Research - Der Pharma Chemica ( 2021) Volume 13, Issue 1
Synthesis and Characterization of Novel Mono Carbonyl Curcumin Analogues of Pyrazole Derivatives
Maddineni A Kumari1*, Chunduri V Rao2 and Begari N Raju22Department of Chemistry, Sri Venkateswara University, Tirupati 517 502, Andhra Pradesh, India
Maddineni A Kumari, Department of Chemistry, IIIT Ongole, RGUKT, Andhra Pradesh, India, Email: maddineniaruna84@gmail.com
Received: 25-Feb-2020 Accepted Date: Jan 22, 2021 ; Published: 28-Jan-2021
Abstract
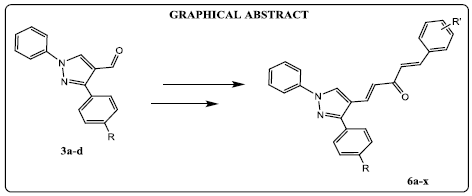
(E)-4-aryl-1-phenyl-1H-pyrazol-4-yl)but-3-en-2-one derivatives were (4a-d) synthesized by the condensation of 3-aryl-1-phenyl-1H-pyrazole-4- carbaldehyde derivatives (3a-d) with acetone in the presence of sodium hydroxide. Compounds (4a-d) on condensation with different aldehydes give mono carbonyl curcumin analogues (MACs) of pyrazole derivatives (6a-x) in good yield.
Keywords
Mono-carbonyl analogs of curcumin, Synthesis, Pyrazoles, Chalcone, Acetone
Introduction
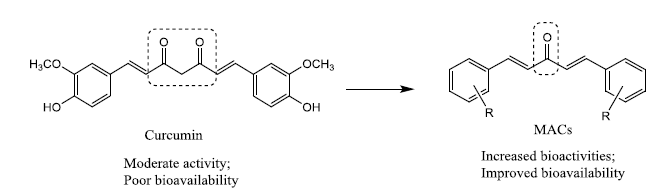
Curcumin, 5-hydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,4,6-trien-3-one (diferuloylmethane), is a secondary metabolite of the well-known Indian spice turmeric, derived from the rhizomes of Curcuma longa, of the Zingiberaceae family. Curcumin is a polyphenol, and it is the main component of the yellow-pigmented fraction of turmeric [1]. To date, no studies in either animals or humans have discovered toxicity associated with the use of curcumin, even when tested at high doses [2].
Curcumin and its derivatives owns a broad spectrum of therapeutic activities viz., antibacterial [3], antifungal [4], antiviral [5], anti-HIV [6], anti-inflammatory [7], anti-Parkinson’s [8], anti-Alzheimer’s [9], anti-angiogenesis [10], free radical scavenging activity [11], antirheumatic [12], antimalarial [13], anticancer [14], antiprotozoan [15], antimutagenic [16], wound treatment [17], hepatoprotective activity [18], anti-leishmanial activity [19] and amyloid heart disease [20]. Among the active curcumin analogs, mono-carbonyl analogs of curcumin (MACs) are important which were designed by deleting the reactive β-diketone moiety. This class of analogs has received much attention due to their improved chemical stability and pharmacokinetic profiles.
Materials and Methods
All the reactions were monitored by thin layer chromatography (TLC) on precoated silica gel 60 F254 (mesh); spots were visualized with UV light. Merck silica gel (60–120 mesh) was used for column chromatography. 1H NMR (400 MHz), and 13C NMR (100 MHz) spectra were recorded on a Bruker AMX 400 MHz NMR spectrometer in CDCl3/DMSO solution using TMS as an internal standard. All chemical shifts are reported in δ (ppm) using TMS as an internal standard. Elemental analysis was determined using a Vario Microcube Elemental Analyzer. Melting points were determined in open capillaries on a Mel Temp apparatus and are uncorrected.
General procedure for the synthesis of acetophenone phenyl hydrazones (2a-d)
A mixture of appropriate ketone (1a-e) (1 mmol) and phenyl hydrazine (1 mmol) in ethanol was refluxed in the presence of few drops of glacial acetic acid for 2 h. The progress of reaction was monitored by TLC using n-hexane: ethyl acetate (7:3) as a mobile phase. The mixture was cooled and the solid product obtained was filtered, washed with water and recrystallized from ethanol.
Z)-1-(1-(4-methoxyphenyl)ethylidene)-2-phenylhydrazine (2a): White solid; Yield: 89%; mp: 125-127 °C; 1H NMR (400 MHz, DMSO-d6): δ = 2.23 (s, 3H), 4.12 (s, 3H), 7.25-7.34 (m, 5H), 7.54 (s, 1H), 7.68 (d, J = 8.72 Hz, 2H), 7.75 (d, J = 8.75 Hz, 2H); 13C NMR (100 MHz, DMSO-d6): δ = 26.9, 114.8, 122.5, 123.7, 125.8, 129.4, 137.5, 145.3, 147.8, 160.2; LCMS (positive ion mode) (m/z): 241.4 [M+H]+ for C15H16N2O.
(Z)-1-(1-(4-chlorophenyl)ethylidene)-2-phenylhydrazine (2b): White solid; Yield: 92%; mp: 120-122 °C; 1H NMR (400 MHz, DMSO-d6): δ = 2.25 (s, 3H), 7.14-7.26 (m, 5H), 7.65 (s, 1H), 7.69 (d, J = 8.56 Hz, 2H), 7.84 (d, J = 8.57 Hz, 2H); 13C NMR (100 MHz, DMSO-d6): δ = 26.3, 113.2, 120.6, 123.5, 124.2, 129.5, 136.5, 144.6, 145.7, 160.1; LCMS (positive ion mode) (m/z): 245.2 [M+H]+ for C14H13ClN2.
