.16, 42.17, 27.10, 25.11, 20.78; ESI-MS: m/z 490 (M+1)+.
K-2 [4-(5,7-dihydroxy-2-methyl-4-oxo-4H-chromen-8-yl)-1-methylpiperidin-3-yl-4-nitrobenzenesulfonate]: Compound K-2 was prepared from rohitukine and 4-nitrobenzene-1-sulfonylchloride using representative procedure to afford pure compound through elution with 80% chloroform in hexane. Yield: 72%; 1H-NMR: (300 MHz, CDCl3) δ: 13.16 (s, 1H), 8.19 (d, J=9.2 Hz, 2H), 7.87 (d, J=9.2 Hz, 2H), 6.59 (s, 1H), 6.17 (s, 1H), 5.02-4.95 (m, 1H), 3.18-3.11 (m, 1H), 2.80-2.73 (m, 3H), 2.62-2.59 (m, 1H), 2.54 (s, 3H), 2.38 (s, 3H), 1.84-1.79 (m, 1H), 1.76- 1.72 (m, 1H); 13C-NMR (200 MHz, CDCl3) δ: 182.91, 166.29, 160.49, 160.16, 159.41, 155.14, 152.95, 132.15, 131.98, 125.14, 125.08, 111.25, 110.40, 106.04, 97.24, 67.11, 57.92, 57.22, 43.12, 26.34, 24.82, 20.22; ESI-MS: m/z 490 (M+1)+
K-3 [4-(5,7-dihydroxy-2-methyl-4-oxo-4H-chromen-8-yl)-1-methylpiperidin-3-yl-3-nitrobenzenesulfonate]: Compound K-3 was prepared from rohitukine and 4-nitrobenzene-1-sulfonylchloride using representative procedure to afford pure compound through elution with 90% chloroform in hexane. Yield: 76%; 1H-NMR: (300 MHz, CDCl3) δ: 12.90 (s, 1H), 8.48 (s, 1H), 8.12 (d, J=9 Hz, 1H), 7. 87 (d, J=9 Hz, 1H), 7.62-7.58 (m, 1H), 6.30 (s, 1H), 6.00 (s, 1H), 5.10-5.04 (m, 1H), 3.10-3.03 (m, 1H), 2.72-2.66 (m, 3H), 2.59-2.55 (m, 1H), 2.48 (s, 3H), 2.34 (s, 3H), 1.72-1.68 (m, 1H), 1.60-1.54 (m, 1H); 13C-NMR (200 MHz, CDCl3) δ: 184.51, 168.59, 163.24, 162.56,161.84, 152.14, 144.26, 138.82, 132.24, 132.12, 128.24, 112.08, 110.40, 107.11, 99.24, 67.38, 57.44, 56.82, 42.12, 26.84, 26.32, 22.38; ESI-MS: m/z 490 (M+1)+.
K-4 [4-(5,7-dihydroxy-2-methyl-4-oxo-4H-chromen-8-yl) -1-methylpiperidin-3-yl 4-chlorobenzenesulfonate]: Compound K-4 was prepared from rohitukine and 4-chlorobenzene-1-sulfonylchloride using representative procedure to afford pure compound through elution with 80% chloroform in hexane. Yield: 68%; 1H-NMR: (300 MHz, CDCl3) 12.85 (s, 1H), 7.58 (d, J=7.8 Hz, 2H), 7.47 (d, J=7.8 Hz, 2H), 6.44 (s, 1H), 6.25 (s, 1H), 4.89-4.82 (m, 1H), 2.94-2.87 (m, 1H), 2.68-2-64 (m, 3H), 2.62-2.58 (m, 1H), 2.30 (s, 3H), 2.26 (s, 3H), 2.00-192 (m, 1H), 1.78-1.74 (m, 1H); 13C-NMR: (200 MHz, CDCl3) δ: 183.64, 165.96, 162.10, 161.65, 161.02, 150.24, 139.18, 133.25, 132.95, 130.60, 130.24, 110.94, 109.67, 106.24, 99.74, 67.34, 57.98, 56.38, 43.10, 26.10, 25.85, 20.78; ESI-MS: m/z 479 (M+1)+.
