Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 6
Synthesis and some Reactions of [4-Oxo-4-(3,4,5-Trimethoxy-Benzylidene)-4-Dihydro-Thiazole-2-Yl]-Acetonitrile with Expected Biological Activity
Mahmoud NA*
Chemistry Department, Faculty of Science, Al-Azhar University, Nasr City, Cairo, Egypt
- *Corresponding Author:
- Mahmoud NA
Chemistry Department
Faculty of Science
Al-Azhar University
Nasr City, Cairo, Egypt
Abstract
4-oxo-5-(3,4,5-trimethoxy-benzylidene)-4,5-dihydrothiazol-2-yl)-acetonitrile 1 was prepared and treatment with sodium azide, phenyl isothiocyanate, 5-bromosalicylaldehyde, thioacetamide and ethylacetate to give the corresponding 2-substitute-4-oxo-5-(3,4,5-Trimethoxy benzylidene-4,5-dihydrothiazole-2-yl) derivatives 2,3,5,6 and 8. Treatment of 3 and 6 with phenacylbromide gave 4 and 7a also 4-oxo-2-[4-oxo- 5-(3,4,5-trimethoxy benzylidene)-4,5-dihydro-thiazol-2-yl)-butyronitrile 8 when reacted with malononitrile, 2-cyanoacetohydrazide, hydrazine hydrate, benzaldehyde and 2-amino-2-(hydroxylmethyl) propane-1,3-diol afforded 9,13,15, 16 and 17. Treatment of compound 9 with sulfur gave 10 which in turn treated with p-nitrobenzaldehyde to give Schiff base 11, which on further treatment with thioglycollic acid gave 12. Cyclization of compound 13 with acetic acid gave 14. The newly synthesized compounds screened for their in vitro antitumor activity against human cancer cell line.
Keywords
Organic synthesis, Thiazole derivatives, Addition-condensation, Cyclization, Anticancer, HePG-2, MCF-7, HT-29
Introduction
Thiazole derivatives play an important role in nature and a diverse range of biological effects such as antitumor, antifungal, antimicrobial, anti-inflammatory, syk inhibitor and antiviral activity [1-14].
Moreover, Thiazoles and their analogues assist as precursors for the preparation of biologically active compounds. There is a growing body of literature that recognizes the importance of Thiazole derivatives as antimicrobial analgesic, anticonvulsant, cardiotonic, anticancer, antitubercular and anthelmintic effect [15].
Based on these interesting biological activities, Thiazoles have become a central issue for a great interest in a wide range of fields. Herein, we reported the synthesis of new class of thiazole derivatives which were evaluated for using in vitro as cytoxic activity against three human cancer cell lines including human liver cancer (HePG-2), human colon cancer (HT-29) and human breast cancer cell (MCF-7) cell lines by standard SRB.
Results and Discussion
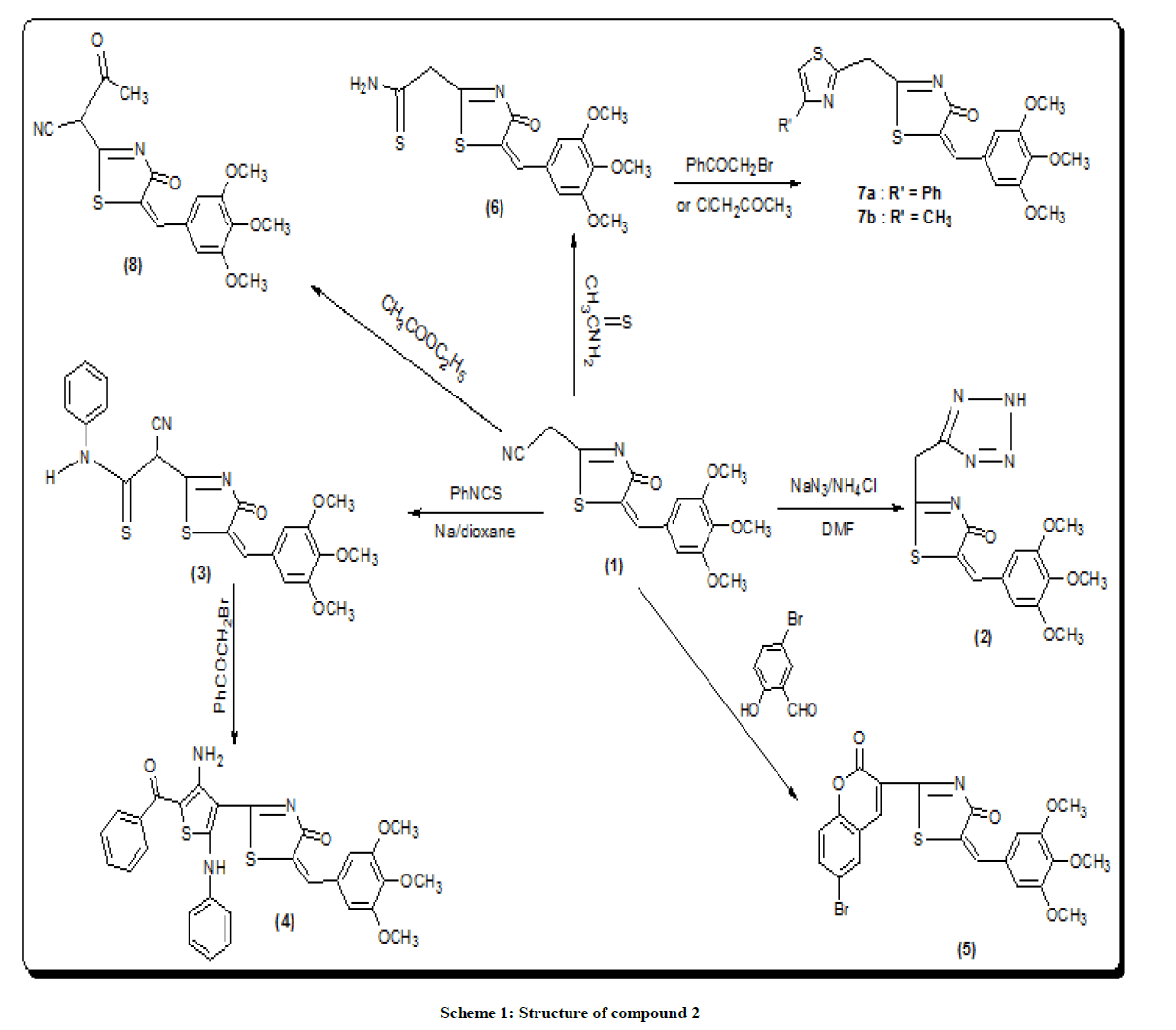
[4-oxo-5-(3,4,5-trimethoxy-benzylidene)-4,5-dihydro-thiazol-2-yl)-acetonitrile (1) was prepared by the reaction of the trimethoxy benzaldehyde with malononitrile and thioglycolic acid (2:2: 1 ratio) [16]. The treatment of 1 with sodium azide and ammonium chloride in DMF [17] afforded the structural 5-(3,4-dimethoxy-5-methoxy methyl-benzylidene-2-(2H-tetrazol-5-yl-methyl)-thiazol-4-one (2) (scheme 1). Infrared Radiation (IR) spectrum of 2 lacked the absorption band due to C≡N and showed strong band at 3340 cm-1 corresponding to NH group. Proton Nuclear Magnetic Resonance (1H-NMR) of 2 should singlet at δ 4.5 ppm corresponding to CH2 and deuterium oxide exchangeable singlet at δ 8.5 ppm corresponding to NH proton.