(Z)-1-phenyl-2-(1-phenylethylidene)hydrazine (2c): White solid; Yield: 88%; mp: 117-119 °C; 1H NMR (400 MHz, DMSO-d6): δ= 2.25 (s, 3H), 7.02 (t, J = 7.50 Hz, 1H), 7.32-7.35 (m, 4H), 7.42-7.45 (m, 1H), 7.45 (s, 1H), 7.56 (d, J = 8.76 Hz, 2H), 7.73 (d, J = 8.74 Hz, 2H); 13C NMR (100 MHz, DMSO-d6): δ= 25.4, 112.6, 120.5, 122.9, 124.5, 129.8, 136.7, 145.3, 146.4, 160.2; LCMS (positive ion mode) (m/z): 211.3 [M+H]+ for C14H14N2.
(Z)-1-(1-(4-fluorophenyl)ethylidene)-2-phenylhydrazine (2d): White solid; Yield: 91%; mp: 118-120 °C; 1H NMR (400 MHz, DMSO-d6): δ= 2.27 (s, 3H), 7.15 (d, J = 7.56 Hz, 2H), 7.25-7.32 (m, 3H), 7.67 (s, 1H), 7.75 (d, J = 8.46 Hz, 2H), 7.83 (d, J = 8.47 Hz, 2H); 13C NMR (100 MHz, DMSO-d6): δ = 27.3, 115.7, 121.4, 123.9, 125.3, 129.7, 137.2, 145.2, 146.5, 160.5; LCMS (positive ion mode) (m/z): 229.3 [M+H]+ for C14H13FN2.
General procedure for the synthesis of 3-aryl-1-phenyl-1H-pyrazole-4-carbaldehyde (3a-d)
To an ice cold dimethyl formamide (10 ml), POCl3 (3 mmol) was added drop wise with continuous stirring over a period of 30 min. Stirring was continued for further 60 min, keeping the reaction temperature at 0°C. Acetophenone phenyl hydrazone derivative (2a-d) (1 mmol) was then added and the reaction mixture was allowed to attain room temperature. The mixture was then refluxed for 5 h, allowed to cool and poured onto ice. The mixture was neutralized with saturated sodium bicarbonate solution. The solid product obtained was filtered, washed with water and recrystallized from methanol. The completion of reaction was monitored by TLC using n-hexane: ethyl acetate (7:3) as a mobile phase.
3-(4-methoxyphenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde (3a): Off-white solid; Yield: 74%; mp: 136-138°C; 1H NMR (400 MHz, DMSO-d6): δ = 3.87 (s, 3H), 7.03 (d, J = 8.69 Hz, 2H), 7.38 (d, J = 7.39 Hz, 1H), 7.51 (t, J = 7.85 Hz, 2H), 7.78-7.80 (m, 4H), 8.52 (s, 1H), 10.04 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ= 55.9, 114.8, 120.3, 122.9, 124.4, 128.4, 130.2, 130.8, 131.8, 139.6, 155.1, 161.1, 185.7; LCMS (positive ion mode) (m/z): 279.15 [M+H]+, 301.10 [M+Na]+ for C31H23ClN6O.
3-(4-chlorophenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde (3b): Off-white solid; Yield: 75%; mp: 128-130°C; 1H NMR (400 MHz, DMSO-d6): δ= 7.13 (d, J = 8.69 Hz, 2H), 7.57 (d, J = 7.85 Hz, 2H), 7.91-7.94 (m, 5H), 8.45 (s, 1H), 10.07 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ= 116.8, 121.5, 123.2, 124.8, 127.4, 129.5, 130.8, 131.7, 138.6, 156.7, 160.6, 185.2; LCMS (positive ion mode) (m/z): 283.10 [M+H]+ for C16H11ClN2O.
1,3-diphenyl-1H-pyrazole-4-carbaldehyde (3c): Off-white solid; Yield: 82%; mp: 129-131°C; 1H NMR (400 MHz, DMSO-d6): δ = 7.14-7.19 (m, 3H), 7.36 (d, J = 7.92 Hz, 2H), 7.53-7.58 (m, 5H), 8.63 (s, 1H), 10.19 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ = 114.8, 121.5, 122.5, 123.5, 129.8, 130.1, 130.3, 131.9, 136.6, 158.7, 161.1, 185.1; LCMS (positive ion mode) (m/z): 249.05 [M+H]+ for C16H12N2O.
3-(4-fluorophenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde (3d): Off-white solid; Yield: 79%; mp: 134-136°C; 1H NMR (400 MHz, DMSO-d6): δ= 7.15 (d, J = 8.69 Hz, 2H), 7.63 (d, J = 7.85 Hz, 2H), 7.81-7.85 (m, 5H), 8.57 (s, 1H), 10.12 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ= 115.9, 120.5, 123.9, 124.4, 127.4, 129.5, 130.8, 131.7, 138.6, 156.7, 160.6, 185.2; LCMS (positive ion mode) (m/z): 267.10 [M+H]+ for C16H11FN2O.
General procedure for the synthesis of (E)-4-aryl-1-phenyl-1H-pyrazol-4-yl)but-3-en-2-one (4a-d):
To a stirred solution of pyrazole derivatives (3a-d) (1 mmol) in ethanol (3 mL) was added 0.5 mL of acetone and 15% aqueous NaOH (1 mL) solution at 0°C. The reaction was allowed to stir at room temperature till it was completed. The reaction was quenched by the addition of water and the formed precipitate was filtered and dried. Further, it was purified by column chromatography (Silica gel, 60-120 mesh, 9:1 hexane/ethyl acetate) to obtain pure chalcone(4a-d).
(E)-4-(3-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)but-3-en-2-one (4a): Yellow solid; Yield: 75%; mp: 143-145 °C; 1H NMR (400 MHz, DMSO-d6): δ= 2.31 (s, 3H), 3.87 (s, 3H), 6.56 (d, J = 16.4 Hz, 1H), 7.03 (d, J = 8.8 Hz, 2H), 7.33 (t, J = 7.6 Hz, 1H), 7.48 (t, J = 7.6 Hz, 2H), 7.53 (s, 1H), 7.57-7.61 (m, 2H), 7.76 (d, J = 8.4 Hz, 2H), 8.24 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ= 27.2, 55.4, 114.3, 117.4, 119.3, 124.6, 126.3, 126.6, 127.2, 129.4, 129.9, 134.3, 139.4, 153.5, 160.1, 198.2. Anal.calcd for C20H18N2O2: C, 75.45%; H, 5.70%; N, 8.80%. Found: C, 75.63%; H, 5.41%; N, 8.59%.