K-5 [4-(5,7-dihydroxy-2-methyl-4-oxo-4H-chromen-8-yl)-1-methylpiperidin-3-yl 2,4-dichlorobenzenesulfonate]: Compound K-5 was prepared from rohitukine and 2,4-dichlorobenzene-1-sulfonylchloride using representative procedure to afford pure compound at 90% chloroform in hexane as eluent. Yield: 75%; 1H-NMR (300 MHz, CDCl3) δ: 13.02 (s, 1H), 8.04 (s, 1H), 7.85 (d, J=9 Hz, 1H), 7.78 (d, J=9 Hz, 1H), 6.28 (s, 1H), 6.14 (s, 1H), 5.02-4.97 (m, 1H), 3.18-3.11 (m, 1H) 2.68-2-59 (m, 3H), 2.57-2.54 (m, 1H), 2.41 (s, 3H), 2.34 (s, 3H), 1.87-1.70 (m, 2H), 13C-NMR (200 MHz, CDCl3) δ: 183.42, 167.29, 162.4, 161.6, 158.41, 140.62, 138.23, 135.4, 134.34, 131.19, 129.24,112.78, 110.65, 107.26, 98.84, 68.57, 57.92, 56.24, 43.47, 27.3, 25.12, 19.98; ESI-MS: m/z 513 (M+1)+.
K-6 [4-(5,7-dihydroxy-2-methyl-4-oxo-4H-chromen-8-yl)-1-methylpiperidin-3-yl-4-methylbenzenesulfonate]: Compound K-6 was prepared from rohitukine and 4-methylbenzene-1-sulfonylchloride using representative procedure to afford pure compound at 80% chloroform in hexane as eluent. Yield: 70%; 1H-NMR (300 MHz, CDCl3) δ: 12.90 (s, 1H), 7.73 (d, J=6 Hz, 2H), 7.44 (d, J=6 Hz, 2H), 6.33 (s, 1H), 6.02 (s, 1H), 4.98-4.92 (m, 1H), 3.10-3.06 (m, 1H), 2.96-2.89 (m, 3H), 2.41 (s, 3H), 2.35 (s, 3H), 2.30 (s, 3H), 2.25-2.21 (m, 1H), 1.84-171 (m, 2H); 13CNMR (200 MHz, CDCl3) δ: 182.94, 166.40, 162.92, 161.46, 158.62, 145.24, 142.25, 131.15, 130.98, 130.24, 130.04, 112.02, 110.90, 107.08, 99.04, 68.10, 58.96, 57.92, 43.12, 25.23, 24.84 22.56, 20.12; ESI-MS: m/z 459 (M+1)+.
K-7 [4-(5,7-dihydroxy-2-methyl-4-oxo-4H-chromen-8-yl)-1-methylpiperidin-3-yl 2,4,6-trimethylbenzenesulfonate]: Compound K-7 was prepared from rohitukine and 2,4,6-trimethylbenzene-1-sulfonyl chloride using representative procedure to afford pure compound at 90% chloroform in hexane as eluent. Yield: 68%; mp: 1H-NMR (300 MHz, CDCl3) δ: 13.06, 7.10 (s, 2H), 6.29 (s, 1H), 6.02 (s, 1H), 4.96-4.91 (m, 1H), 3.16-3.10 (m, 1H), 2.81-2.76 (m, 3H), 2.56 (s, 6H), 2.46-2.42 (m, 1H), 2.40 (s, 3H), 2.35 (s, 3H), 2.29 (s, 3H), 1.90-1.82 (m, 2H), 13C-NMR (200 MHz, CDCl3) δ:184.12, 166.72, 163.24, 163.12, 161.24, 142.12, 138.84, 137.23, 137.19, 130.42, 130.40, 112.78, 110.65, 105.82, 96.48, 67.95, 63.22, 5812, 56.82, 44.38, 24.42, 23.24, 23.21, 22.12, 19.44; ESI-MS: m/z 487 (M+1)+.
K-8 [4-(5,7-dihydroxy-2-methyl-4-oxo-4H-chromen-8-yl)-1-methylpiperidin-3-yl-2,4,6-triisopropylbenzenesulfonate]: Compound K-8 was prepared from rohitukine and 2,4,6-triisopropylbenzene-1-sulfonyl chloride using representative procedure to afford pure compound at 80% chloroform in hexane as eluent. Yield: 77%; 1H-NMR (300 MHz, CDCl3) δ: 12.98 (s, 1H), 7.60 (s, 2H), 6.20 (s, 1H), 6.04 (s, 1H), 5.02-4.96 (m, 1H), 3.21-3.14 (m, 2H) 2.82-2.64 (m, 6H), 2.36 (s, 3H), 2.29 (s, 3H), 1.84-1.75 (m, 2H) 1.30 (s, 6H), 1.27 (s, 6H), 1.24 (s, 6H); 13C-NMR (200 MHz, CDCl3) δ: 184.12, 167.82, 162.44, 162.16, 160.10, 156.12, 156.08, 154.15, 126.24, 124.82, 124.77, 111.78, 110.65, 106.12, 99.48, 67.15, 59.42, 57.22, 43.12, 36.46, 36.44, 35.82, 26.12, 25.90, 24.80, 24.77, 24.42, 24.40, 24.04, 24.02, 21.24; ESI-MS: m/z 571 (M+1)+.
