Research Article - Der Pharma Chemica ( 2021) Volume 13, Issue 4
Synthesis, Characterization and Antioxidant Activity of pyrimidinone Derivatives
Jyothi N Rao1* and Sujatha K22Department of Chemistry, Karnataka University, Dharwad-580003 Karnataka, India
Jyothi N Rao, Department of PG Chemistry, St Aloysius College (Autonomous) Mangaluru-575003 Karnataka, India, Email: jyothirao@staloysius.edu.in
Received: 10-Dec-2020 Accepted Date: Apr 22, 2021 ; Published: 30-Apr-2021
Abstract
Three component Biginelli reactions is carried out using aromatic aldehydes, aliphatic amides and 1, 3-dicarbonyl compound to afford corresponding dihydropyrimidinones in the presence of MgBr2 as an efficient catalyst. The advantage of this method includes high yield, environment friendly, reduced reaction time and operational simplicity. Representative compounds of the synthesized products were tested and evaluated as antioxidant agents. Most of the synthesised compounds have potent antioxidant activity.
Keywords
Biginelli reaction, Dihydropyrimidinones, Scavenging activity, Green synthesis, Antioxidant, DPPH assay
Introduction
In the year 1893, Italian Chemist PietroBiginelli proposed a multicomponent reaction. He used ethylacetoacetate, benzaldehyde and urea in ethanol to synthesize dihydropyrimidinones. The acid used here was hydrochloric acid [1-3]. Even though he synthesised this heterocyclic compound, the yield was very less. So scientist started to think about new methodologies for the effective synthesis of this compound.
The main reagents used for this reaction was aromatic aldehyde, urea or thiourea and 1, 3 -carbonyl compound (mainly esters). The product formed from this reaction was Dihydropyrimidinones or DHPMs and their derivatives. These are very important pharmacologically active molecule with a wide range of application in that field. Some application involves antihypertensive agents, inhibitors of fatty acid transporters, calcium channel blockers, α1a - adrenergic antagonists, in mitotic kinesin inhibition, etc. A potent anti- HIV activity (HIVgp-120-CD4 inhibition) is shown by batzelladine alkaloids which contains a dihydropyrimidinone core.
Variations in the reagents, i.e. the change in the substitutions of aromatic aldehyde, using different 1,3 - carbonyl compounds, extend the scope of the original multicomponent resulting in the preparation of many variety of dihydropyrimidinone molecules. Several alkaloids containing this molecule unit have been isolated from marine sources which exhibit so many biological activities [4].
Its biological investigation shows that these varieties of molecules shows different activities like antiviral, antitumor, anti-inflammatory, antiproliferative, antibacterial, antitubercular, antifungaletc. Similarly, the structural core of quinoline is frequently associated with medicinal applications such as anticancer, antimicrobial, HIV-1 integrase inhibition, HIV protease inhibitors, antileishmanial activity, NK-3 receptor antagonists, PLT antagonists, and antimalarial activity [5].
Fundamental targets of organic synthesis are, to synthesis or perform a chemical transformation using three or more components in a single reaction by a catalytic process, avoiding stoichiometric toxic reagents, avoiding expensive purification technique and to avoid usage of large amount of solvents [6]. Therefore, this reaction has received many reviewed interest.
Several improved procedures are reported. Substituted hydropyrimidinones synthesis can be done by using various protic and Lewis acids like, AcOH [7], HCl [8], LiBr [9] etc. Most recently catalysts like polyphosphosphate ester (PPE)[10] ionic liquids [11] montmorillonite KSF [12], lanthanide triflate[13] etc were used for the one pot solvent free synthesis of the molecules. Some of the above processes like reaction using HCl, AcOH catalyst are microwave irradiated to accelerate the reaction. However, many of these reactions involve expensive reagents, needed longer period of time (to complete reaction), strongly acidic conditions, unsatisfactory yield etc, in spite of their potential utility [14].