(E)-4-(1,3-diphenyl-1H-pyrazol-4-yl)but-3-en-2-one (4b):Yellow solid; Yield: 78%; mp: 142-144 °C; 1H NMR (400 MHz, DMSO-d6): δ= 2.27 (s, 3H), 6.58 (d, J = 16.4 Hz, 1H), 7.05 (d, J = 8.8 Hz, 2H), 7.38 (t, J = 7.6 Hz, 1H), 7.50 (t, J = 7.6 Hz, 2H), 7.54 (s, 1H), 7.58-7.63 (m, 3H), 7.78 (d, J = 8.4 Hz, 2H), 8.23 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ = 27.5, 114.9, 117.5, 119.6, 125.2, 126.3, 126.8, 128.3, 129.6, 131.2, 133.4, 138.4, 153.2, 159.2, 198.0. Anal.calcd for C19H16N2O: C, 79.14%; H, 5.59%; N, 9.72%. Found: C, 79.43%; H, 5.41%; N, 9.89%.
(E)-4-(3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)but-3-en-2-one (4c): Off-white solid; Yield: 77%; mp: 146-148 °C; 1H NMR (400 MHz, DMSO-d6): δ= 2.25 (s, 3H), 6.50 (d, J = 16 Hz, 1H), 7.29 (t, J = 7.2 Hz, 1H), 7.39-7.46 (m, 5H), 7.54 (d, J = 8.4 Hz, 2H), 7.68 (d, J = 8.0 Hz, 2H), 8.18 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ= 27.4, 117.6, 118.9, 119.4, 126.5, 127.0, 127.5, 129.0, 129.6, 130.7, 133.5, 134.8, 139.2, 152.4, 198.0. Anal.calcd for C19H15ClN2O: C, 70.70%; H, 4.68%; N, 8.68%. Found: C, 70.50%; H, 4.92%; N, 8.34%.
(E)-4-(3-(4-fluorophenyl)-1-phenyl-1H-pyrazol-4-yl)but-3-en-2-one (4d): Off-white solid; Yield: 75%; mp: 145-147 °C; 1H NMR (400 MHz, DMSO-d6): δ = 2.27 (s, 3H), 6.56 (d, J = 16 Hz, 1H), 7.31 (t, J = 7.2 Hz, 1H), 7.42-7.47 (m, 5H), 7.62 (d, J = 8.4 Hz, 2H), 7.68 (d, J = 8.0 Hz, 2H), 8.20 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ= 27.6, 118.2, 118.9, 120.5, 125.6, 127.2, 127.9, 129.2, 129.6, 131.2, 133.8, 135.4, 141.2, 152.6, 198.5. Anal.calcd for C19H15FN2O: C, 74.50%; H, 4.94%; N, 9.14%. Found: C, 74.63%; H, 4.61%; N, 9.09%.
General procedure for the synthesis of compounds (6a-x)
To a stirred solution of chalcone (4a-d) (0.5 mmol) in ethanol (5 mL) was added 15% aqueous NaOH (2 mL) solution and appropriate aldehyde (5a-f) (0.5 mmol) at 0 °C. The resulting solution was stirred at room temperature till the completion of the reaction. The reaction mixture was quenched by the addition of water and the formed precipitate was filtered and dried. Further, it was purified by column chromatography (Silica gel, 60-120 mesh, 9:1 hexane/ethyl acetate) to obtain pure compounds (6a-x).
(1E,4E)-1-(3,4-dimethoxyphenyl)-5-(3-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)penta-1,4-dien-3-one (6a): Off-white solid; Yield: 72%; mp: 176-178 °C; 1H NMR (400 MHz, DMSO-d6): δ = 3.98-4.00 (m, 9H), 6.96 (d, J = 15.89 Hz, 1H), 7.03 (d, J = 15.77 Hz, 1H), 7.34 (s, 1H), 7.45 (t, J = 7.34 Hz, 1H), 7.56-7.61 (m, 5H), 7.67 (d, J = 15.89 Hz, 1H), 7.74 (d, J = 8.19 Hz, 2H), 7.82-7.87 (m, 4H), 8.40 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ= 57.0, 61.8, 106.3, 118.9, 120.2, 125.7, 125.9, 127.6, 128.2, 129.8, 130.4, 130.8, 131.0, 131.6, 134.1, 135.6, 137.8, 139.6, 140.1, 141.2, 144.0, 153.3, 154.3, 189.0. Anal.calcd for C29H26N2O4: C, 74.66%; H, 5.62%; N, 6.00%. Found: C,74.74%; H, 5.82%; N, 6.12%.
(1E,4E)-1-(3-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)-5-(3,4,5-trimethoxyphenyl)penta-1,4-dien-3-one (6b): Light yellow solid; Yield: 74%; mp: 175-177 °C; 1H NMR (400 MHz, DMSO-d6): δ= 3.87 (s, 3H), 3.90-3.91 (m, 9H), 6.81 (s, 2H), 6.89 (d, J = 15.89 Hz, 1H), 6.94 (d, J = 15.89 Hz, 1H), 7.03 (d, J = 8.56 Hz, 2H), 7.35 (t, J = 7.34 Hz, 1H), 7.59 (d, J = 15.77 Hz, 2H), 7.64 (d, J = 8.56 Hz, 2H), 7.76-7.82 (m, 4H), 8.31 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ= 55.3, 56.1, 61.0, 105.4, 114.2, 118.0, 119.3, 124.5, 124.8, 125.1, 126.5, 127.2, 129.5, 130.0, 130.3, 134.0, 139.4, 140.2, 142.9, 153.4, 153.6, 160.1, 188.4. Anal.calcd for C30H28N2O5: C, 72.56; H, 5.68%; N, 5.64%. Found: C, 72.64%; H, 5.72%; N, 5.81%.