2-(4-Amino-5-benzoyl-2-phenyl amino-thiophen-3-yl) derivative (4) was produced by the reaction of 1 with phenyl isothiocyanate in dioxan to give 2-cyano-2-[4-oxo-5—(3,4,5-trimethoxy-benzylidene)-4,5-dihydro-thiazole-3-yl]-N-phenyl-thioacetamide (3). IR spectrum of 3 showed band at 3337 cm-1 to phenyl-NH while IR spectrum of 4b showed band at 3340 cm–1 due to NH2 group. 1H-NMR spectrum exhibits a broadened singlet at δ 8.9 ppm belonging to the (NH) group. The Carbon-13 (C13) Nuclear Magnetic Resonance (13C- NMR) spectrum of 3 agrees with their assigned structures.
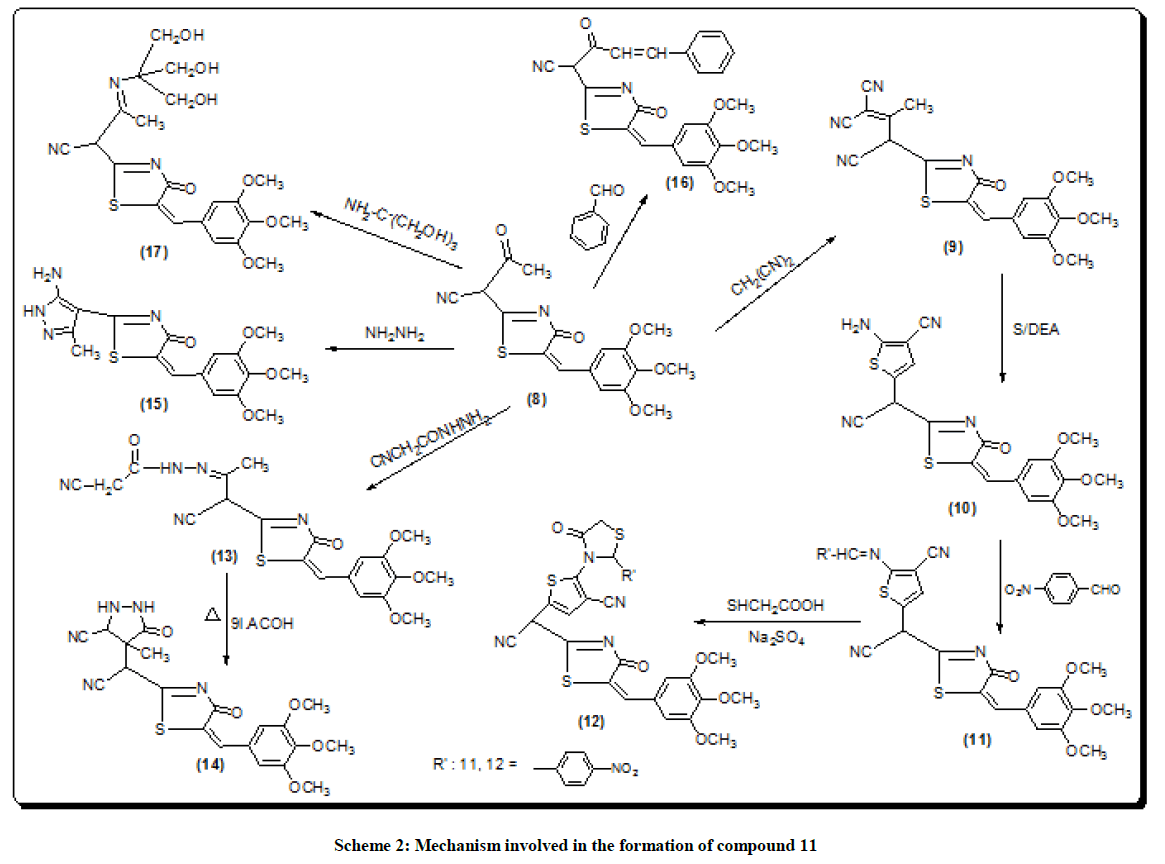
The structural data of 6 revealed the disappearance of NH2 group indicating their involvement in cyclization process. The stretching band at 3321 cm-1. Shows the occurrence of NH2 group thermal condensation of 1 with 5-bromosalicylaldehyde was carried out to prepared the corresponding 2-(7-bromo-2-oxo-2H-chromen-4-yl)-5-(3,4,5-trimethoxy benzylidene) thiazol-4-one (5) (scheme 1) IR spectrum showed evidence for this cyclization by exhibiting absorption band at 1702 cm-1 corresponding to coumarin carbonyl. 1H-NMR of 5 should singlet at δ 5.8 ppm corresponding to (CH=C chromen) reaction of 1 with ethyl acetate [18] gave the corresponding 4-oxo-2-[4-oxo-5-(3,4,5, tri-methoxy benzyliden)-4,5-dihydro-thiazol-2-yl]-butronitrile (8). The IR spectrum of the compound 8 revealed the presence of the carboxyl absorption band at 1704 cm-1. Compound 9 is obtained from reaction of compound 8 with malononitrile in the presence of piperidine as a catalyst led to the formation of 3,3-dicyano-2-[3-oxo-5-(3, 4,5-tri-methoxy benzylidene)-4-5-dihydro-thiazol-2-yl)-butyro-nitrile (9). IR spectrum of compound 9 showed characteristic absorption band at 2218, 2220 (2CN). Ring closure of compound 9 via the reaction with sulfur in the presence of diethyl-amine gave 2-amino-5-(cyano-[4-oxo-5-tri-methoxy-benzylidene)-4,5-dihydro-thiazol-2-yl]-methyl-thio-carbo-nitrile (10).