Magnesium bromide, in recent years, in additives or activated complexes is noticed as a powerful catalyst for the effective transformations such as condensation [15], cyclopolymerisation reactions [16-17] etc. The challenge for a sustainable environment calls for clean procedures that can avoid using harmful organic solvents [18]
Here the reaction we are preceding is the solvent free, i.e., we are not using an external solvent to precede the reaction. Recently, researcher has tried to find an environmentally friendly improved procedure for the Biginelli reaction to achieve high yields under solvent free conditions and they found that magnesium bromide which is an inexpensive and easily available chemical, which can be used as a catalyst result in high yield of the final product for this Biginelli reaction with a short period of time [19].
Experimental
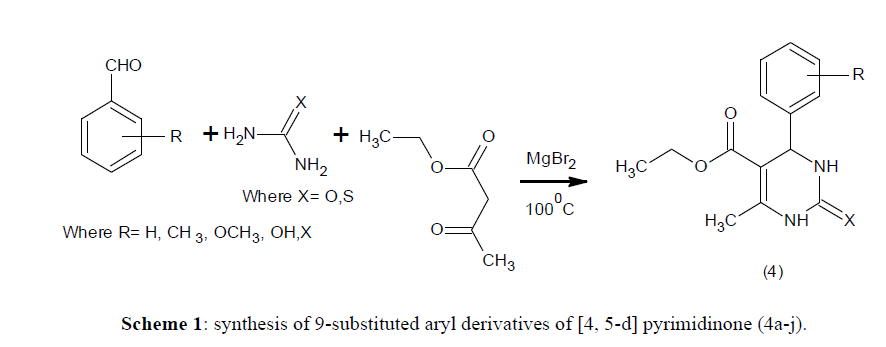
All reactions were carried out using oven dried glasswares. All the chemicals (reagent and solvent) were purchased from Ranbaxy Chemicals Ltd. The purity of these chemicals was 90-99% and was used without further purification and distillation. 1H-NMR and 13C NMR (400 MHz) spectra were recorded on a Bruker Advance Spectrophotometer. The chemical shift in 1H NMR and 13 C NMR were reported using ppm and deuterated chloroform (CDCl3) was used as NMR solvent. Thin layer chromatography (TLC) was analysed on thin layer aluminium plate of 0.2 mm Merck - precoated silica gel. The spots obtained were observed under UV light (254 nm). Column chromatography (CC) was performed using silica gel Merck 70-230mesh. Recrystallization of the compounds was performed using ethanol. Melting points of individual compound was recorded using Leica Galen III Kofler melting point apparatus and were uncorrected. (Scheme 1)
General procedure for the synthesis of 9-substituted aryl derivatives of [4, 5-d] pyrimidinone (4a-j)
A mixture of ethyl acetoacetate (2 mmol), substituted aromatic aldehydes (2 mmol) aliphatic amides (2 mmol) and magnesium bromide (0.2 mmol) was heated at 100°C with stirring until the mixture turned to solid mass (45 min). After completion (monitored by TLC), the solid was cooled to room temperature and poured onto crushed ice (20 g) and stirred for 10 min. The crude product was filtered, washed with cold water and dried then recrystallized from ethanol.
Results and Discussions
The compounds were characterised by IR (Infra-red) Spectroscopy, 1H-NMR (Proton Nuclear Magnetic Resonance) Spectroscopy and 13C-NMR (Carbon -13 Nuclear Magnetic Resonance) and Mass Spectroscopy and DPPH Scavenging Activity.