(1E,4E)-1-(4-(dimethylamino)phenyl)-5-(3-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)penta-1,4-dien-3-one (6c):Pale yellow solid; Yield: 74%; mp: 175-176 °C; 1H NMR (400 MHz, DMSO-d6): δ= 3.03 (s, 6H), 3.88 (s, 3H), 6.68 (d, J = 8.31 Hz, 2H), 6.80 (d, J = 15.53 Hz, 1H), 6.92 (d, J = 15.65 Hz, 1H), 7.03 (d, J = 7.95 Hz, 2H), 7.33 (t, J = 7.17 Hz, 1H), 7.47-7.49 (m, 4H), 7.64-7.68 (m, 3H), 7.73-7.79 (m, 3H), 8.28 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ= 40.1, 55.3, 111.8, 114.2, 118.3, 119.2, 120.9, 122.6, 125.0, 125.4, 126.3, 127.0, 129.5, 130.0, 130.2, 132.6, 139.5, 143.9, 151.9, 153.4, 160.0, 188.5. Anal.calcd for C29H27N3O2: C, 77.48%; H, 6.05%; N, 9.35%. Found: C, 77.21%; H, 6.32%; N, 9.47%.
(1E,4E)-1-(3-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)-5-(4-nitrophenyl)penta-1,4-dien-3-one (6d):Yellow solid; Yield: 75%; mp: 179-180 °C; 1H NMR (400 MHz, DMSO-d6): δ= 3.92 (s, 3H), 6.92 (d, J = 15.89 Hz, 1H), 6.99 (d, J = 15.89 Hz, 1H), 7.12 (d, J = 8.34 Hz, 2H), 7.35-7.39 (m, 3H), 7.49-7.53 (m, 4H), 7.61-7.67 (m, 4H), 7.77-7.82 (m, 2H), 8.32 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ= 55.3, 114.3, 117.4, 119.3, 124.2, 124.6, 126.3, 126.6, 127.2, 128.8, 128.9, 129.5, 129.6, 129.9, 130.1, 134.3, 139.4, 139.6, 153.5, 160.1, 187.8. Anal.calcd for C27H21N3O4: C, 71.83%; H, 4.69%; N, 9.31%. Found: C, 71.95%; H, 4.79%; N, 9.13%.
(1E,4E)-1-(4-chlorophenyl)-5-(3-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)penta-1,4-dien-3-one (6e): Light yellow solid; Yield: 72%; mp: 175-177 °C; 1H NMR (400 MHz, DMSO-d6): δ= 3.88 (s, 3H), 6.89 (d, J = 15.89 Hz, 1H), 6.95 (d, J = 15.89 Hz, 1H), 7.04 (d, J = 8.07 Hz, 2H), 7.33-7.38 (m, 3H), 7.47-7.52 (m, 4H), 7.60-7.65 (m, 3H), 7.77-7.81 (m, 3H), 8.29 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ= 55.4, 114.3, 117.9, 119.3, 124.7, 125.9, 126.6, 127.2, 129.2, 129.4, 129.5, 129.9, 130.0, 133.4, 134.4, 136.2, 139.4, 141.4, 153.6, 160.1, 188.3. Anal.calcd for C27H21ClN2O2: C, 73.55%; H, 4.80%; N, 6.35%. Found: C, 73.62%; H, 5.01%; N, 6.49%.
(1E,4E)-1-(4-fluorophenyl)-5-(3-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)penta-1,4-dien-3-one (6f): Light yellow solid; Yield: 74%; mp: 177-179 °C; 1H NMR (400 MHz, DMSO-d6): δ = 3.97 (s, 3H), 6.98 (d, J = 15.89 Hz, 1H), 7.04 (d, J = 15.89 Hz, 1H), 7.13 (d, J = 8.07 Hz, 2H), 7.42-7.47 (m, 3H), 7.56-7.60 (m, 4H), 7.69-7.74 (m, 3H), 7.86-7.90 (m, 3H), 8.38 (s, 1H);13C NMR (100 MHz, DMSO-d6): δ= 55.4, 114.4, 118.0, 119.4, 124.8, 125.9, 126.7, 127.3, 129.3, 129.5, 129.6, 130.0, 130.1, 133.4, 134.4, 136.3, 139.5, 141.4, 153.7, 160.2, 188.4. Anal.calcd for C27H21FN2O2: C, 76.40%; H, 4.99%; N, 6.60%. Found: C, 76.23%; H, 5.14%; N, 6.52%.
(1E,4E)-1-(3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-(3,4-dimethoxyphenyl)penta-1,4-dien-3-one (6g):Yellow solid; Yield: 72%; mp: 177-179 °C; 1H NMR (400 MHz, DMSO-d6): δ= 3.93 (s, 6H), 6.85 (d, J = 15.77 Hz, 1H), 6.93 (d, J = 16.13 Hz, 1H), 7.11-7.26 (m, 3H), 7.37 (t, J = 7.45 Hz, 1H), 7.47-7.53 (m, 4H), 7.61-7.67 (m, 3H), 7.72-7.79 (m, 3H), 8.31 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ= 55.9, 109.9, 111.2, 118.2, 119.4, 123.0, 123.8, 125.2, 126.7, 127.4, 127.7, 129.0, 129.6, 130.0, 130.9, 132.9, 134.8, 139.3, 143.2, 149.3, 151.4, 152.4, 188.3. Anal.calcd for C28H23ClN2O3: C, 71.41%; H, 4.92%; N, 5.95%. Found: C, 71.63%; H, 4.71%; N, 6.09%.
(1E,4E)-1-(3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-(3,4,5-trimethoxyphenyl)penta-1,4-dien-3-one (6h): Yellow solid; Yield: 76%; mp: 179-180 °C; 1H NMR (400 MHz, DMSO-d6): δ = 3.90-3.92 (m, 9H), 6.82 (s, 2H), 6.88 (d, J = 15.77 Hz, 1H), 6.95 (d, J = 15.77 Hz, 1H), 7.37 (t, J = 7.34 Hz, 1H), 7.48-7.53 (m, 5H), 7.59 (d, J = 15.89 Hz, 1H), 7.66 (d, J = 8.19 Hz, 2H), 7.77-7.79 (m, 2H), 8.32 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ= 56.2, 61.0, 105.5, 118.1, 119.4, 124.9, 125.1, 126.8, 127.4, 129.0, 129.6, 130.0, 130.2, 130.8, 133.3, 134.8, 139.3, 140.4, 143.2, 152.5, 153.4, 188.2. Anal.calcd for C29H25ClN2O4: C, 69.53%; H, 5.03%; N, 5.59%. Found: C, 69.73%; H, 5.19%; N, 6.05%.