K-9 [4-(5,7-dihydroxy-2-methyl-4-oxo-4H-chromen-8-yl)-1-methylpiperidin-3-yl-4-methoxy benzenesulfonate]: Compound K-9 was prepared from rohitukine and 4-methoxybenzene-1-sulfonyl chloride using representative procedure to afford pure compound at 80% chloroform in hexane as eluent. Yield: 74%; 1H-NMR (300 MHz, CDCl3) δ: 12.98 (s, 1H), 7.42 (d, J=8.52 Hz, 2H), 6.96 (d, J=8.52 Hz, 2H), 6.23 (s, 1H), 6.05 (s, 1H), 4.97-4.91 (m, 1H), 3.37 (s, 3H), 3.18-3.10 (m, 1H), 2.74-2.66 (m, 3H), 2.41 (s, 3H), 2.35 (s, 3H), 2.29-2.25 (m, 1H), 2.02-184 (m, 2H); 13C-NMR (200 MHz, CDCl3) δ: 185.22, 168.48, 165.94, 160.59, 160.16,159.12, 141.67, 135.12, 135.12, 117.28, 117.28, 112.04, 110.02, 106.12, 99.14, 67.17, 58.74, 56.34, 55.40, 43.87, 25.74, 25.24 21.28; ESI-MS: m/z 475 (M+1)+.
K-10 [4-(5,7-dihydroxy-2-methyl-4-oxo-4H-chromen-8-yl)-1-methylpiperidin-3-yl-naphthalene-1-sulfonate]: Compound K-10 was prepared from rohitukine and 4 naphthalene-1-sulfonyl chloride through representative procedure to afford pure compound at 90% chloroform in hexane as eluent. Yield: 80%; 1H-NMR (300 MHz, CDCl3) δ: 12.90 (s, 1H), 8.70 (d, J=8.49, 1H), 8.13 (d, J=7.71, 1H), 8. 11 (d, J=6.96, 1H), 7. 94 (d, J=8.01, 1H) 7.71, (t, J=7.92), 7.63-7.49 (m, 2H) 6.10 (s), 5.98 (s), 4.95-4.90 (m, 1H), 3.07-3.01 (m, 1H), 2.74-2.68 (m, 3H), 2.57-2.52 (m, 1H), 2.32 (s, 3H), 2.26 (s, 3H) 2.12-1.96 (m, 2H); 13C-NMR (200 MHz, CDCl3) δ: 183.22, 167.35, 163.23, 163.02, 160.46, 144.74, 135.91, 132.82, 130.80, 130.14, 128.82, 128.71, 126.90, 126.74, 125.51, 111.72, 110.10, 106.86, 98.94,69.06, 58.22, 55.12, 42.32, 25.83, 25.24, 20.54; ESI-MS: m/z 495 (M+1)+.
Cell viability assay
MCF-7 and MDA-MB-231 cell lines were used for screening of anti- breast cancer activity. Cell viability was evaluated by the trypan blue exclusion method. Cells treated with or without the test compounds were harvested by trypsinization. Cells were incubated in 0.04% trypan blue (Sigma-Aldrich) for 4 min and counted under a Hemocytometer. The total number of cells and number of cells that retained the dye (Nonviable cells) were calculated.
Antiproliferative activity assay
The antiproliferative effect was determined through 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) assay by Botta [17]. 1 × 104 cells/well were seeded in 96-well micro culture plates in 100 μl Dulbecco's Modified Eagle Medium (DMEM) media supplemented with 10% FBS in each well and incubated in a CO2 incubator for 24 h at 37°C. All the compounds were dissolved in Dimethyl Sulfoxide (DMSO) to prepare 10 mM stock solutions and subsequently diluted to the desired concentrations in the culture medium in the wells with respect to the vehicle control. After 48 h of incubation, media were removed and 100 μl MTT (5 mg/ml) was added to each well and plates were further incubated for 4 h. Supernatant was removed carefully from each well, 100 μl of DMSO was added to each well to dissolve formazon crystals and the absorbance was recorded at 570 nm using microtiter reader (Bio-Tek, USA).
Analysis of Mitochondrial Membrane Potential (MMP)
The MMP was measured by the uptake of unique fluorescent cationic dye, JC-1 (Excitation at 488 nm and emission at 525 nm), to signal the loss of MMP [18]. This fluorescent probe exists as a green fluorescent monomer (Emission 527 nm) at low MMP. Mitochondrial depolarization is indicated by an increase in green fluorescence (FL-1). The MCF-7 cells (0.2 × 106 cells) were seeded in a 6-well plate and exposed to compounds at varying concentrations for 36 h. After that the cells were washed and finally harvested in chilled PBS containing JC-1 (1 μM). The samples were incubated at 37°C for 30 min in the dark, washed twice with chilled Phosphate-buffered Saline (PBS) and finally resuspended in 200 μl PBS. Mitochondrial permeability transition was subsequently quantified on Fluorescence Activated Cell Sorter (FACS).
