Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 5
Synthesis, Characterization and Antibacterial Activities of Novel Thieno, Pyrazol Pyridines and Pyrazolopyrimidine Derivatives
Hussein SH Mohamed*, Mohamed NM Gad, Ali M El-zanaty and Sayed A Ahmed
Department of Chemistry, Faculty of Science, Beni-Suef University, Beni-Suef, 62511, Egypt
- *Corresponding Author:
- Hussein SH Mohamed
Department of Chemistry
Faculty of Science
Beni-Suef University
Beni-Suef, 62511, Egypt
Abstract
Series of new heterocyclic compounds, Theinopyridines, Pyrazolopyridines and pyrazolopyrimidines derivatives were obtained by the reaction of sodium salt of 1-(4,5-diphenyl-1H-pyrrol-3-yl)ethanone with aminopyrazole derivatives, cyanothioacetaamide and cyanoacidhydrazide. The obtained compounds were confirmed by spectroscopic analysis such as Infrared Radiation (IR) and Proton Nuclear Magnetic Resonance (1H-NMR) and mass spectra. The newly synthesized compounds screened for antimicrobial activity against Gram-positive, Gram-negative bacteria and fungi. Pyrazolopyridines and Pyrazolopyrimidines showed anti-microbial, anti-bacterial and anti-fungal activities.
Keywords
1-(4,5-diphenyl-1H-pyrrol-3-yl)ethanone, Thienopyridines, Pyrazolopyridines, Antimicrobial
Introduction
Pyrazolopyridines are medically used as an antimalarial [1-4]. Pyrazolopyridines have antifungal activity [5,6], antibacterial activity [7,8], mantiviral activity and it can be used as a medication to HSV-infected patients [9]. Pyrazolopyridines are also used as an anti-inflammatory activity [10,11], dopamine D3 partial agonists [12], growth inhibition activity against Leishmania amazonensis promastigotes. Also Pyrazolopyridines have an antileishmanial activity [13], anxiolytic activity [14], antiproliferative activity [15], an anti-herpetics [16], antiangiogenic effects against Endothelial Cells (EC) proliferation, migration [17]. Pyrazolopyridines are showing good anti-tumor activity [18]. Amino-pyrazolopyridine has cytotoxic activity against erythroleukemia [19]. Thienopyridines can be used as anti-bacterial agents [20], antimicrobial activity [21,22], antifungal activity [5,23], anti-inflammatory agents [24], anticancer activities [25-27], acytotoxic agent against atumorigenic cell line (potent anti-tumor) [28], Thienopyridines are used as an anti-tumor in three representative human tumor cell lines [29]. Thienopyridines are preventive toleukaemiatumour cell lines [30], have neurotropic activity [31], Lck inhibitors, where Lck is important for initial steps of T cell receptor signaling that excite the production of cytokines [32]. Thienopyridines are reducing conversion of prothrombin to thrombin by inhibition of coagulation enzyme factor Xa [33]. Thioxopyridine stimulate macrophage growth where Macrophagesare a type of white blood cell that engulfs foreign substances and microbes [34]. Thienopyridines are inhibiting VEGFR-2 kinase where VEGF receptors are receptors for vascular endothelial growth factor [35], inhibitor for elongation factor-2 kinase [36], inhibit Herpes Simplex Virus-1 [37], Thienopyridines have antidiabetic activity namely type 2 diabetes [38], have coronary vasodilator and antihypertensive activities [39], anti Hepatocellular carcinoma (HCC) activity and low hepatotoxicity [40]. Thienopyridinesare inhibiting enzymatic and cellular c-Src activities [41]. Thienopyridine derivatives are stimulating osteoblastic differentiation by increasing Alkaline Phosphatase (ALPase) activity [42]. Also Thienopyridines have highly efficacious agents for reducing platelet activation and aggregation [43]. Thienopyridines are inhibiting Hepatitis C Virus HCV [44], and used as CHK1 inhibitors where CHK1 is Serine/threonine-protein kinase [45]. Pyridine-thiones and relate Glycosides are showing no activity against different types of tumor virus [46]. Pyrazolopyrimidines have anti-inflammatory agent [47], decreasing leishmanistatic activity [48]. Pyrazolopyrimidines are potent and selective GPR119 agonists where GPR119 agonists treat of type-2 diabetes and obesity [49]. Pyrazolopyrimidines have antibacterial agents [50], antimicrobial activity [51,52], reducing proliferation of SH-SY5Y human neuroblastoma cells where Neuroblastoma (NB) is a type of cancer that forms in certain types of nerve tissue [53]. Pyrazolopyrimidines have anticancer inhibition activity [54,55], anticancer activity namely anti-proliferative effects on leukemia [56]. Pyrazolopyrimidines are reducing GLUT1 where GLUT1 facilitates the transport of glucose across the plasma membranes of mammalian cells [57]. Pyrazolopyrimidines are acting as antiviral activity toward hepatitis-A virus (HAV) [55] also towards herpes simplex virus type-1 [58]. Pyrazolopyridopyrimidinedione derivatives have antibacterial activity [59]. Aminoquinoline pyrazolopyrimidine are showing antimalarial activities [60].
In this work, we used sodium salt of methyl ketone to obtain the interesting novel Pyrazolopyrimidine, Aminopyridine, Pyrazolopridine and Thienopyridine with anti-microbial activity.
Experimental
Instrumentation
The determination of melting points in open capillary tubes was obtained by Gallenkamp melting point apparatus. The IR spectra (KBr discs) were obtained with a Vertex 70 spectrometer. Proton Nuclear Magnetic Resonance (1H-NMR) spectral was recorded on Bruker AVANCE III 400 MHz spectrometer in Dimethyl sulfoxide (DMSO) solutions and with tetramethylsilane as an internal standard, Mass spectra were recorded on Mass Lynx SCN781 spectrometer TOF mode.