(1E,4E)-1-(3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-(4-(dimethylamino)phenyl)penta-1,4-dien-3-one (6i):Orange solid; Yield: 69%; mp: 179-181 °C; 1H NMR (400 MHz, DMSO-d6): δ= 3.03 (s, 6H), 6.69 (d, J = 8.68 Hz, 2H), 6.79 (d, J = 15.77 Hz, 1H), 6.93 (d, J = 15.77 Hz, 1H), 7.47-7.52 (m, 7H), 7.67 (d, J = 8.44 Hz, 2H), 7.71 (d, J = 15.89 Hz, 2H), 7.78 (d, J = 8.19 Hz, 2H), 8.30 (s,1H); 13C NMR (100 MHz, DMSO-d6): δ= 40.1, 111.8, 118.4, 119.3, 120.7, 122.4, 125.9, 126.6, 127.3, 129.0, 129.6, 130.0, 130.1, 130.3, 130.9, 131.9, 144.2, 152.0, 152.3, 157.7, 188.4. Anal.calcd for C28H24ClN3O: C, 74.08%; H, 5.33%; N, 9.26%. Found: C, 74.21%; H, 5.52%; N, 9.35%.
(1E,4E)-1-(3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-(4-nitrophenyl)penta-1,4-dien-3-one (6j):Yellow solid; Yield: 71%; mp: 175-177 °C; 1H NMR (400 MHz, DMSO-d6): δ= 6.77 (d, J = 8.68 Hz, 2H), 6.88 (d, J = 15.89 Hz, 1H), 7.01 (d, J = 15.87 Hz, 1H), 7.44 (t, J = 7.45 Hz, 2H), 7.55-7.58 (m, 5H), 7.72-7.76 (m, 3H), 7.81-7.87 (m, 3H), 8.38 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ= 114.3, 117.4, 119.4, 124.2, 124.7, 126.4, 126.7, 127.2, 127.4, 128.8, 129.5, 129.6, 130.0, 130.1, 134.4, 139.4, 139.7, 153.5, 160.1, 187.8. Anal.calcd for C26H18ClN3O3: C, 68.50%; H, 3.98%; N, 9.22%. Found: C, 68.62%; H, 4.03%; N, 9.42%.
(1E,4E)-1-(4-chlorophenyl)-5-(3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)penta-1,4-dien-3-one (6k): Off-white solid; Yield: 73%; mp: 176-178 °C; 1H NMR (400 MHz, DMSO-d6): δ= 6.98 (d, J = 15.89 Hz, 2H), 7.18 (t, J = 8.31 Hz, 2H), 7.45 (t, J = 7.33 Hz, 1H), 7.56-7.60 (m, 4H), 7.64-7.67 (m, 2H), 7.70-7.74 (m, 3H), 7.81-7.87 (m, 3H), 8.39 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ= 116.1, 116.3, 118.2, 119.5, 125.3, 126.9, 127.5, 129.1, 129.7, 130.0, 130.1, 130.3, 130.4, 130.9, 131.1, 133.5, 134.9, 139.4, 141.9, 188.3. Anal.calcd for C26H18Cl2N2O: C, 70.12%; H, 4.07%; N, 6.29%. Found: C, 70.35%; H, 4.21%; N, 6.35%.
(1E,4E)-1-(3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-(4-fluorophenyl)penta-1,4-dien-3-one (6l):Off-white solid; Yield: 71%; mp: 178-180 °C; 1H NMR (400 MHz, DMSO-d6): δ= 6.90 (d, J = 15.89 Hz, 2H), 7.10 (t, J = 8.31 Hz, 2H), 7.37 (t, J = 7.33 Hz, 1H), 7.47-7.51 (m, 4H), 7.56-7.59 (m, 2H), 7.63-7.67 (m, 3H), 7.73-7.79 (m, 3H), 8.31 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ = 116.0, 116.2, 118.1, 119.4, 125.2, 126.8, 127.4, 129.0, 129.6, 129.9, 130.0, 130.2, 130.3, 130.8, 131.0, 133.4, 134.8, 139.3, 141.8, 188.2.Anal.calcd for C26H18ClFN2O: C, 72.81%; H, 4.23%; N, 6.53%. Found: C, 72.99%; H, 4.35%; N, 6.67%.
(1E,4E)-1-(3,4-dimethoxyphenyl)-5-(1,3-diphenyl-1H-pyrazol-4-yl)penta-1,4-dien-3-one (6m):Off-white solid; Yield: 71%; mp: 177-179 °C; 1H NMR (400 MHz, DMSO-d6): δ= 3.93 (s, 6H), 6.86 (d, J = 14.91 Hz, 1H), 6.94 (d, J = 15.89 Hz, 1H), 7.11-7.26 (m, 3H), 7.35 (t, J = 7.33 Hz, 1H), 7.43-7.52 (m, 6H), 7.62 (d, J = 15.89 Hz, 1H), 7.72 (d, J = 7.70 Hz, 2H), 7.78-7.81 (m, 3H), 8.32 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ= 55.9, 109.9, 111.1, 118.2, 119.3, 123.0, 123.7, 125.0, 126.5, 127.2, 127.8, 128.6, 128.7, 128.8, 129.5, 132.4, 133.4, 139.4, 143.0, 149.3, 151.4, 153.7, 188.5. Anal.calcd for C28H24N2O3: C, 77.04%; H, 5.54%; N, 6.42%. Found: C, 77.25%; H, 5.31%; N, 6.62%.