The IR spectrum of compound 10 showed the stretching band of NH2 at 3341 cm-1 and 1H-NMR spectrum showed broad singlet at δ 10.5 ppm belonging to the NH2 group (scheme 2) acid catalyzed reaction of compound 10 with 4-nitro-benzaldehyde gave the corresponding Schiff base 5-(cyano-[4-oxo-5-(3,4,5-tri-methoxy benzylidene)-4,5-dihydro-thiazole-2-yl] methyl-2-[(4-nitro-benzylidene)-amino)]-thiophen-3-carbo-nitrile (11). The 1H-NMR spectra of compound 11 revealed singlet signal at δ 6.5 ppm attributed to anil proton (CH=N). Cyclocondensation of the schiff base a with thioglycolic acid in dry dioxane and in the presence of anhydrous sodium sulphate led to the formation 5-(cyano-[4-oxo-5- (3,4,5-trimethoxy-benzylidene)-4,5-dihydro-thiazol-2-yl]-2-[2-(4-nitro-phenyl-4-oxo-thiazolidin-3-yl]-thiophen-3-carbonitrile (12) (scheme 2). Condensation of compound (8) with 2-cyanoacetohydrazide at room temperature afforded hydrazine derivative 13, which underwent heterocycliztion by heating with acetic acid and product pyrazoline derivative 14 (scheme 2). In addition, compound 8 reacted with hydrazine hydrate in acetic acid and toluene to give 2-(5-amino-3-methyl-H-pyrazol-4-yl)-5-(3,4,5-trimethoxy benzylidene)- thiazol-4-one (15). IR spectrum of compound 15 showed the absence of keto absorption band which appeared for 8 showed new absorption bands at 3310, 3340 for NH and NH2 groups. The 1H-NMR spectrum revealed singals at 10.5 ppm belonging to NH2 and 11.1 ppm belonging to NH group. Claisen-schmidt reaction of ketone 8 with benzaldehyde in ethanol and in the presence of aqueous potassium hydroxide (25%) led to the formation of 3- oxo-2-[4-oxo-5-(3,4,5-trimethoxy benzilidene)-4,5-dihydrothiazol-2-yl)]-5-phenyl-pen-4-ene-nitrile (16) (scheme 2). 1H-NMR spectrum of compound 16 showed doublet band at δ 6.1 ppm due to (CH=CH). Furthermore, reaction of 8 with 2-amino-2-(hydroxy methyl) propane-1,3- diol in glacial acetic acid gave 3-(3-hydroxy-1,1-bis-hydroxymethylethylimino)-2-[4-oxo-5-(3,4,5-trimethoxybenzylidene)-4,5-dihydrothiazol-2- yl)] butyronitrile (17) (scheme 2). IR spectrum of compound 17 showed stretching band of (3OH) at 3314 cm-1 and 1H-NMR spectrum of compound 17 showed appearance a triplet band at δ 10.1 ppm due to (3 CH2OH).
Materials and Methods
Experimental
Melting points were determined in open capillary tubes on an electrothermal 9100 digital melting point apparatus (Buchi, Switzerland) and were uncorrected IR spectra were recorded on a Perkin-Elmer 1600 FTIR (Perkin-Elmer, USA) in KBr discs. 1H and 13C-NMR spectra were measured with a Bruker Avance spectrometer (300 and 125 MHz) (Bruker, Germany) in DMSO-d6 and chemical shifts were recorded in δ ppm to TMS as internal standard solvent. Mass spectrometer, single-phase 200V/60 Hz, 30A (Jeol Ltd. Japan). Elemental analysis was determined using a perkin–Elmer 24°C microanalysis the new synthesized compounds were screened for in antitumor activity at Egyptian organization for Biological Products and Vaccines (Vacsera).
4-ox-5-[(3,4,5-Trimethoxy-benzylidene)-4,5-dihydro-thiazol-2-yl]-acetonitrile (1)
A mixture of Trimethoxy benzaldehyde (0.01 mol) and malononitrile (0.02 mol) and thioglycolic acid (0.01 mol) in ethanol (50 ml) and a catalytic amount of piperidine was heated under reflux for 5 h. The solution was concentrated. Then the product was collected and recrystallized from ethanol yellow powder; yield (79%); M. p.355-360°C; Anal. Calcd (%) for C15H14N2O4S (318) C, 56.6, H, 4.4, N, 8.8 found (%) C, 56.5, H, 4.1, N, 5.8; IR (KBr, cm-1): 1562 (C=C), 1695 (C=O), 2200 (CN), 2839 (CH aliph.); 1H-NMR (DMSO-d6) δ (ppm)=3.68, 3.75, 3.79 (3s, 9H, 30CH3), 4.56 (s, 2H, CH2), 6.69 (s, CH=C), 6.92-7.82 (m, 2H, Ar–H); 13C-NMR: 19.01 (CH3), 56.60 (CH2), 64.02 (CH=C), 117.95 (CN), 139.91-139.99 (C–Ar), 140.19 (C=N), 153.79 (C=O).
5- (3,4-dimethoxy-5-methoxy methyl-benzylidene-2-(2H-tetrazol-5-yl-methyl)-thiazol-4-one (2)
A mixture of compound 1 (0.01 mol), sodium azide (0.01 mol) and ammonium chloride (0.01 mol) in DMF (10 ml) was heated under reflux for 24 h. then diluted with cold water (20 ml). The obtained precipitate was filtered, washed with water, dried and recrystallized from ethanol. Light brown powder; yield (75%); M. p. 170-172°C; Anal-Calcd (%) for C15H15N5O4S (361), C, 49.1, H, 4.1, N, 19.3 Found (%) C, 49.1, H, 4.2, N, 19.1; IR (KBr, cm-1): 1562 (C=C) 1695 (C=O), 2839 (CH-aliph.) 3340(NH); 1H-NMR (DMSO-d6) δ (ppm)=3.68, 3.75, 3.79 (3s, 9H, 3OCH3), 4.5 (s, 2H, CH2) 5.69 (s, H, CH=C), 6.9-7.8 (m, 2H, Ar–H), 8.5 (s, H, NH, D2O exchangeable).
2-Cyano-2-[4-oxo-5-(3,4,5-trimethoxy-benzylidene)-4,5-dihydro-thiazol-2-yl]-N-phenyl-thioacetamide (3)
A mixture of 1 (0.01 mol), finely divided sodium metal (0.01 mol), and phenyl isothiocyanate (0.01 mol) was refluxed for 7 h. in dry dioxan (50 ml), then allowed to cold water and neutralized with dilute HCl the precipitated was filtered off and recrystallized from pot. ether 80-100°C. Brown powder; yield (65%) M. p. 120-123°C; Anal. Calcd. (%) for C22H19N3O4S2 (453), C, 58.2, H, 4.1, N, 9.2 Found. (%) C, 58.1, H, 4.1, N, 9.5; IR (KBr, cm-1): 1567 (C=C) 1692 (C=O), 1242 (C=S), 2199 (CN), 2891 (CH aliph.), 3337(NH).
2-(4-amino-5-benzoyl-2-phenyl amino-thiophen-3-yl)-5-(3,4,5 trimethoxy-benzylidene)-thiazol-4-one (4)
A mixture of 3 (0.01 mol), sodium ethoxide (0.005 mol) and phenacyl bromide (0.01 mol) in 50 ml ethanol was heated under reflux for 22 h. and the reaction mixture was poured into ice and dilute HCl. The product was crystallized from benzene. Dark brown powder; yield (55%); M. p. 155-157°C; Anal. Calcd. (%) for C30H25N3O5S2 (571), C, 63.1, H, 4.3, N, 7.3. Found (%) C, 63.1, H, 4.1, N, 7.2; IR (KBr, cm-1) 1563 (C=C), 1693 (C=O), 3314 (NH), 3340 (NH2); 1H-NMR (DMSO–d6) δ (ppm)=3.71, 3.72, 3.93 (3s, 9H, 3OCH3), 5.57 (s, H, CH=C), 6.9-8.805 (m, 12H, Ar–H), 10.3 (s, 2H, NH2), 11.1 (s, H, NH, D2O exchangeable).