Synthesis
Synthesis of Sodium-3-(4,5-diphenyl-1H-pyrrol-3-yl)prop-3-oxo-1-en-1-olate (2)
[0.01 mol] of sodium methoxide was placed in three necked flask and a mixture 20 ml ether, [0.01 mol] of 1-(2-methyl-4,5-diphenyl-1H-pyrrol- 3-yl)ethanone 1 with [0.01 mol] of ethyl formate was added over it through separating funnel with effective stirring. The produced solid was taken and directly used in the reactions.
Synthesis of 2-mercapto-6-(2-methyl-4,5-diphenyl-1H-pyrrol-3-yl)nicotinonitrile (8)
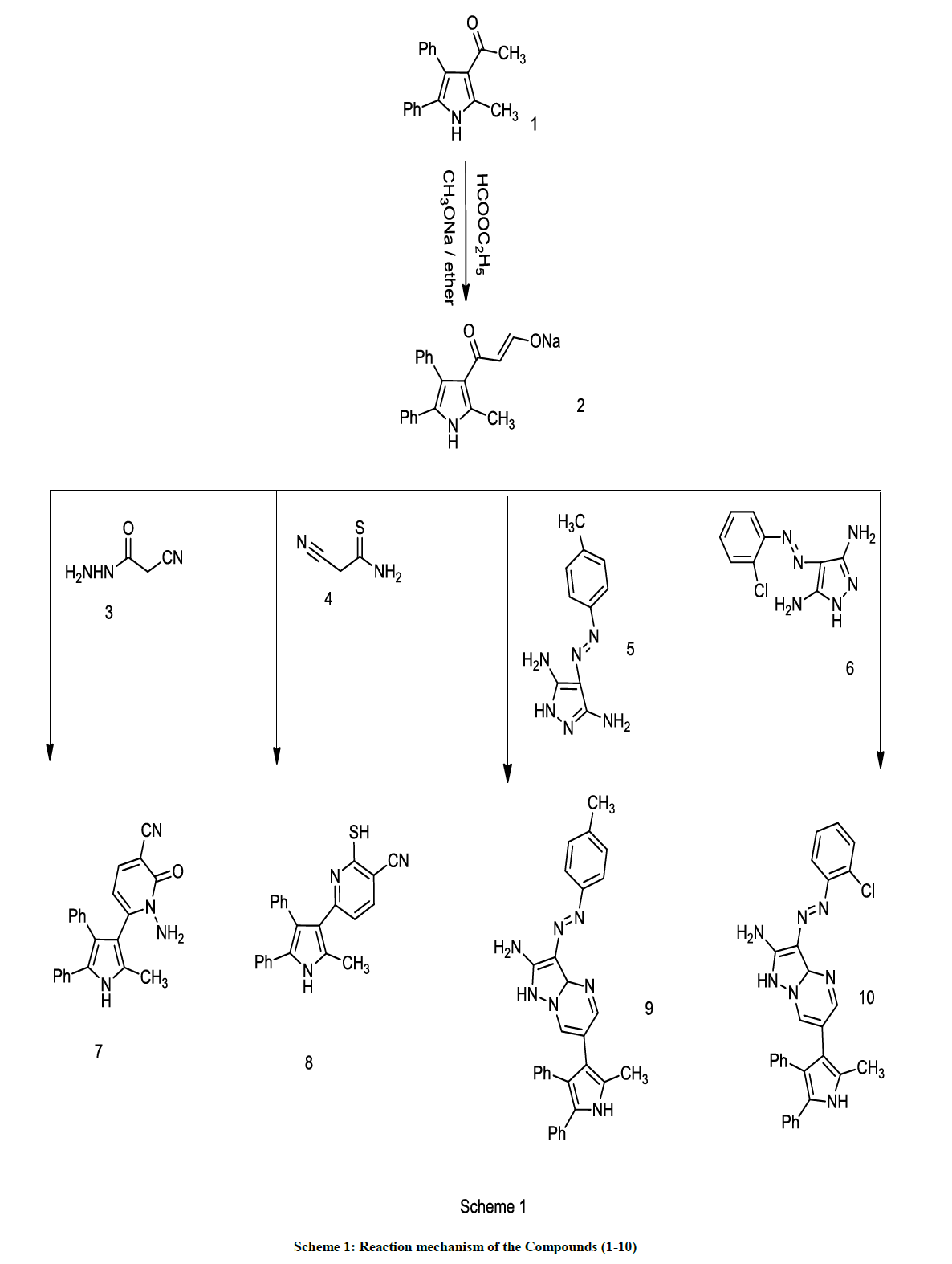
A solution of Sodium-3-(4,5-diphenyl-1H-pyrrol-3-yl)prop-3-oxo-1-en-1-olate 2 refluxed for 10 min with, 2-cyanothioacetamide 4 and piperidine acetate in water. Acetic acid was poured over the hot solution. Finally the produced solid collection was done after filtration and recrystallization from dioxane/ethyl alcohol.
Brown (yield 59 %), m. p. 253-258°C, umax/cm-1 (KBr) 3420 (NH), 3037 (CH aromatic), 2210 (CN). 1H-NMR (DMSO) d = 2.21 (s, 3H, CH3), d = 4.19 (s, 1H, SH), d = 7.22-8.35 (m, 12H, aromatic protons), 9.3 (s, 1H, pyrrole proton, NH). m/z367 (Calcd. for C23H17N3S: C, 75.18; H, 4.66; N, 11.44; S, 8.73%. Found: C, 75.25; H, 4.62; N, 11.40; S, 8.79%.
Procedure for the preparation of compounds 7, 9 and 10
By refluxing a mixture of Sodium-3-(4,5-diphenyl-1H-pyrrol-3-yl)prop-3-oxo-1-en-1-olate 2 and 2-cyanoacetohydrazide 3 or 4-(p-tolyldiazenyl)- 1H-pyrazole-3,5-diamine 5 or 4-((2-chlorophenyl)diazenyl)-1H-pyrazole-3,5-diamine6 and sodium hydroxide in ethanol for 2 h. The produced solid was filtered off and recrystallized from the appropriate solvent.