(1E,4E)-1-(1,3-diphenyl-1H-pyrazol-4-yl)-5-(3,4,5-trimethoxyphenyl)penta-1,4-dien-3-one (6n): Off-white solid; Yield: 74%; mp: 178-180 °C; 1H NMR (400 MHz, DMSO-d6): δ= 3.99-4.01 (m, 9H), 6.91 (s, 2H), 6.97 (d, J = 15.89 Hz, 1H), 7.04 (d, J = 15.89 Hz, 1H), 7.46 (t, J = 7.34Hz, 1H), 7.57-7.62 (m, 5H), 7.68 (d, J = 15.89 Hz, 1H), 7.75 (d, J = 8.19 Hz, 2H), 7.83-7.88 (m, 3H), 8.41 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ= 56.0, 60.9, 105.3, 118.0, 119.2, 124.7, 125.0, 126.6, 127.3, 128.8, 129.5, 129.9, 130.1, 130.7, 133.2, 134.6, 139.1, 140.2, 143.0, 152.4, 153.3, 188.1. Anal.calcd for C29H26N2O4: C, 74.66%; H, 5.62%; N, 6.00%. Found: C, 74.52%; H, 5.75%; N, 6.13%.
(1E,4E)-1-(4-(dimethylamino)phenyl)-5-(1,3-diphenyl-1H-pyrazol-4-yl)penta-1,4-dien-3-one (6o): Orange solid; Yield: 68%; mp: 175-176 °C; 1H NMR (400 MHz, DMSO-d6): δ= 3.03 (s, 6H), 6.67 (d, J = 8.80 Hz, 2H), 6.79 (d, J = 15.77 Hz, 1H), 6.93 (d, J = 15.77 Hz, 1H), 7.34 (t, J = 7.45 Hz, 1H), 7.47-7.51 (m, 6H), 7.65 (d, J = 15.77 Hz, 1H), 7.71-7.74 (m, 3H), 7.76-7.80 (m, 3H), 8.31 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ= 40.1, 111.8, 118.4, 119.3, 119.4, 120.8, 122.5, 125.6, 126.5, 127.1, 128.6, 128.7, 128.8, 129.5, 130.2, 132.4, 139.5, 144.0, 151.9, 153.6, 188.6.Anal.calcd for C28H25N3O: C, 80.16%; H, 6.01%; N, 10.02%. Found: C, 80.37%; H, 6.19%; N, 10.20%.
(1E,4E)-1-(1,3-diphenyl-1H-pyrazol-4-yl)-5-(4-nitrophenyl)penta-1,4-dien-3-one (6p):Yellow solid; Yield: 73%; mp: 179-180 °C; 1H NMR (400 MHz, DMSO-d6): δ= 6.72 (d, J = 8.76 Hz, 2H), 6.72 (d, J = 15.77 Hz, 1H), 6.98 (d, J = 15.77 Hz, 1H), 7.28 (t, J = 7.42 Hz, 1H), 7.45-7.49 (m, 6H), 7.62 (d, J = 15.77 Hz, 1H), 7.75-7.78 (m, 3H), 7.81-7.85 (m, 3H), 8.34 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ= 110.9, 118.7, 119.6, 119.9, 120.4, 121.7, 124.7, 126.8, 127.7, 128.2, 128.4, 129.1, 129.5, 130.0, 132.8, 139.3, 144.9, 151.5, 154.7, 188.3.Anal.calcd for C26H19N3O3: C, 74.10%; H, 4.54%; N, 9.97%. Found: C, 74.27%; H, 4.62%; N, 10.01%.
(1E,4E)-1-(4-chlorophenyl)-5-(1,3-diphenyl-1H-pyrazol-4-yl)penta-1,4-dien-3-one (6q): Off-white solid; Yield: 70%; mp: 177-178 °C; 1H NMR (400 MHz, DMSO-d6): δ= 6.69 (d, J = 8.76 Hz, 2H), 6.71 (d, J = 15.77 Hz, 1H), 6.95 (d, J = 15.77 Hz, 1H), 7.24 (t, J = 7.42 Hz, 2H), 7.42-7.47 (m, 5H), 7.60 (d, J = 15.77 Hz, 1H), 7.73-7.76 (m, 2H), 7.81-7.85 (m, 4H), 8.36 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ= 110.5, 117.9, 119.2, 119.7, 120.2, 121.6, 123.5, 125.8, 127.3, 127.8, 128.8, 129.3, 129.8, 131.3, 132.5, 138.1, 143.6, 149.5, 153.4, 188.7.Anal.calcd for C26H19ClN2O: C, 76.00%; H, 4.66%; N, 6.82%. Found: C, 76.20%; H, 4.80%; N, 6.95%.
(1E,4E)-1-(1,3-diphenyl-1H-pyrazol-4-yl)-5-(4-fluorophenyl)penta-1,4-dien-3-one (6r):Off-white solid; Yield: 70%; mp: 172-174 °C; 1H NMR (400 MHz, DMSO-d6): δ= 6.72 (d, J = 8.76 Hz, 2H), 6.77 (d, J = 15.77 Hz, 1H), 6.98 (d, J = 15.77 Hz, 1H), 7.29 (t, J = 7.42 Hz, 1H), 7.48-7.53 (m, 6H), 7.63 (d, J = 15.77 Hz, 1H), 7.75-7.78 (m, 2H), 7.91-7.95 (m, 4H), 8.38 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ= 111.5, 118.5, 118.9, 119.4, 120.1, 122.4, 123.2, 124.9, 126.9, 128.1, 128.9, 129.6, 129.9, 130.7, 133.5, 138.6, 145.8, 148.5, 152.7, 188.5.Anal.calcd for C26H19FN2O: C, 79.17%; H, 4.86%; N, 7.10%. Found: C, 79.35%; H, 4.97%; N, 7.35%.
(1E,4E)-1-(3,4-dimethoxyphenyl)-5-(3-(4-fluorophenyl)-1-phenyl-1H-pyrazol-4-yl)penta-1,4-dien-3-one (6s):Off-white solid; Yield: 73%; mp: 177-178 °C; 1H NMR (400 MHz, DMSO-d6): δ = 3.95 (s, 6H), 6.87 (d, J = 15.77 Hz, 1H), 7.03 (d, J = 15.77 Hz, 1H), 7.15-7.21 (m, 4H), 7.39 (t, J = 7.47 Hz, 1H), 7.52-7.56 (m, 3H), 7.63-7.66 (m, 3H), 7.72-7.76 (m, 3H), 8.32 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ= 56.2, 110.9, 111.8, 117.1, 120.4, 123.3, 123.8, 124.9, 126.5, 127.4, 128.3, 129.3, 129.7, 130.2, 131.3, 132.7, 135.4, 139.8, 144.2, 149.6, 151.3, 152.9, 188.7. Anal.calcd for C28H23FN2O3: C, 73.99%; H, 5.10%; N, 6.16%. Found: C, 74.02%; H, 5.15%; N, 6.28%.