2-(7-bromo-2-oxo-2H-chromen-4-yl)-5-(3,4,5-trimethoxy-benzylidene) thiazol-4-one (5)
A mixture of the appropriate 1 (0.0 mol) and 5-bromo-salicylaldehyde (0.01 mol) in absolute ethanol (35 ml) containing piperidine (5 drops) was refluxed for 4 h. The reaction mixture was cooled and then acidified with dilute HCl the solid formed was collected and recrystallized from ethanol. Black powder; yield (75%); M. p. 240-242; Anal. Calcd. (%) for C22H16NO6BrS (502) C, 52.6, H, 3.2, N, 2.7. Found (%) C, 52.1, H, 3.1, N, 2.6; IR (KBr, cm-1) 1566 (C=C), 1695 (C=O cyclic), 1022 (C-O–C), 1702 (C=O chromen); 1H-NMR (DMS-d6) δ (ppm)=3.83, 3.84, 3.85 (3s, 9H, 3OCH3), 5.6 (s, H, CH=C thiazole), 5.8 (s, H, CH=C chromen), 6.5-7.9 (m, 5H, Ar–H).
2-[4-oxo-5-(3,4,5-trimethoxy benzylidene-4,5-dihydro-thiazol-2-yl] thioacetamide (6)
A solution of 1 (0.01 mol) and thioacetamdie (40 ml) in DMF (75 ml) was heated in an oil bath to 90-100°C for about 2 h, while HCl gas was being bubbled into the reaction mixture. The solvent was evaporated in vacuo, and the residue was triturated with acetone to give crude product. This material was recrystallized from benzene. Light brown powder; yield (70%); M. p. 200-202°C; Anal. Calcd. (%) for C15H16N2O4S2 (352), C, 51.1, H, 4.5, N, 7.9. Found (%) C, 51.1, H, 4.4, N, 7.7; IR (KBr, cm-1), 1565 (C=C), 1695(C=O), 1249 (C=S), 3321 (NH2); 1H-NMR (DMSO-d6) δ (ppm)=3.30, 3.31, 3.32 (3s, 9H, 3OCH3), 4.5 (s, 2H, CH2), 5.6 (s, H, CH=C), 6.6-7.9 (m, 2H, Ar–H), 9.8 (s, 2H, NH2, D2O exchangeable).
2-(4-phenyl-thiazol-2-yl methyl)-5-(3,4,5-trimethoxy-benzylidene)-thiazol-4-one(7a) and 2-(4-methyl-thiazol-2-yl methyl)-5-(3,4,5-trimethoxy-benzylidene)- thiazol-4-one (7b)
A solution of 6 (0.01 mol) and phenacyl bromide or chloro acetone (0.01 mol) in ethanol (25 ml) was heated at reflux for 6 h. After the starting material had been consumed as judged by Thin Layer chromatography (TLC), the reaction mixture was cooled and the crude product was collected by filtration. Chromatography on silica (90: 9: 1, CHCl3: MeOH: NH4OH) yielded a brown oil, which was dissolved in MeCN and treated with diethyl ether. It was collected and recrystallized from ethanol.
7a: yellowish-brown crystals; yield (55%) M. p. 260-262°C; Anal. Calcd. (%) for C23H20N2O4S2 (452), C, 61, H, 4.4, N, 6.1 Found (%) C, 61.1, H, 4.3, N, 6.2; IR (KBr, cm-1): 1567 (C=C), 1694 (C=O). MS, m/z (%): 452 (M+) 98 (18), 64(51), 80(100).
7b: Dark brown powder; yield (45%) M. p. 230-232°C; Anal. Calcd. (%) for C18H18N2O4S2 (366), C, 55. 3, H, 4.6, N, 7.1 Found (%) C, 55.1, H, 4.5, N, 7.2; IR (KBr, cm-1): 1568 (C=C), 1695 (C=O).
4-oxo-2-[4-oxo-5(3,4,5-trimethoxy-bezylidene)-4,5-dihydro-thiazol-2-y]-butyronitrile (8)
A solution of 1 (0.01 mol) in ethyl acetate (200 ml), sodium metal (0.28 mol) was added portion wise at room temperature. The mixture was stirred at room temperature for 48 h. the collected solid was washed with copious amounts of diethyl ether and recrystallized from ethanol. Dark brown powder; yield (65%) M. p. 200-202°C; Anal. Calcd. (%) for C17H16N2O5S (360), C, 56.6, H, 4.4, N, 7.7, Found (%) C, 56.1, H, 4.3, N, 7.6; IR (KBr, cm-1): 1564 (C=C), 1693 (C=O cyclic), 1704 (C=O ketone) 2201 (CN); 1H-NMR (DMSO-d6) δ (ppm)=3.50, 3.51, 3.52 (3s, 9H, 3OCH3), 2.26 (s, 3H, CH3), 5.2 (s, H, CH=C), 4.5 (s, H, CH–CN), 6.9-7.7 (m, 2H, Ar–H).
3,3-Dicyano-2[3-oxo-5-(3,4,5-trimethoxy-benzylidene)-4,5-dihydro-thiazol-2-yl]-butyronitrile(9)
A mixture of 8 (0.01 mol) and malononitrile (0.01 mol) in absolute ethanol (20 ml) containing few drops of piperidine was refluxed for 8 h. After cooling, the reaction mixture was poured onto ice-water (50 ml) and the solid that formed was filtered off, air dried and crystallized from ethanol. Black powder; yield (65%) M. p. 110-111°C; Anal. Calcd. (%) for C19H16N4O4S (396), C, 57.5, H, 4.04, N, 14.1, Found (%) C, 57.3, H, 4.1, N, 14.2; IR (KBr, cm-1): 1566 (C=C), 1690 (C=O), 2190-2218 (3CN).
2-Amino-5-(cyano-[4-oxo-5-trimethoxy-benzylidene)-4,5-dihysro-thiazol-2-yl]-methyl thio carbonitrile (10)
To a solution of compound 9 (0.05 mol) and sulfur (0.05 mol) in absolute ethanol (50 mol), diethylamine (0.05 mol) was added dropwise at 15°C and then the reaction mixture was stirred for 2 h at 65°C. After evaporation of all solvent, the residue was dissolved in absolute ethanol (50 ml). Followed by further stirring for 30 min in an ice off, washed with water, air dried and crystallized from methanol. Yellow brown powder; yield (75%) M. p. 200-201°C; Anal. Calcd. (%) for C20H16N4O4S2 (440), C, 54.5, H, 3.6, N, 12.7, Found (%) C, 54.4, H, 3.5, N, 12.6; IR (KBr, cm-1): 1578 (C=C), 1688 (C=O), 2219-2220 (2CN), 3341(NH2); 1H-NMR (DMSO-d6) δ (ppm)=3.61, 3.65, 3.68 (3s, 9H, 3OCH3), 4.6 (s, H, CH– CN), 5.8 (s, H, CH=C), 6.7-7.9 (m, 2H, Ar-H), 8.1 (s, H, thiophene), 10.5 (s, 2H, NH2, D2O exchangeable).