1-amino-6-(2-methyl-4,5-diphenyl-1H-pyrrol-3-yl)-1H-pyridin-2-one (7): Yellow (yield 64 %), M. P. 140°C, umax/cm-1 (KBr) 3520, 3324 (NH2), 3027, 2997 (CH), 1714 (CO). 1H-NMR (DMSO) d = 2.11 (s, 3H, CH3 ), d = 6.95-7.66(m, 13H, aromatic protons), d = 7.8-7.94 (s, 2H, NH2) and 9.63 (s, 1H, NH pyrrole proton), m/z 341 (Calcd. for C22H19N3O : C, 77.40; H, 5.61; N, 12.31; O, 4.69 %. Found: C, 77.42; H, 5.59; N, 12.30; O, 4.71 %.
7-(2-methyl-4,5-diphenyl-1H-pyrrol-3-yl)-3-(p-tolyldiazenyl)-1,3a-dihydropyrazolo[1,5-a]pyrimidin-2-amine (9): Pale yellow (yield 79 %), m. p. 270°C, umax/cm-1 (KBr) 3515, 3420 (NH2), 3020 (CH aromatic), 1630 (C=N), 1650, 1410 (C=C aromatic). 1H NMR (DMSO) d = 2.10 (s, 3H, CH3), d = 2.38 (s, 3H, CH3), d = 6.43 (s, 2H, NH2), d= 5.67 (s, 1H, NH pyrrole), d = 7.14-8.72 (m, 17H, aromatic protons), 8.93 (s, 1H, pyrazole proton, NH), m/z 485 (Calcd. for C30H27N7 : C, 74.20; H, 5.60; N, 20.19%. Found: C, 74.25; H, 5.58; N, 20.16 %.
3-((2-chlorophenyl)diazenyl)-7-(2-methyl-4,5-diphenyl-1H-pyrrol-3-yl)-1,3a-dihydropyrazolo[1,5-a]pyrimidin-2-amine (10): Yellow (yield 73 %), M. P. 310°C, umax/cm-1 (KBr) 3513, 3298 (NH2), 3045 (CH aromatic), 1626 (C=N), 1652, 1405 (C=C aromatic). 1H-NMR (DMSO) d = d = 2.13 (s, 3H, CH3), d = 5.56 (s, 2H, NH2), d= 6.67 (s, 1H, NH pyrrole), d = 7.22-8.91 (m, 17H, aromatic protons), 9.13 (s, 1H, pyrazole proton, NH), m/z 506 (Calcd. for C29H24ClN7 : C, 68.84; H, 4.78; N, 19.38; Cl, 7.01 %. Found: C, 68, 88; H, 4.73; N, 19.41; Cl, 6.99%.
Procedure for the preparation of compounds 12 and 14
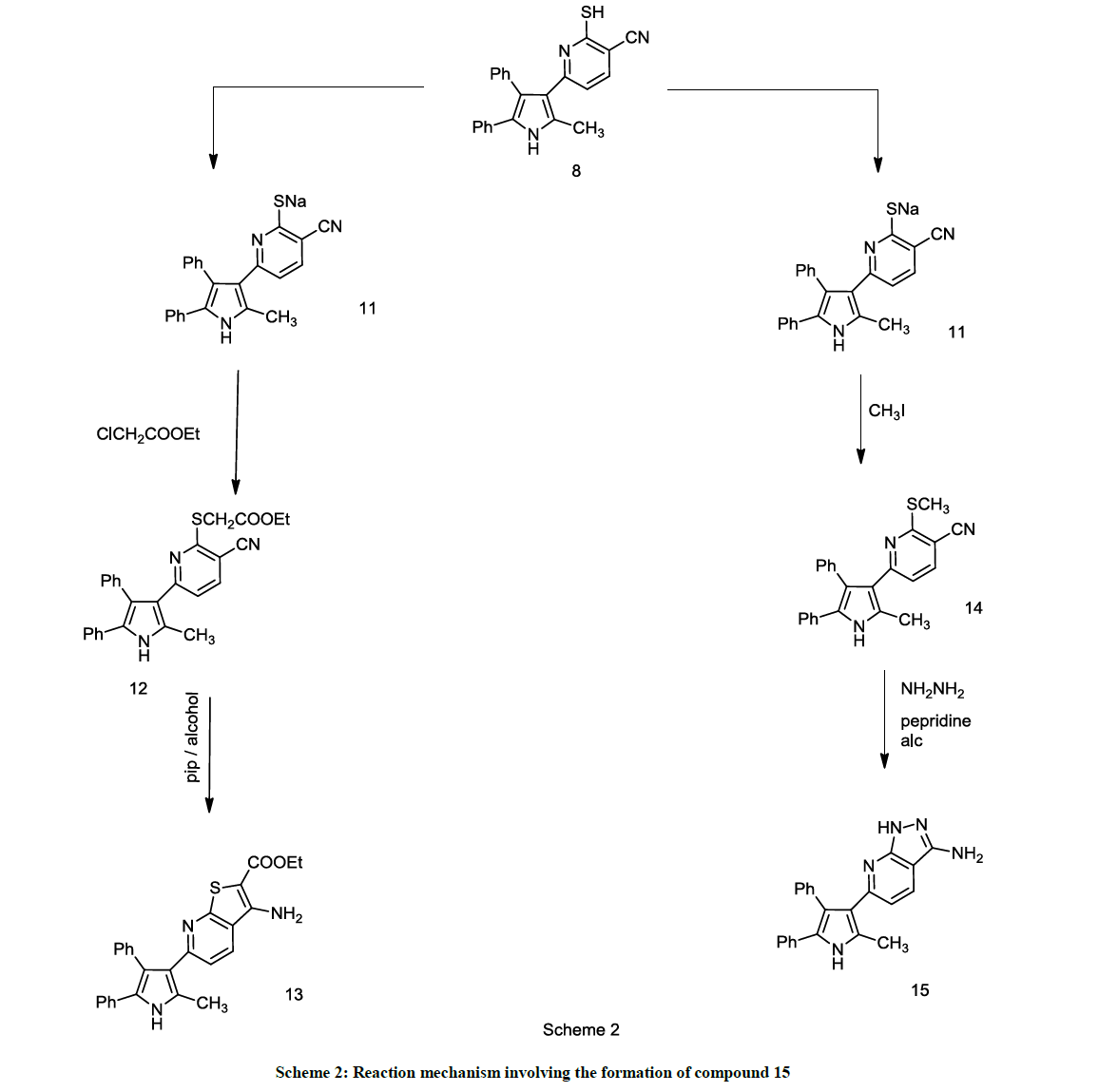
A solution of (0.01 mol.) potassium hydroxide in 20 ml methanol, stirring for completely soluble then add (0.01 mol.) 2-mercapto-6-(2-methyl- 4,5-diphenyl-1H-pyrrol-3-yl)nicotinonitrile 8 was Left stirring until completely soluble then added (0.01 mol.) Ethylchloroacetate and Iodomethane drop by drop with stirring, the mixture was stirred overnight and added over ice/water, finally the resulting solid was filtered then recrystallized from ethanol.