(1E,4E)-1-(3-(4-fluorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-(3,4,5-trimethoxyphenyl)penta-1,4-dien-3-one (6t): Off-white solid; Yield: 75%; mp: 178-179 °C; 1H NMR (400 MHz, DMSO-d6): δ = 3.98-4.03 (m, 9H), 6.82 (s, 2H), 6.91 (d, J = 15.77 Hz, 1H), 6.97 (d, J = 15.77 Hz, 1H), 7.25 (t, J = 7.84 Hz, 1H), 7.51-7.55 (m, 5H), 7.61 (d, J = 15.89 Hz, 1H), 7.69 (d, J = 8.19 Hz, 2H), 7.79-7.83 (m, 2H), 8.37 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ = 56.4, 61.5, 106.7, 117.6, 120.4, 124.4, 125.3, 126.4, 128.4, 129.2, 129.6, 130.2, 130.8, 131.8, 133.3, 135.7, 138.9, 141.4, 144.7, 151.5, 154.5, 188.8. Anal.calcd for C29H25FN2O4: C, 71.89%; H, 5.20%; N, 5.78%. Found: C, 72.01%; H, 5.41%; N, 5.96%.
(1E,4E)-1-(4-(dimethylamino)phenyl)-5-(3-(4-fluorophenyl)-1-phenyl-1H-pyrazol-4-yl)penta-1,4-dien-3-one (6u): Orange solid; Yield: 68%; mp: 179-180 °C; 1H NMR (400 MHz, DMSO-d6): δ= 3.08 (s, 6H), 6.65 (d, J = 8.74 Hz, 2H), 6.81 (d, J = 15.77 Hz, 1H), 6.95 (d, J = 15.77 Hz, 1H), 7.51-7.54 (m, 7H), 7.68 (d, J = 8.43 Hz, 2H), 7.75 (d, J = 15.89 Hz, 2H), 7.82 (d, J = 8.19 Hz, 2H), 8.35 (s,1H); 13C NMR (100 MHz, DMSO-d6): δ= 40.4, 112.5, 117.6, 119.8, 120.9, 122.6, 126.1, 126.8, 127.6, 129.2, 129.7, 130.2, 130.5, 130.8, 131.3, 132.5, 144.6, 152.1, 153.5, 157.9, 188.7. Anal.calcd for C28H24FN3O: C, 76.87%; H, 5.53%; N, 9.60%. Found: C, 76.52%; H, 5.65%; N, 9.76%.
(1E,4E)-1-(3-(4-fluorophenyl)-1-phenyl-1H-pyrazol-4-yl)-5-(4-nitrophenyl)penta-1,4-dien-3-one (6v):Yellow solid; Yield: 70%; mp: 177-179 °C; 1H NMR (400 MHz, DMSO-d6): δ= 6.81 (d, J = 8.65 Hz, 2H), 6.92 (d, J = 15.89 Hz, 1H), 7.12 (d, J = 15.87 Hz, 1H), 7.46 (t, J = 7.37 Hz, 2H), 7.63-7.65 (m, 5H), 7.75-7.78 (m, 3H), 7.84-7.87 (m, 3H), 8.34 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ = 114.6, 118.3, 120.1, 123.5, 124.9, 126.4, 126.9, 127.4, 127.8, 129.1, 129.4, 129.8, 130.3, 131.7, 135.8, 139.5, 139.7, 154.6, 159.8, 188.2. Anal.calcd for C26H18FN3O3: C, 71.06%; H, 4.13%; N, 9.56%. Found: C, 71.21%; H, 4.27%; N, 9.31%.
(1E,4E)-1-(4-chlorophenyl)-5-(3-(4-fluorophenyl)-1-phenyl-1H-pyrazol-4-yl)penta-1,4-dien-3-one (6w): Off-white solid; Yield: 70%; mp: 175-176 °C; 1H NMR (400 MHz, DMSO-d6): δ= 6.94 (d, J = 15.89 Hz, 2H), 7.23 (t, J = 8.45 Hz, 2H), 7.47 (t, J = 7.35 Hz, 1H), 7.54-7.57 (m, 3H), 7.65-7.69 (m, 3H), 7.70-7.74 (m, 4H), 7.81-7.87 (m, 2H), 8.41 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ= 116.5, 117.2, 118.5, 120.7, 125.8, 126.5, 127.8, 128.9, 129.6, 129.8, 130.2, 130.3, 130.6, 130.9, 132.0, 133.6, 135.4, 139.8, 142.6, 188.7. Anal.calcd for C26H18ClFN2O: C, 72.81%; H, 4.23%; N, 6.53%. Found: C, 72.96%; H, 4.45%; N, 6.72%.
(1E,4E)-1-(4-fluorophenyl)-5-(3-(4-fluorophenyl)-1-phenyl-1H-pyrazol-4-yl)penta-1,4-dien-3-one (6x):Off-white solid; Yield: 71%; mp: 174-176 °C; 1H NMR (400 MHz, DMSO-d6): δ = 6.92 (d, J = 15.89 Hz, 2H), 7.10 (t, J = 8.34 Hz, 2H), 7.39 (t, J = 7.33 Hz, 1H), 7.47-7.51 (m, 3H), 7.58-7.61 (m, 3H), 7.65-7.69 (m, 3H), 7.82-7.87 (m, 3H), 8.38 (s, 1H); 13C NMR (100 MHz, DMSO-d6): δ = 116.7, 117.1, 118.5, 119.9, 124.8, 125.8, 127.7, 129.2, 129.5, 129.9, 130.1, 130.2, 130.6, 130.9, 132.5, 133.8, 134.6, 140.1, 142.8, 188.5. Anal.calcd for C26H18F2N2O: C, 75.72%; H, 4.40%; N, 6.79%. Found: C, 75.91%; H, 4.72%; N, 6.86%.