5-(cyano-[4-oxo-5-(3,4,5-trimethoxy-benzylidene)-4,5-dihydro-thiazol)-2-yl]methyl-2-[(4-nitro-benzylidene)-amino]-thiphene-3-carbonitrile (11)
A mixture of the compounds 10 (0.01 mol) and 4-nitrobenzaldehyde (0.01 mol) in glacial acetic acid (20 ml) was refluxed for 6 h. after cooling, the reaction mixture was poured onto ice-water (50 ml) the solid that formed was filtered off, air dried and crystallized from benzene. Brown powder; yield (55%) M. p. 190-19°C; Anal. Calcd (%) for C27H19N5O6S2 (573), C, 56.5, H, 3.3, N, 12.2. Found (%) C, 56.3, H, 3.2, N, 12.1. IR (KBr, cm-1): 1576 (C=C), 1692 (C=O), 2212(CN); 1H-NMR (DMSO-d6) δ (ppm)=3.65, 3.67, 3.69 (3s, 9H, 3OCH3), 4.6 (s, H, CH-CN), 5.8 (s, H, CH=C), 6.5 (s, H, CH=N), 6.9-7.7 (m, 6H. Ar–H), 8.9 (s, H, thiophene); 13C-NMR: 22.64, 23.30, 25.79 (3, CH3), 63.75-63.24 (CH=C), 42.50 (CH), 156.88-114.71 (Ar–C), 117.94, 116.85 (2CN), 161.99 (CH=N), 164.52 (C=N), 165.23 (C=O).
5-(cyano-[4-oxo-5-(3,4,5-trimethoxy-benzylidene)-4,5-dihydro-thiazol-2-yl)-methyl)-2-[2-(4-nitro-thiazol-2-yl)-methyl)-2-[2-(4-nitro-phenyl)-4- oxo-thiazolidin-3-yl]-thiophene-3-carbonitrile (12)
To a stirred solution of compounds 11 (0.01 mol) in dry dioxane (25 ml), thioglycollic acid (0.01 mol) was added. The reaction mixture was stirred for 4 h then, anhydrous sodium sulphate (30 g) was added and refluxed for 6 h, and then reaction filtered on hot. After cooling the solid that formed was filtered off, air dried and crystallized from chloroform. Light brown powder; yield (65%) M. p. 120-122°C; Anal. Calcd (%) for C29H21N5O7S3 (647), C, 53.7, H, 3.2, N, 10.8, Found (%) C, 53.1, H, 3.1, N, 10.7; IR (KBr, cm-1): 1566 (C=C), 1663, 1694 (2C=O), 2210(CN).
Isocyano-acetic acid (2-cyano-1-methyl-2-5-(3,4,5-trimethoxy-benzylidene)-4,5-dihydro thiazol-yl)-ethylidene)-hydrazide (13)
To a solution of 8 (0.01 mol) in glacial acetic acid (10 ml), was added 2-cyanoacetohydrazide (0.0 mol) the reaction mixture was stirred at room temperature for 30 min the solid formed was filtered recrystallized from absolute ethanol. Brown powder yield (55%) M. p. 160-162°C; Anal. Calcd (%) for C20H19N5O5S (441), C, 54.4, H, 4.3, N, 15.8, Found (%) C, 54.2, H, 4.2, N, 15.7. IR (KBr, cm-1): 1560 (C=C), 1690 (C=O), 2210, 2220 (2CN), 3330 (NH); 1H-NMR (DMSO-d6) δ (ppm)=3.5 (s, 3H,CH3), 3.66, 3.68, 3.69 (3s, 9H, 3OCH3), 4.4 (s, 2 H, CH2CN), 4.9 (s, H, CHCN), 5.6 (s, H, CH=C), 6.7-7.9 (m, 2H, Ar–H), 10.1 (s, H, NH, D2O exchangeable).
3-(3-isocyano-5-oxo-4,5-dihydro-1H-pyrazol-4-yl)-2-[4-oxo-5-(3,4,5-trimethoxy-benzylidene)-4,5-dihydro-thiazol-2-yl]-butyronitrile (14)
Compound 13 (0.01 mol) was heated and refluxed in glacial acetic acid for 5 h. The reaction mixture was then concentrated to 1/2 of its volume under vacuum and left to cool at refrigerator. The product that formed was collected by filtration washed with water, air dried and recrystallized from aqueous ethanol. Black powder yield (45%) M. p. 140-142°C; Anal. Calcd (%) for C21H18N5O5S (452), C, 55.6, H, 4.1, N, 13.2, Found (%) C, 55.5, H, 4.2, N, 13.1; IR (KBr, cm-1): 1566 (C=C), 1690, 1698 (2C=O), 2210, 2221 (2CN), 3330 (NH); 1H-NMR (DMSO-d6) δ (ppm)=3.5,(s, 3H,CH3), 3.65, 3.66, 3.68 (3s, 9H, 3OCH3), 4.8 (s, H, CH–CN), 5.5 (s, H, CH=C), 6.8-7.9 (m, 2H, Ar–H), 10.6 (s, H, NH, D2O exchangeable).
2-(5-amino-3-methyl-H-pyrazol-4-yl)-5-(3,4,5-trimethoxy-benzylidene)-thiazol-4-one (15)
A mixture of 8 (0.01 mol), hydrazine hydrate (0.01 mol), acetic acid (10 ml) and toluene (100 ml) was heated at reflux for 6 h. The reaction mixture was cooled to room temperature. The solid was collected and recrystallized from benzene. Light brown powder yield (65%) M. p. 175- 177°C; Anal. Calcd (%) for C17H18N4O4S (373), C, 54.5, H, 4.8, N, 14.9, Found (%) C, 54.3, H, 4.5, N, 14.5; IR (KBr, cm-1): 1655 (C=C), 1692 (C=O), 3310 (NH), 3340 (NH2); 1H-NMR (DMSO-d6) δ (ppm)=3.5(s, 3H,CH3), 3.67, 3.68, 3.77 (3s, 9H, 3OCH3), 6.5-7.9 (m, 7H, Ar–H), 10.5 (s, 2H, NH2), 11.1 (s, H, NH, D2O exchangeable).
3-oxo-2-[4-oxo-5-(3,4,5-trimethoxy-benzylidene)-4,5-dihydro-thiazol-2-yl]-5-(phenyl)pen-4-enenitrile (16)
To a solution of 8 (0.01 mol) in ethanol (10 ml) containing potassium hydroxide solution (5 ml, 25%) benzaldehyde (0.01 mol) was added. The reaction mixture was stirred for 2 h at room temperature and then left overnight at refrigerator. The reaction mixture was neutralized with dilute HCl (1: 1) and the solid that formed was filtered, washed with water, air dried and recrystallized from benzene. Brown powder yield (55%) M. p. 145-147°C; Anal. Calcd (%) for C27H26N5O8S (538), C, 60.2, H, 4.8, N, 5.2, Found (%) C, 60.1, H, 4.2, N, 5.3; IR (KBr, cm-1): 1567 (C=C), 1705 (C=O), 2206 (CN); 1H-NMR (DMSO-d6) δ (ppm), 3.66, 3.75, 3.78 (3s, 9H, 3OCH3), 4.4 (s, H, CH–CN), 5.8 (s, H, CH=C), 6.1 (d, 2H, CH=CH), 6.5-7.6 (m, 4H, Ar–H).