Ethyl 2-((3-cyano-6-(2-methyl-4,5-diphenyl-1H-pyrrol-3-yl)pyridin-2-yl)thio)acetate (12): Page (yield 68%), M. P. 160°C, umax/cm-1 (KBr) 3498 (NH), 3021 (CH aromatic), 2215 (CN), 1739 (CO), 1690 (C=N), 1600, 1436 (C=C aromatic), 1147 (C-O). 1H-NMR (DMSO) d = 1.43 (t, 3H, CH3CH2 ), d= 2.55 (s, 3H, CH3), d= 4.19 (q, 2H, CH2CH3), d= 4.57 (s, 2H, SCH2CO), d = 6.23 (s, 1H, NH pyrrole proton), d = 7.36-8.67 (m, 12H, aromatic protons), m/z 453 (Calcd. for C27H23N3O2S: C, 71.50; H, 5.11; N, 9.26; O, 7.06; S, 7.07 %. Found: C, 71.47; H, 5.13; N, 9.25; O, 7.04; S, 7.09 %.
6-(2-methyl-4,5-diphenyl-1H-pyrrol-3-yl)-2-(methylthio)nicotinonitrile (14): White (yield 66%), M. P. 190°C, umax/cm-1 (KBr) 3490 (NH), 3043 (CH aromatic), 2217(CN), 1678(C=N), 1616, 1423 (C=C aromatic). 1H-NMR (DMSO) d = 2.09 (s, 3H, CH3), d = 2.47 (s, 3H, SCH3), d = 6.09 (s, 1H, NHpyrroleproton), d = 7.13-8.89 (m, 12H, aromatic protons), m/z 381 (Calcd. for C24H19N3S: C, 75.56; H, 5.02; N, 11.02; S, 8.41%. Found: C, 75.53; H, 5.05; N, 11.07; S, 8.39%.
Synthesis of Ethyl 3-amino-6-(2-methyl-4,5-diphenyl-1H-pyrrol-3-yl)thieno[2,3-b]pyridine-2-carboxylate (13)
A solution of (0.01 mol) Ethyl 2-((3-cyano-6-(2-methyl-4,5-diphenyl-1H-pyrrol-3-yl)pyridin-2-yl)thio)acetate 12 was refluxed in ethanol and small catalytic amount of piperidine for 2-3 h. The produced solid was collected and finally recrystallized from ethanol.
Off white (yield 66 %), M. P. 250°C, umax/cm-1 (KBr) 3518, 3322 (NH2), 3035 (CH aromatic), 1732 (CO), 1654 (C=N), 1625, 1409 (C=C aromatic). 1H-NMR (DMSO) d = 2.03 (t, 3H, CH3CH2), d = 2.43 (s, 3H, CH3), d= 4.23 (q, 2H, CH2CH3), d= 5.53 (s, 2H, NH2), d = 6.34 (s, 1H, NH pyrrole proton), d = 7.253-8.97 (m, 12H, aromatic protons), m/z 453 (Calcd. for C27H23N3O2S: C, 71.50; H, 5.11; N, 9.26; S, 7.07; O, 7.06 %. Found: C, 71.47; H, 5.13; N, 9.27; S, 7.08; O, 7.04 %.
Synthesis of 6-(2-methyl-4,5-diphenyl-1H-pyrrol-3-yl)-1H-pyrazolo[3,4-b]pyridin-3-amine (15)
By refluxing a mixture of 6-(2-methyl-4,5-diphenyl-1H-pyrrol-3-yl)-2-(methylthio)nicotinonitrile 14 and excess of hydrazine hydrate and pepridine for 2 h. the reaction solution was added over cold water, and the resulting solid was collected and finally recrystallized from ethanol. Yellow (yield 55 %), M. P. 290°C, umax/cm-1 (KBr) 3543, 3415 (NH2), 3041 (CH aromatic), 1678 (C=N), 1614, 1411 (C=C aromatic). 1H-NMR (DMSO) d = 2.15 (s, 3H, CH3 ), d= 5.53 (s, 2H, NH2), d = 6.04 (s, 1H, CH pyrazole proton), d= 6.77 (s, 1H, NH pyrrole), d = 7.22-8.07 (m, 12H, aromatic protons), m/z 365 (Calcd. for C23H19N5 : C, 75.59; H, 5.24; N, 19.16%. Found: C, 75.55; H, 5.26; N, 19.18%.