Results and Discussions
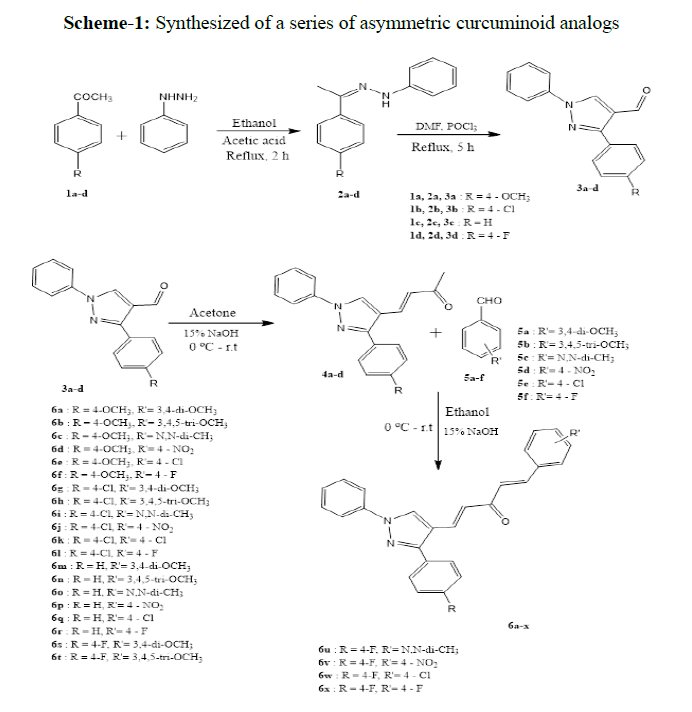
In the present study, we have synthesized a series of asymmetric curcuminoid analogs (6a-x) by taking two core structural elements aldehydes (3a-d) and chalcones (4a-d). Initially, for the preparation of aldehyde derivatives (3a-d), the appropriate phenyl hydrazones were prepared by treating different aldehydes with phenyl hydrazine in the presence of acetic acid. Then they were refluxed with VilsmeierHaack reagent (DMF+POCl3) at 80 °C to yield 3-(substituted phenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde (3a-d). Chalcones (4a-d) were prepared by NaOH-madiatedClaisen-Schmidt condensation of aldehydes (3a-d) with acetone[21]. Finally, NaOH-madiatedClaisen-Schmidt condensation of chalcones (4a-d) and substituted benzaldehydes (5a-f) furnished the target compounds 6a-x in good yields (Scheme 1).
All the synthesized compounds were purified by column chromatography and well characterized by spectroscopic techniques such as 1H NMR, 13C NMR spectra and elemental analysis. The formation of compounds 3a-d was confirmed by the appearance of aldehyde proton in the range of δ 10.04-10.19 ppm. The 1H NMR spectrum of compound 6c showed a singlet of two methyl groups on nitrogen at δ 3.03 ppm, a singlet of methoxy protons at δ 3.88 ppm and pyrazole –CH proton was assigned at δ 8.28 ppm. All the aromatic protons and four olefinic protons appeared in the region of δ 6.68-8.28 ppm. In the 13C NMR spectrum, the aliphatic carbons appeared in the region between δ 40.1-55.3 ppm while the aromatic carbons and four olefinic carbons were assigned in the region of δ 119.8-160.0 ppm. The most deshielded carbon at δ 188.5 ppm was assigned to carbonyl carbon. Almost similar patterns were observed in 1H and 13C NMR spectra of rest of the compounds (6a-x).
Conclusion
In summary, we have synthesized a series of curcumin inspired pyrazole derivatives (6a-x) by using Claisen Schmidt reaction. Intiallychalcones (4a-d) were prepared from pyrazolecarbaldehyde derivatives (3a-d) by treating with acetone in the presence of potassium carbonate. Finally monocarbonyl analogs of curcumins were synthesized by Claisen Schmidt condensation of chalcones with benzaldehyde derivatives (5a-f). All the MCAs were characterized by 1H NMR, 13C NMR spectra and elemental analysis.
References
- M Akram, AA Shahab-Uddin, K Usmanghani et al., J Biol Plant Biol. 2010, 55(2): p. 65.
- A Goel, AB Kunnumakkara, BB Aggarwal, Biochem Pharmacol. 2008, 75 (4): p. 787.
- R De, P Kundu, SSwarnakar et al., Agents Chemother. 2009, 53: p. 1592.
- JY Cho, GJ Choi, SW Lee et al., Plant Pathol J. 2006, 22: p. 94.
- SK Abraham, L Sharma and PC Keshvan, Mutat Res 1993, 303: p. 109.
- G Liang, S Yang, H Zhou et al., Eur J Med Chem. 2009, 44: p. 915.
- GK Jayaprakash, LJ Rao and KK Sakariah, Food Chem. 2005, 98: p. 720.
- MS Wang, S Boddapati, SEmadi et al., BMC Neurosci. 2010, 11: p. 11.
- R Narlawar, S Pichardt, K Leuchtenberger et al., Chem Med Chem. 2008, 3: p. 165.
- JFolkman, Proc. Natl. Acad Sci U.S.A. 2001, 98: p. 398.
- MK Kim, W Jeong, J Kang et al., Med Chem. 2011, 19: p. 3793.
- S Mishra, K Karmodiya, NSurolia et al., Bioorg Med Chem. 2008, 16: p. 2894.
- PV.Leyon and GKuttam, J Exp Clin Cancer Res. 2003, 22: p. 77.
- B Ku, W Zhou, F Yu et al., Patent. 2001, 18: p. 3945.
- YShukla, AArora and PTaneja, Mutat Res. 2002, 515: p. 197.
- SK Das, H Hand P Cohly, U. S. Patent. 1995, 541: p. 1504.
- AB Hegge, T Andersen, JEMelvik et al., J Pharma Sci. 2011, 100: p. 174.
- NK Gupta and VK.Dixit, J Pharma Sci. 2011, 100: p. 1987.
- ED Roberson and LMucke, Science. 2006, 314: p. 781.
- M Ashok, BS Holla and NSKumari, Eur J Med Chem. 2001, 42: p. 380.
- Q Nan, L Chun-Bao, J Mei-Na et al., Eur J Med Chem. 2011, 46: p. 5189.