3-(3-hydroxy-1,1-bis-hydroxymethyl-ethylimino)-2-[4-oxo-5-(3,4,5-trimethoxy-benzylidene-4,5-dihydro-thiazol-2-yl]-butronitrile (17)
To a solution of 8 (0.01 mol) in glycial acetic acid (10 ml) was added 2-amino-2-(hydroxyl methyl) propane-1,3 diol (0.01 mol). The solid formed was filtered off, washed water, air dried and recrystallized from absolute ethanol. Light brown powder yield (55%) M. p. 155-157°C; Anal. Calcd (%) for C21H25(415), C, 60.7, H, 6.02, N, 15.4, Found (%) C, 60.6, H, 5.9, N, 15.1; IR (KBr, cm-1): 1550 (C=C), 1620 (C=N), 1697 (C=O), 3414 (OH); 1H-NMR (DMSO-d6) δ (ppm)=3.30 (s, 3H, CH3), 3.65, 3.67, 3.69 (3s, 9H, 3OCH3), 4.5 (s, H, CH–CN), 5.6 (s, H, CH=C), 6.6-7.9 (m, 2H, Ar–H), 10.1 (3t, 9H, 3CH2OH).
Antiproliferative activity
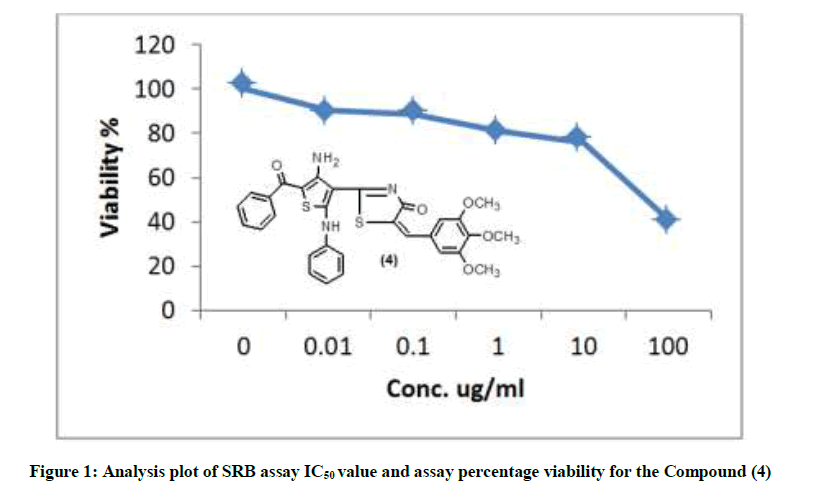
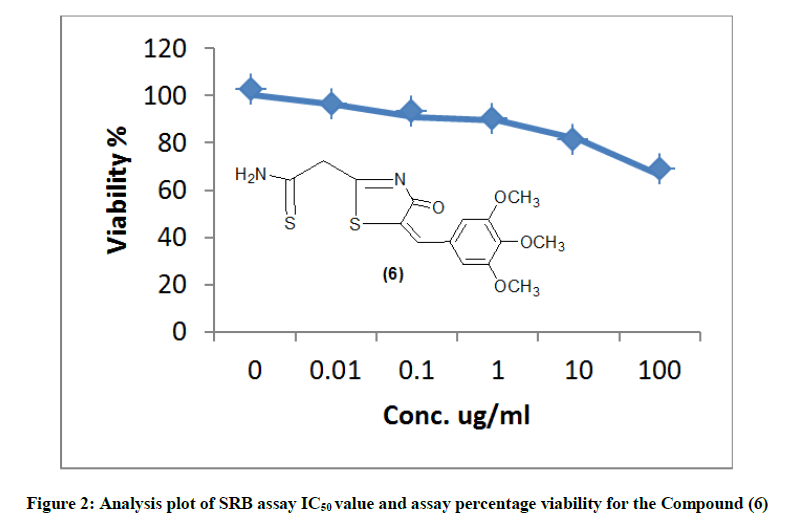
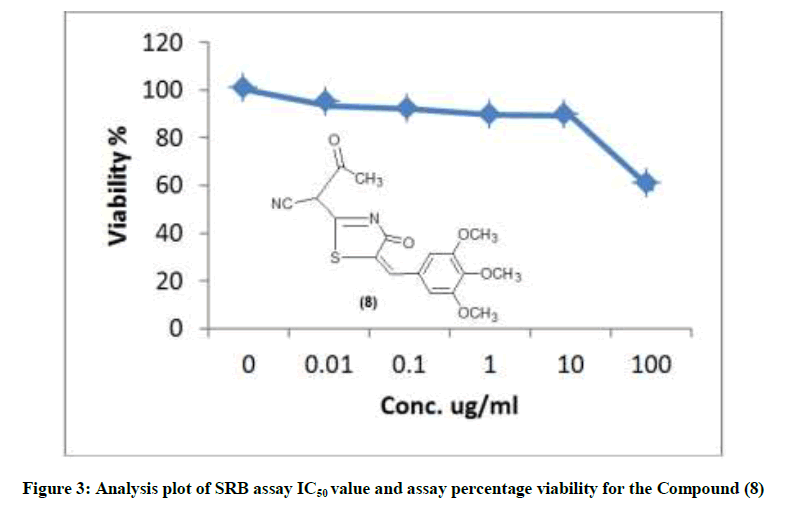
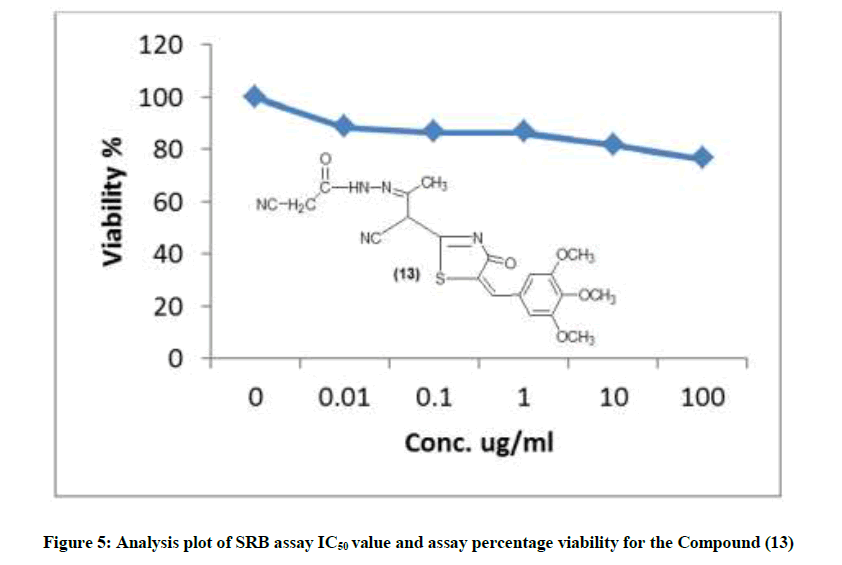
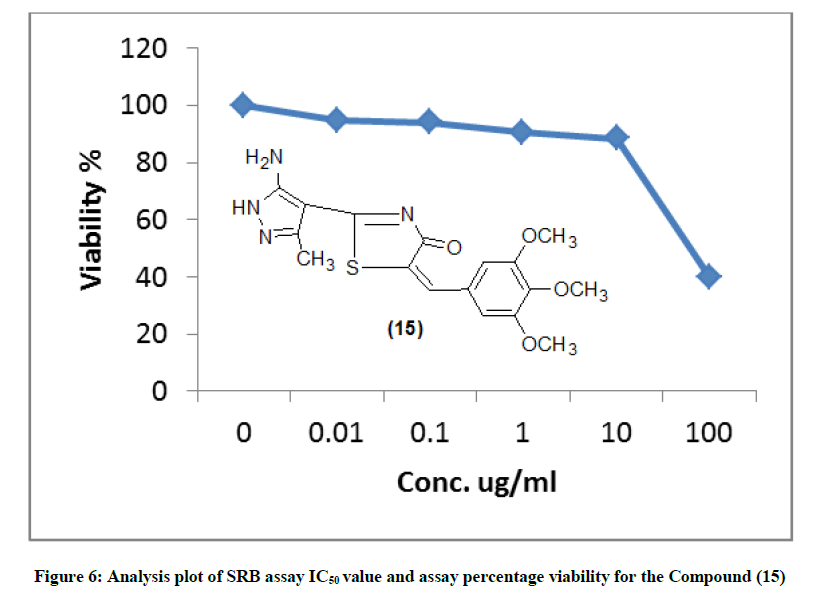
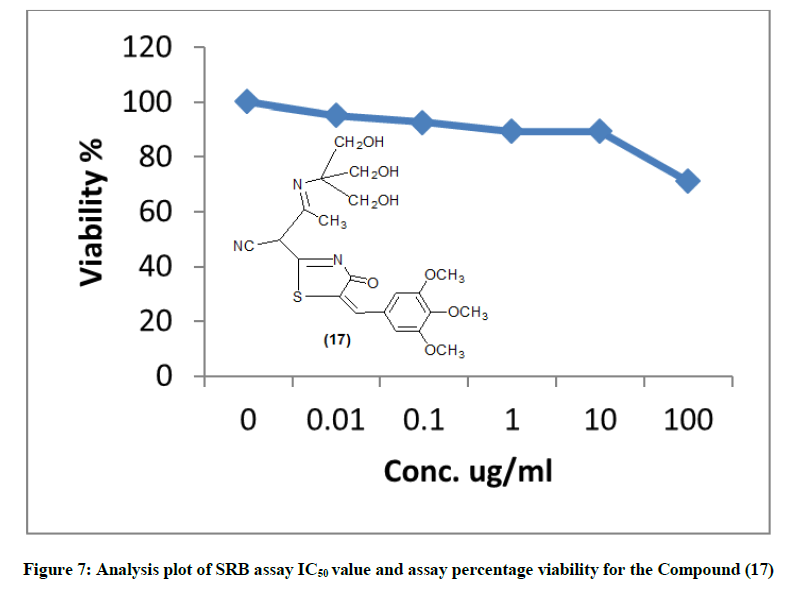
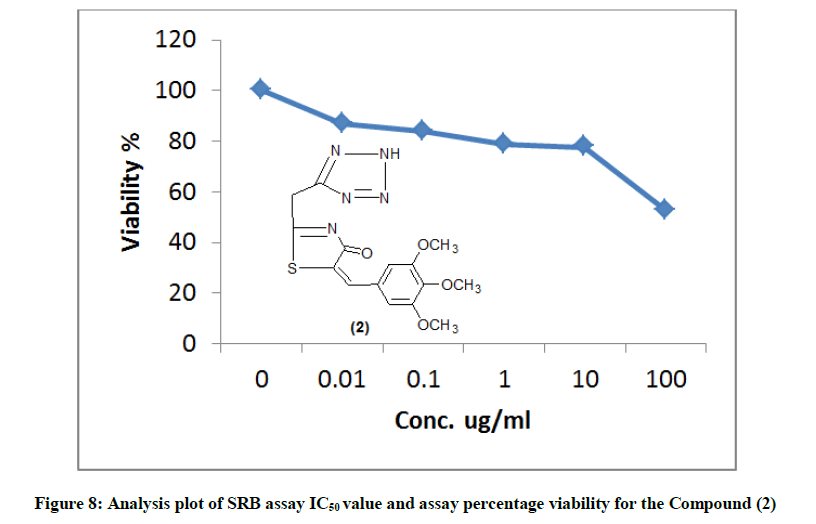
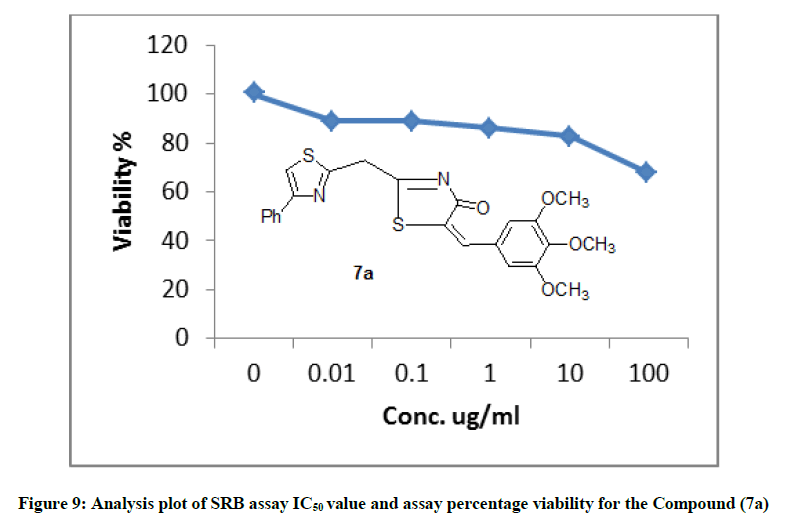
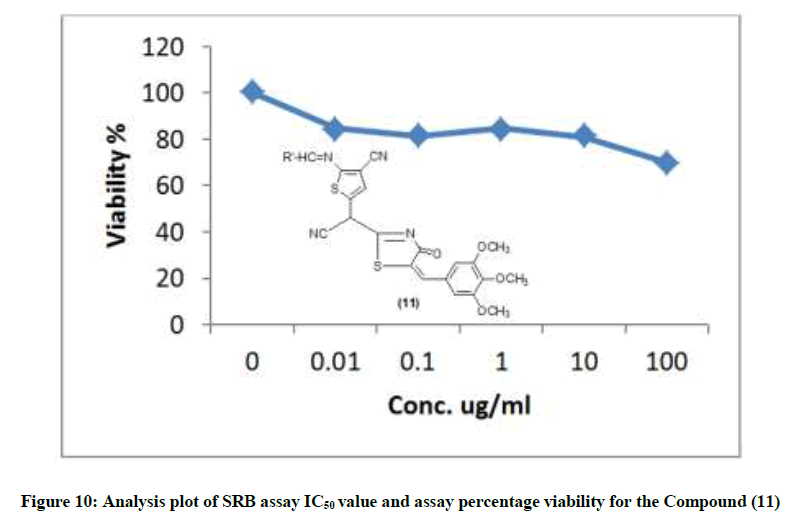
The newly synthesized compounds were evaluated for their in vitro cytotoxic effects against human liver cancer cell (HePG-2), human colon cancer cell (HT-29) and human breast cancer cell (MCF-7) cell lines by the standard SRB [19]. The method is based on the ability of the protein dye sulforhodamine B to bind electrostatically and pH dependent on protein basic amino acid residues of trichloroacetic acid-fixed cells. Under mild acidic conditions, it binds to and under mild basic conditions it can be extracted from cells and solubilized for measurement. Results of the SRB assay were linear with cell number and cellular protein measured at cellular densities ranging from 1 to 100% of confluence. Its sensitive is comparable with that of several fluorescence assays and superior to that of Lowry or Brodford. The signal to noise ratio is favorable and the resolution is 1000-2000 cells/well. SRB assay is used to determine the drug concentration required to inhibit the growth of human cancer cells by 50% (IC50). The results of SRB assay IC50 values and the results of the assay percentage viability are show in (Figures 1-10) and (Tables 1-3) in order to investigate the structure activity. The compounds (10, 4) showed to be the most active have IC50 values 41.78, 65.17 ug/ml against (HEPG-2) cell line while the compounds 8, 6 less activity whereas in case of (HCT-116) cell line the compounds are recorded IC50% 66.53 ug/ml found to be most potent than compounds 17 and 13. Over all the compound 2 revealed the best activity against MCF-7 cell line which recorded IC50% 181.27 ug/ml than other compounds 11, 7a.