Antimicrobial screening
The synthesized compounds were investigated by the exploratory antimicrobial activity to promote the selectivity of these derivatives against test microorganisms. The Regional Center for Mycology and Biotechnology supplied us by all microbial strains from culture collection at Al- Azhar University, Cairo, Egypt. The synthesized compounds tested against Two Gram-positive bacterial species (Bacillus subtilis, Staphylococcus aureus), two Gram negative bacterial species (Escherichia coli, Salmonella typhimurium), two types of fungi (Aspergillus flavus, Syncephalastrum racemosum) and two yeasts ((Candida albicans, Creptococcusneoformans) using a modified well diffusion method (Table 1). Briefly we developed 100 μl bacteria/fungi in 10 ml of new media until bacteria/fungi are 108 cells/ml for bacteria or 105 cells/ml for fungi [61,62]. According to well diffusion method on Mueller-Hinton and Sabaroudagar we developed one hundred μl of microbial onto agar plates. One hundred μL of each sample (at 5 mg/ml) was added in each 10 mm diameter holes cut in the agar gel. We incubate the agar plates at 37°C for 48 h at 28°C (for filamentous fungi) and for 24-48 h (for bacteria and yeast). After that the outgrowth of microorganism will start. We measured the diameters of inhibition region in millimeters. This measurement can be used as standard for the antimicrobial activity. The region containing the chemical will not appear any effect for the organism. The region in which no effect was shown around the disc is termed "Zone of inhibition" or "Clear zone". The regions size in which no outgrowth is to the inhibition activity of the compound under observation. We dissolved the synthesized compounds in (DMSO) which is Negative solvent controls. (DMSO) does not show any effect on development of these microorganisms. Standard antibacterial and antifungal drugs used were Gentamycin and ketoconazole (Sigma Aldrich, USA) at 30 and 50 ug/ml, respectively.
| Tested microorganisms | Sample code | 10 | 7 | 9 | 15 | 13 | Control |
|---|---|---|---|---|---|---|---|
| FUNGI | Ketoconazole | ||||||
| Aspergillus flavus (RCMB 002002) | 14 | NA | 15 | NA | NA | 16 | |
| Candida albicans RCMB 005003 (1) ATCC 10231 | NA | NA | NA | NA | NA | 20 | |
| Cryptococcus neoformas RCMB 0049001 | 15 | 12 | 18 | 16 | 14 | 25 | |
| Syncephalastrum racemosum RCMB 016001 (1) | NA | 15 | 10 | 15 | 15 | 15 | |
| Gram Positive Bacteria | Gentamycin | ||||||
| Staphylococcus aureus (RCMB010010) | 16 | 15 | 11 | 15 | NA | 24 | |
| Bacillus subtilis RCMB 015 (1)NRRL B-543 | 18 | 12 | 15 | 13 | 9 | 26 | |
| Gram Negatvie Bacteria | Gentamycin | ||||||
| Salmonella typhimurium RCMB 006 (1) ATCC 14028 | 17 | 13 | 16 | 14 | NA | 17 | |
| Escherichia coli (RCMB 010052)ATCC 25955 | 16 | 11 | 14 | 13 | NA | 30 | |
Table 1: Antimicrobial activity of the synthesized compounds versus the organisms expressed as inhibition diameter regions in millimeters based on well diffusion assay
Results and Discussion
Chemistry
Reaction of 1-(2-methyl-4,5-diphenyl-1H-pyrrol-3-yl) ethanone 1 [63] with ethyl formate in presence of sodium methoxide afforded Sodium-3- (4,5-diphenyl-1H-pyrrol-3-yl)prop-3-oxo-1-en-1-olate 2 [64,65] which reacted with 2-cyanothioacetamide 4 to form 2-mercapto-6-(2-methyl- 4,5-diphenyl-1H-pyrrol-3-yl)nicotinonitrile 8. Structure of 8 was established by elemental analysis and spectral data. Thus compound 8 is identified by its mass spectrum and showed a molecular formula of C23H17N3S (M+) = 367, 1H-NMR (DMSO) d = 2.21 (s, 3H, CH3), d = 4.19 (s, 1H, SH), d = 7.22-8.35 (m, 12H, aromatic protons), 9.3 (s, 1H, pyrrole proton, NH). Also compound 4 was reacted with 2-cyano-acetohydrazide 3, 4-(p-tolyl-diazenyl)-1H-pyrazole-3,5-diamine 5 or 4-((2-chloro-phenyl)diazenyl)-1H-pyrazole-3,5-diamine 6to produce 1-amino-6-(2-methyl- 4,5-diphenyl-1H-pyrrol-3-yl)-1H-pyridin-2-one 7, 7-(2-methyl-4,5-diphenyl-1H-pyrrol-3-yl)-3-(p-tolyldiazenyl)-1,3a-dihydro-pyrazolo[1,5- a]pyrimidin-2-amine 9 and 3-((2-chloro-phenyl)diazenyl)-7-(2-methyl-4,5-diphenyl-1H-pyrrol-3-yl)-1,3a-dihydro-pyrazolo[1,5-a]pyrimidin-2- amine 10. Compound 10 shows molecular formula C29H24ClN (M+) = 506 and confirmed by 1H NMR (DMSO) d = d = 2.13 (s, 3H, CH3), d = 5.56 (s, 2H, NH2), d= 6.67 (s, 1H, NH pyrrole), d = 7.22-8.91 (m, 17H, aromatic protons), 9.13 (s, 1H, pyrazole proton, NH) (Scheme 1).
2-mercapto-6-(2-methyl-4,5-diphenyl-1H-pyrrol-3-yl)nicotinonitrile 8 reacted with Ethyl-chloroacetate and Iodomethane in presence of KOH with stirring overnight and afforded Ethyl 2-((3-cyano-6-(2-methyl-4,5-diphenyl-1H-pyrrol-3-yl)pyridin-2-yl)thio)acetate 12 and 6-(2-methyl- 4,5-diphenyl-1H-pyrrol-3-yl)-2-(methylthio)nicotinonitrile 14. Compound 14 was confirmed by elemental analysis and spectral analysis which showed molecular formula C24H19N3S (M+) = 381 and confirmed by 1H-NMR (DMSO) d = 2.09 (s, 3H, CH3), d = 2.47 (s, 3H, SCH3), d = 6.09 (s, 1H, NH pyrrole proton), d = 7.13-8.89 (m, 12H, aromatic protons). Ethyl 2-((3-cyano-6-(2-methyl-4,5-diphenyl-1H-pyrrol-3-yl)pyridin-2- yl)thio)acetate 12 was refluxed in ethanol in presence of piperidine for 3 h to yield Ethyl 3-amino-6-(2-methyl-4,5-diphenyl-1H-pyrrol-3- yl)thieno[2,3-b]pyridine-2-carboxylate 13, also 6-(2-methyl-4,5-diphenyl-1H-pyrrol-3-yl)-2 (methylthio) nicotine nitrile 14 refluxed in ethanol with hydrazinehydrate in presence of piperedine for 2 h to form 6-(2-methyl-4,5-diphenyl-1H-pyrrol-3-yl)-1H-pyrazolo[3,4-b]pyridin-3- amine 15. Compound 15 shows molecular formula C23H19N5 (M+) = 365 and 1H-NMR (DMSO) d = 2.15 (s, 3H, CH3), d= 5.53 (s, 2H, NH2), d = 6.04 (s, 1H, CH pyrazole proton), d= 6.77 (s, 1H, NH pyrrole), d = 7.22-8.07 (m, 12H, aromatic protons) (Scheme 2).