Assessment of apoptosis by flow cytometry
The percentage of cells undergoing apoptosis was determined using Annexin V FITC Assay kit (BD Biosciences). Flurochrome-labeled Annexin V is used for the detection of exposed Phosphatidylserine (PS) and Propidium Iodide (PI) for the differentiation of necrotic cells using flow cytometry. The MCF-7 cells were cultured and treated with different doses of compounds for 24 h. At the end of this time period cells were washed twice with PBS, collected in 1X binding buffer and centrifuged at 1200 rpm for 5 min. Afterwards cell pellet was treated with 5 μl Annexin V and 5 μl of PI, incubated for 15 min. in dark. Finally 200 μl of binding buffer was added to the cell pellet and analyzed by flow cytometry (BD Biosciences).
Statistical analysis
Data were analyzed by one-way Analysis of Variance (ANOVA-F) with Newman-Keüls post analysis test using Graph Pad Prism version 5.00 and P<0.05 was considered statistically significant.
Conclusion
The synthesis of these derivatives from rohitukine have serve their purpose to an extent and taking lead from them, other derivatives with better potential as well as novel mechanism of action can be derived to combat cancer.
Acknowledgements
Authors are thankful to the Director, CDRI, Lucknow, India for constant encouragement for the drug developing program on anticancer agents and Council of Scientific and Industrial Research for senior research fellowship. We are grateful to the Sophisticated analytical instrument Facility (CDRI) for spectral data.
References
- D.M. Parkin, Lancet Oncol., 2001, 2, 533-543.
- T. Hochhauser, J. Tobias, Cancer and Its Management, 6th Edi., Wiley-Blackwell, Oxford, UK, 2009.
- W. Tao, V.J. South, Y. Zhang, J.P. Davide, L. Farrell, N.E. Kohl, L. Sepp-Lorenzino, R.B. Lobell, Cancer Cell., 2005, 8(1), 49-55.
- S.J. Park, C.H. Wu, J.D. Gordon, X. Zhong, A. Emami, A.R. Safam, J. Biol. Chem., 2004, 279(49), 51057-51067.
- A.D. Kinghorn, Drug discovery from natural products. In T.L. Lemke, D.A. Williams, editors. Foye’s Principles of Medicinal Chemistry, 6th Edi., Philadelphia, PA, USA: Wolters Kluwer/Williams & Wilkins, 2008, 12-25.
- G.M. Cragg, D.G.I. Kingston, D.J. Newman, Editors, Anticancer Agents from Natural Products, Boca Raton, FL, USA, CRC Press Taylor & Francis, 2005.
- D.M. Parkin, P. Pisani, J. Ferlay, Cancer J. Clin., 1999, 49, 33-64.
- F. Labrie, C. Labrie, A. Belanger, J. Simard, S. Gauthier, V. Luu-The, Y. Mérand, V. Giguere, B. Candas, S. Luo, C. Martel, S.M. Singh, M. Fournier, A. Coquet, V. Richard, R. Charbonneau, G. Charpenet, A. Tremblay, G. Tremblay, L. Cusan, R.J. Veilleux, Steroid Biochem. Mol. Biol., 1999, 69, 51-84.
- L.J. Lerner, V.C. Jordan, Cancer Res., 1990, 50, 4177-4189.
- R.B. Lawrence, L.C. Hartmann, N. Engl. J. Med., 2003, 348, 618-629.
- G. Keshri, R.M. Oberoia, V. Lakshmi, K. Pandey, M.M. Singh, Contraception., 2007, 76, 400-407.
- R.J. Naik, S.L. Kattige, S.V. Bhat, B. Alreja, N.J. DeSouza, R.H. Rupp, Tetrahedron., 1988, 44, 2081-2086.
- E.E. Schweizer, D. Meeder-Nycz, In Heterocyclic Compounds: Chromenes; Ellis, G.P., Ed.; Wiley: New York, 2000, 11-139.
- C. Menut, J.M. Bessiere, H. Ntalani, P. Verin, A.T. Henriques, R. Limberger, Phytochemistry., 2000, 53, 975-979.
- B. Havsteen, Biochem. Pharmacol.,1983,32, 1141-1148.
- E. Middleton, K. Chithan, The impact of plant flavonoids on mammalian biology: implications for immunity, inflammation and cancer. In The Flavonoids: Advances in research since 1986, Harborne J.B. (Ed.), Chapman and Hall, London, 1993, 619-652.
- M. Botta, S. Armaroli, D. Castagnolo, G. Fontana, P. Perad, E. Bombardelli, Bioorg. Med. Chem. Lett.,2007,17, 1579-1583.
- A. Srivastava, M. Tiwari, R.A. Sinha, J. Biol. Chem., 2006, 19762-19771.