| Comp. No. | Viability % | IC50 (μg/ml) HePG-2 | |||||
|---|---|---|---|---|---|---|---|
| Sample concentration (μg/ml) | |||||||
| 100 | 10 | 1 | 0.1 | 0.01 | 0 | ||
| 4 | 41.01 | 75.67 | 84.14 | 88.72 | 90.27 | 100 | 65.17 |
| 6 | 66.51 | 81.28 | 89.52 | 90.97 | 96.11 | 100 | 339.14 |
| 8 | 58.55 | 89.05 | 89.61 | 92.14 | 93.21 | 100 | 271.4 |
| 10 | 12.34 | 96.16 | 96.77 | 96.95 | 92.95 | 100 | 41.78 |
Table 1: Drug concentration required to inhibit the growth of human cancer cells by 50% (IC50) of the tested compounds against HePG-2 cell line
| Comp. No. | Viability % | IC50 (μg/ml) HCT-116 | |||||
|---|---|---|---|---|---|---|---|
| Sample concentration (μg/ml) | |||||||
| 100 | 10 | 1 | 0.1 | 0.01 | 0 | ||
| 13 | 76.48 | 81.6 | 86.58 | 86.58 | 88.52 | 100 | 8442.66 |
| 15 | 39.83 | 88.24 | 90.45 | 93.91 | 94.74 | 100 | 66.53 |
| 17 | 71.23 | 89.07 | 89.34 | 92.66 | 95.02 | 100 | 462.33 |
Table 2: Drug concentration required to inhibit the growth of human cancer cells by 50% (IC50) of the tested compounds against HCT-116 cell line
| Comp. No. | Viability % | IC5 (ug/ml) MCF-7 | |||||
|---|---|---|---|---|---|---|---|
| Sample concentration (ug/ml) | |||||||
| 100 | 10 | 1 | 0.1 | 0.01 | 0 | ||
| 2 | 52.83 | 77.48 | 78.64 | 84.02 | 86.76 | 100 | 181.27 |
| 7a | 67.5 | 82.77 | 85.97 | 88.9 | 89.04 | 100 | 368.72 |
| 11 | 69.91 | 80.91 | 81.33 | 84.67 | 84.86 | 100 | 279.34 |
Table 3: Drug concentration required to inhibit the growth of human cancer cells by 50% (IC50) of the tested compounds against MCF-7 Cell Line
Conclusion
In summary, we have synthesized novel thiazole derivatives and their structures were characterized on the basis of the spectral data and elemental analyses. Most of the synthesize compounds were evaluated for their cytotoxic activity against human cancer cell lines in vitro. It was observed that The compounds (10, 4) showed to be the most active have IC50 values 41.78, 65.17 ug/ml against (HEPG-2) cell line while the compounds 8, 6 less activity whereas in case of (HCT-116) cell line the compounds is recorded IC50% 66.53 ug/ml found to be most potent than compounds 17 and 13. Over all the compound 2 revealed the best activity against MCF-7 cell line which recorded IC 50% 181.27 ug/ml than other compounds 11, 7a which concluded from their IC50% value.
Acknowledgement
The author wishes to thank Dr. Ing Eman S Zarie, Material science Department Aalto University, Finland for carrying out NMR analysis and Egyptian company for biologics and vaccines for biological screening. This work was financially supported by Faculty of Science, (Girls), Al- Azhar University, Nasr City, Cairo, Egypt.
References
- N. Siddigui, S.K. Arya, W. Ahsan, B. Azad, Aretrospect., 2011, 3, 4, 0975-93441.
- L. Yurttas, Y. Ozkay, H.K. Gencer, U. Acar, J. Chem., 2015, 4643, 7.
- A. Sonone, W. Devendar, S. Mujahid, A. Durrani, J. Medic. Chem. Drug Discove., 2017, 02, 03, 80-87.
- S.M. Gomha, T.A. Salah, A.O. Abdelhamid, Monath Chem., 2015, 146, 149-158.
- S.M. Gomha, T.A. Salah, H.M.E. Hassaneen, H. Abelaziz, M.A. Khder, Molecules., 2016, 21, 1-17.
- F. Sultana, S.R. Bonam, V.G. Reddy, V.L. Nayak, R. Akunuri, S.R. Routhu, A. Alarifi, M.S.K. Halmuthur, A. Kamal, Bioorganic Chem., 2018, 76, 1-12.
- L.J. Zhange, M.Y. Yang, H. Sunz, Tancx, T.Q. Weng, Wulk, Liuxh, Lett. Drug. Discov., 2014, 11, 1107-1111.
- S.M. Gomha, Bull. Koren. Chem. Soc., 2012, 33, 2985-2990.
- P. Karegoudarp, M.S. Karthikeyan, D.J. Prasad, M. Mahalinga, B.S. Holla, N.S. Kumari, Eur. J. med. Chem., 2008, 43, 261-267.
- B. Ghasemi, M. Najimi, Iranian J. Vet. Med., 2016, 10(1), 47-52.
- S.K. Mohanty, A. Khuntia, M.S.Harika, S.P. Sarangi, D.J. Susmitha, Synthesis Characterization and Antimicrobial Activity of some Oxazole and thiazole derivatives, Human J., 2015, 2(3), 60-66.
- P.K. Deb, R. Kaur, B. Chandrawken, R. Mailavaram, Med. Chem. Res., 2014, 23, 2780-2792.
- G. Thoma, S. Veenstra, R. Strang, J. Blanz, E. Vangrevelinghe, J. Berghausen, C.C. Lee, S.H.G. Zerwe, Bioorg. Med. Chem. Lett., 2015, 25, 20, 4642-4647.
- J.S. Barradas, M.I. Errea, N.B. DAccorso, C.S. Sepulveda, E.B. Damonte, Eur. J. Med. Chem., 2011, 46, 259-264.
- D.S.N. Bikobo, D.C. Vodnar, A. Stana, B. Tiperciuc, C. Nastasa, M. Douchet, O. Oniga, J. Saudi Chem Soc., 2017 21, 861-868.
- M.S. Al-Thebeili, IL Farmaco, 2000, 55, 109-118.
- W.A. El-Sayed, S.M. El-Kosy, O.M. Ali, H.M. Emselm, A.A.H. Abdel Rahman, Acta Poloniae Pharmaceutica-drug Research., 2012, 69, 4, 669-677.
- A.Y. Hassan, Phosphorus, Sulfur, and Silicon., 2009, 184, 2856-2869.
- E.A. Orellana, A.L. Kasinski, Bioprotoc., 2016, 16, 21, 1-7.