Antimicrobial activity
The study and investigation for antibacterial and antifungal agents is always renewed; because of the rapid evolution of the resistance between bacteria and fungi. We have produced different series of antibacterial and antifungal agents. These synthesized compounds were tested for their expected effects against the selected Fungi species, Gram-positive and Gram-negative bacteria.
Antibacterial and antifungal activities
The synthesized products were screened for their antimicrobial activities in vitro towards two Gram-positive bacterial species (B. subtilis, S. aureus), two Gram negative bacterial species (E. coli, S. typhimurium), two types of fungi (A. flavus, S. racemosum) and two yeasts (C. albicans, C. neoformans). Under the same conditions using Gentamycin and ketoconazole as standard antibacterial and antifungal drugs at 30 and 50 ug/ml, respectively. Pyrazolopyrimidine 9, 10 and aminopyridine 7 exhibit high inhibitory effects against Aspergillus flavus. Aminoprydine 7, pyrazolopridine 15 and thienopyridine 13 have no inhibitory effect towards A. flavus. All synthesized compounds have no inhibitory effect towards C. albicans. Pyrazolopyrimidine 9, 10 and pyrazolopridine 15 have moderate effect against C. neoformas. Thienopyridine 13 and pyrazolopridine 15 exhibit high inhibitory effects against S. racemosum, while Pyrazolopyrimidine 9 has moderate effect against S. racemosum. Pyrazolopyrimidine 10 has no inhibitory effect towards S. racemosum. Thienopyridine 13 has no inhibitory effect towards S. aureus, while Pyrazolopyrimidine 9, 10 and pyrazolopridine 15 and aminopyridine 7 have moderate effect against S. aureus. Pyrazolopyrimidine 9, 10 and pyrazolopridine 15 and aminopyridine 7 have moderate effect against B. subtilis. Pyrazolopyrimidine 9, 10 exhibit high inhibitory effects against S. typhimurium while aminopyridine 7 and pyrazolopridine 15 have moderate effect against S. typhimurium. Pyrazolopyrimidine 9, 10 and aminopyridine 7 have moderate effect against E. coli while thienopyridine 13 has no inhibitory effect towards E. coli.
Conclusion
In summary, we have achieved a synthesis of interesting novel, Theinopyridines, Pyrazolopyridines and pyrazolopyrimidines derivatives were synthesized by reaction of sodium salt of 1-(4,5-diphenyl-1H-pyrrol-3-yl)ethanone with aminopyrazole derivatives, cyanothioacetaamide and cyanoacidhydrazide. The newly synthesized compounds were confirmed by spectroscopic analysis and screened for antimicrobial activity. The newly synthesized compounds show anti-microbial, anti-bacterial and anti-fungal activities.
Acknowledgement
The authors are grateful to Professor Ahmed Hafez Elghandour for helping.
References
- C. Le Manach, T. Paquet, C. Brunschwig, M. Njoroge, Z. Han, D.G. Cabrera, S. Bashyam, R. Dhinakaran, D. Taylor, J. Reader, M. Botha, A. Churchyard, S. Lauterbach, T.L. Coetzer, L.M. Birkholtz, S. Meister, E.A. Winzeler, D. Waterson, M.J. Witty, S. Wittlin, M.B. Jiménez-Díaz, M. Santos Martínez, S. Ferrer, I. Angulo-Barturen, L.J. Street, K. Chibale, J. Med. Chem., 2015, 58(21), 8713-22.
- C.M.S. Menezes, C.M.R.S.A., C.R. Rodrigues, E.J. Barreiro, J. Mol. Struct., 2002, 579(1-3), 31-39.
- D. Saini, S. Jain, A. Kumar, N. Jain, EXCLI J., 2016, 15, 730-737.
- T.B. Silva, A.M.R. Bernardino, M.L.G. Ferreira, K.R. Rogerio, L.J.M. Carvalho, N. Boechat, L.C.S. Pinheiro, Bioorg. Med. Chem., 2016, 24(18), 4492-4498.
- N.M. Rateb, S.H. Abdelaziz, H.F. Zohdi, Int. J. Adv. Res., 2014, 2(1), 446-455.
- S. Mohamed Gomha, A. Sami Shawali, A. Osman Abdelhamid, Turk. J. Chem., 2014, 38, 865-879.
- N. Panda, S. Karmakar, A. Jena, Chem. Heterocycl. Compd., 2011, 46(12), 1500.
- E.B. Talaat I. El-Emary, Pharmazie., 1999, 54(2), 106-111.
- B.A. Johns, K.S. Gudmundsson, E.M. Turner, S.H. Allen, V.A. Samano, J.A. Ray, G.A. Freeman, F.L. Boyd Jr, C.J. Sexton, D.W. Selleseth, K.L. Creech, K.R. Moniri, Bioorg. Med. Chem., 2005, 13(7), 2397-2411.
- P.K. Sharma, K. Singh, S. Kumar, P. Kumar, S.N. Dhawan, S. Lal, H. Ulbrich, G. Dannhardt, Medicinal Chemistry Research., 2010, 20(2), 239-244.
- S.B. Bharate, T.R. Mahajan, Y.R. Gole, M. Nambiar, T.T. Matan, A. Kulkarni-Almeida, S. Balachandran, H. Junjappa, A. Balakrishnan, R.A. Vishwakarma, Bioorg. Med. Chem., 2008, 16(15), 7167-7176.
- L. Bettinetti, K. Schlotter, H. Hübner, P. Gmeiner, J. Med. Chem., 2002, 45(21), 4594-4597.
- H. de Mello, A. Echevarria, A.M. Bernardino, M. Canto-Cavalheiro, L.L. Leon, J. Med. Chem., 2004, 47(22), 5427-5432.
- J.B. Patel, J.B. Malick, A.I. Salama, M.E. Goldberg, Pharmacol. Biochem. Behav., 1985, 23, 675-680.
- V. Giannouli, N. Lougiakis, I.K. Kostakis, N. Pouli, P. Marakos, A.L. Skaltsounis, S. Nam, R. Jove, D. Horne, R. Tenta, H. Pratsinis, D. Kletsas, Bioorg. Med. Chem. Lett., 2016, 26(21), 5229-5233.
- B.A. Johns, K.S. Gudmundsson, E.M. Turner, S.H. Allen, D.K. Jung, C.J. Sexton, F.L. Boyd Jr, M. Peel, Tetrahedron., 2003, 59(45), 9001-9011.
- M. Michailidou, V. Giannouli, V. Kotsikoris, O. Papadodima, G. Kontogianni, I.K. Kostakis, N. Lougiakis, A. Chatziioannou, F.N. Kolisis, P. Marakos, N. Pouli, H. Loutrari, Eur. J. Med. Chem., 2016, 121, 143-157.
- M.A. Elneairy, S.M. Eldine, A.S. Mohamed, Der Pharma Chemica., 2015, 7(5), 284-295.
- B. Orlikova, W. Chaouni, M. Schumacher, M. Aadil, M. Diederich, G. Kirsch, Eur. J. Med. Chem., 2014, 85, 450-457.
- H.M.F. Madkour, M.A.I. Salem, M.I. Marzouk, M.E. Azab, N.F.H. Mahmoud, American-Eurasian J. Scientific Res., 2007, 2(2), 161-169.
- A.M. Hussein, F.A. Abu-shanab, E.A. Ishak, Phosphorus. Sulfur. Silicon. Relat. Elem., 2000, 159(1), 55-68.
- F.A. Attaby, M.A. Elneairy, M. Elsayed, Arch. Pharm. Res., 1999, 22(2), 194-201.
- N.M. Rateb, S.H. Abdelaziz, H.F. Zohdi, J. Sulphur. Chem., 2011, 32(4), 345-354.
- K. Madhusudana, B. Shireesha, V.G. Naidu, S. Ramakrishna, B. Narsaiah, A.R. Rao, P.V. Diwan, Eur. J. Pharmacol., 2012, 678(1-3), 48-54.
- S.M. GOMHA, I.M. ABBAS, M.A.A. ELNEAIRY, M.M. ELAASSER, B.K.A. MABROUK, J. Serbian Chemical Society., 2015, 80(10), 1251-1264.
- S.A. Al-Trawneh, M.M. El-Abadelah, J.A. Zahra, S.A. Al-Taweel, F. Zani, M. Incerti, A. Cavazzoni, P. Vicini, Bioorg. Med. Chem., 2011, 19(8), 2541-2548.
- X.X. Zeng, R.L. Zheng, T. Zhou, H.Y. He, J.Y. Liu, Y. Zheng, A.P. Tong, M.L. Xiang, X.R. Song, S.Y. Yang, L.T. Yu, Y.Q. Wei, Y.L. Zhao, L. Yang, Bioorg. Med. Chem. Lett., 2010, 20(21), 6282-6285.
- Hayakawa I., Thienopyridine and benzofuran derivatives as potent anti-tumor agents possessing different structure-activity relationships. Bioorg. Med. Chem. Lett., 2004, 14(13), 3411-3414.
- Queiroz M.J. Novel 6-[(hetero)arylamino]thieno[3,2-b]pyridines: synthesis and antitumoral activities. Eur J Med Chem, 2010. 45(12): pP: 5732-8.
- L. Feng, I. Reynisdottir, J. Reynisson, Eur. J. Med. Chem., 2012, 54, 463-469.
- A. Krauze, S. Ģērmane, O. Eberliņš, I. Šturms, V. Klusā, G. Duburs, 1999, 34(4), 301-310.
- L. Abbott, P. Betschmann, A. Burchat, D.J. Calderwood, H. Davis, P. Hrnciar, G.C. Hirst, B. Li, M. Morytko, K. Mullen, B. Yang, Bioorg. Med. Chem. Lett., 2007, 17(5), 1167-1171.
- M.R. Becker, W.R. Ewing, R.S. Davis, H.W. Pauls, C. Ly, A. Li, H.J. Mason, Y.M. Choi-Sledeski, A.P. Spada, V. Chu, K.D. Brown, D.J. Colussi, R.J. Leadley, R. Bentley, J. Bostwick, C. Kasiewski, S. Morgan, Bioorg. Med. Chem. Lett., 1999, 9(18), 2753-2758.
- N.A. Hamdy, A.M. Gamal-Eldeen, Eur. J. Med. Chem., 2009, 44(11), 4547-4556.
- M.J. Munchhof, J.S. Beebe, J.M. Casavant, B.A. Cooper, J.L. Doty, R.C. Higdon, S.M. Hillerman, C.I. Soderstrom, E.A. Knauth, M.A. Marx, A.M. Rossi, S.B. Sobolov, J. Sun, Bioorg. Med. Chem. Lett., 2004, 14(1), 21-24.
- J.W. Lockman, M.D. Reeder, K. Suzuki, K. Ostanin, R. Hoff, L. Bhoite, H. Austin, V. Baichwal, J.A. Willardsen, Bioorg. Med. Chem. Lett., 2010, 20(7), 2283-2286.
- L.C.S. Pinheiro, J.C. Borges, C.D. Oliveira, V.F. Ferreira, G.A. Romeiro, I.P. Marques, P.A. Abreu, Izabel C. P.P. Frugulheti, C.R. Rodrigues, M.G. Albuquerque, H.C. Castro, A.M.R. Bernardino, Arkivoc., 2008, 14, 77-87.
- R.H. Bahekar, M.R. Jain, P.A. Jadav, V.M. Prajapati, D.N. Patel, A.A. Gupta, A. Sharma, R. Tom, D. Bandyopadhya, H. Modi, P.R. Patel, Bioorg. Med. Chem., 2007, 15(21), 6782-6795.
- I. Adachi, T. Yamamori, Y. Hiramatsu, K. Sakai, S. Mihara, M. Kawakami, M. Masui, O. Uno, M. Ueda, Chem. Pharm. Bull., 1988, 36(11), 4389-4402.
- R.M. Abreu, I.C. Ferreira, R.C. Calhelha, R.T. Lima, M.H. Vasconcelos, F. Adega, R. Chaves, M.J. Queiroz, Eur. J. Med. Chem., 2011, 46(12), 5800-5806.
- I. Pevet, C. Brulé, A. Tizot, A. Gohier, F. Cruzalegui, J.A. Boutin, S. Goldstein, Bioorg. Med. Chem., 2011, 19(8), 2517-2528.
- K. Saito, A. Nakao, T. Shinozuka, K. Shimada, S. Matsui, K. Oizumi, K. Yano, K. Ohata, D. Nakai, Y. Nagai, S. Naito, Bioorg. Med. Chem., 2013, 21(7), 1628-1642.
- P.P. Toth, A. Armani, Current Atherosclerosis Reports., 2009, 11(5), 364-370.
- N.Y. Wang, W.Q. Zuo, Y. Xu, C. Gao, X.X. Zeng, L.D. Zhang, X.Y. You, C.T. Peng, Y. Shen, S.Y. Yang, Y.Q. Wei, L.T. Yu, Bioorg. Med. Chem. Lett., 2014, 24(6), 1581-1588.
- L. Zhao, Y. Zhang, C. Dai, T. Guzi, D. Wiswell, W. Seghezzi, D. Parry, T. Fischmann, M.A. Siddiqui, Bioorg. Med. Chem. Lett., 2010, 20(24), 7216-7221.
- A.M. Attia, G.H. Elgemeie, L.A. Shahada, Tetrahedron., 1997, 53(51), 17441-17448.
- A.H. Abdelazeem, S.A. Abdelatef, M.T. El-Saadi, H.A. Omar, S.I. Khan, C.R. McCurdy, S.M. El-Moghazy, Eur. J. Pharm. Sci., 2014, 62, 197-211.
- J.L. Avila, M.A. Polegre, A. Avila, R.K. Robins, Comparative Biochemistry and Physiology Part C: Comparative Pharmacology., 1986, 83(2), 285-289.
- M. Azimioara, P. Alper, C. Cow, D. Mutnick, V. Nikulin, G. Lelais, J. Mecom, M. McNeill, P.Y. Michellys, Z. Wang, E. Reding, M. Paliotti, J. Li, D. Bao, J. Zoll, Y. Kim, M. Zimmerman, T. Groessl, T. Tuntland, S.B. Joseph, P. McNamara, H.M. Seidel, R. Epple, Bioorg. Med. Chem. Lett., 2014, 24(23), 5478-5483.
- G.M. Goshu, D. Ghose, J.M. Bain, P.G. Pierce, D.W. Begley, S.N. Hewitt, H.S. Udell, P.J. Myler, R. Meganathan, T.J. Hagen, Bioorg. Med. Chem. Lett., 2015, 25(24), 5699-5704.
- C.N. Khobragade, R.G. Bodade, S.G. Konda, B.S. Dawane, A.V. Manwar, Eur. J. Med. Chem., 2010, 45(4), 1635-1638.
- B.S. Holla, M. Mahalinga, M.S. Karthikeyan, P.M. Akberali, N.S. Shetty, Bioorg. Med. Chem., 2006, 14(6), 2040-2047.
- N. Michele, M. Celano, M. Jessica, D. Russo, Toxicol Lett., 2007, 172, S232.
- M.M. Kandeel, L.W. Mohamed, M.K. Abd El Hamid, A.T. Negmeldin, Sci. Pharm., 2012, 80(3), 531-545.
- A. Rahmouni, S. Souiei, M.A. Belkacem, A. Romdhane, J. Bouajila, H. Ben Jannet, Bioorg. Chem., 2016, 66, 160-168.
- S. Schenone, C. Brullo, O. Bruno, F. Bondavalli, L. Mosti, G. Maga, E. Crespan, F. Carraro, F. Manetti, C. Tintori, M. Botta, Eur. J. Med. Chem., 2008, 43(12), 2665-2676.
- H. Siebeneicher, M. Bauser, B. Buchmann, I. Heisler, T. Müller, R. Neuhaus, H. Rehwinkel, J. Telser, L. Zorn, Bioorg. Med. Chem. Lett., 2016, 26(7), 1732-1737.
- A.E. Rashad, M.I. Hegab, R.E. Abdel-Megeid, N. Fathalla, F.M. Abdel-Megeid, Eur. J. Med. Chem., 2009, 44(8), 3285-3292.
- A. Bazgir, M.M. Khanaposhtani, A.A. Soorki, Bioorg. Med. Chem. Lett., 2008, 18(21), 5800-5803.
- M. Kannan, A.V. Raichurkar, F.R. Khan, P.S. Iyer, Bioorg. Med. Chem. Lett., 2015, 25(5), 1100-1103.
- M.A. Pfaller, L. Burmeister, M.S. Bartlett, M.G. Rinaldi, J. Clin. Microbiol., 1988, 26(8), 1437-1441.
- H.S. Ibrahim, W.M. Eldehna, H.A. Abdel-Aziz, M.M. Elaasser, M.M. Abdel-Aziz, Eur. J. Med. Chem., 2014, 85, 480-486.
- S.I. Bhat, D.R. Trivedi, Tetrahedron Lett., 2013, 54(41), 5577-5582.
- S.A. Ahmed, H.S. Elgendy, Int. J., 2014, 2(5), 865-876.
- S.A. Ahmed, O.M. Ahmed, H.S. Elgendy, J. Pharm. Res., 2014, 8(9), 1303-1313.