9-phenyl- [4,5-d] pyrimidinione (4a)
Colourless solid, yield 0.16g (91%), mp 54-56°C IR (KBr/cm-1): 2983 (C H), 1894,1700 ( 2 C=O), 1591(C - C), 1490, 3402 (N–H)
1H NMR ( 400MHz DMSO-d6 ppm) 1.06(t,3H,CH3 of ethyl group), 4.01 (q,2H,CH2 of ethyl group), 2.45 (s,3H,CH3 group of pyrimidine), 6.5-8.2 ( m,9H,Ar-H),10.2 and 11.2( 2 br, 2H,-NH )
13C-NMR (400 MHz, DMSO-d6 ppm) 165,160,155,148,142,,120,90,55,50,42,32
Mass: Molecular ion peak was observed at m/z 258 (M+ + 1)
9-(4-methoxy phenyl) - [4,5-d] pyrmidinione (4b)
Yellow Solid, Yield 0.17 g (94%), mp 89-90°C IR (KBr/cm-1) 3423, 3210 ( 2 -NH), 1725, 1670 (2 C=O), 1268 (O-R)
1H-NMR (400MHz,DMSO-d6 ppm) 1.03 (t,3H,CH3 of ethyl group), 3.89 (q,2H,CH2 of ethyl group), 2.45 (s,3H,CH3 group of pyrimidine), 3.78 (s,3H,- Ar-OCH3),7.0-8.0 (m,8H,Ar-H)11.2 and 11.4 (2bs,2H,-NH)
13C-NMR (400 MHz, DMSO-d6 ppm) 164,162,155,148,142,129,126,90,55,50,42,30
Mass: Molecular ion peak was observed at m/z 289 (M+ + 1)
9-(4-bromophenyl)- [ 4,5-d] pyrimidinione (4c)
Colourless solid, Yield 0.19g (95%) mp 156-58°C
IR (KBr/cm-1) 3512, 3140 (2 -NH), 1680, 1610 (2 C=O);
1H-NMR (400MHz, DMSO-d6 ppm) 1.56 (t, 3H,CH3of ethyl group), 3.74 (q,2H,CH2 of ethyl group), 2.89 (s,3H,CH3 group of pyrimidine), , 7.2- 8.5 (m, 8H, Ar-H), 10.2 and 11.4 (2 br, 2H,-NH);
13C-(400MHz DMSOd6,ppm) 166,165,164,160,150,148,130,132,126,90,50,45,40,30
Mass: Molecular ion peak was observed at m/z 337 (M+ + 1)
9-(4-fluorophenyl) – [4,5-d] pyrimidinone (4d)
Colourless solid, Yield 1.1g (78%) MP 196-97°C
IR (KBr/cm-1) 3271,3150 (2-NH), 1670, 1620 (2 C=O);
1H-NMR (400MHz,DMSO-d6,ppm ) 1.88 (t,3H,CH3 of ethyl group), 3.32(q,2H,CH2 of ethyl group), 2.46 (s,3H,CH3 group of pyrimidine), 7.1-8.4 (m,8H,Ar-H),10.1 and11.2 (2 br,2H,-NH)
13C NMR (400MHz,DMSO-d6/ppm),166,162,160,155,145,135,130,120,90,55,40,32
Mass: Molecular ion peak was observed at m/z 293 (M+ + 1)
Antioxidant Activity
DPPH (1, 1-diphenyl-2-picrylhydrazyl) radical scavenging activity
The reaction cocktail was prepared by mixing individual newly synthesised organic compounds is added equal volume of 0.1mM solution of DPPH radical in absolute ethanol. After 20 minutes of incubation at room temperature, the DPPH reduction was calculated by reading absorbance at 517nm using UV-Visible spectrophotometer. Ascorbic acid (1mM) was used as reference compound. The compound (4d and 4j) shows remarkable antioxidant activity against DDPH radical scavenging activity with reference of ascorbic acid (91.4 ± 0.021) (Table 1).
| Entry | Compound Code | % Radical scavenging activity | |
|---|---|---|---|
| DPPH radical Scavenging | OH radical scavenging | ||
| 1 | 4a | 45.4 ± 0.65 | 52.0 ± 1.01m |
| 2 | 4b | 62.6 ± 0.66 | 64.2 ± 1.42 |
| 3 | 4c | 60.9 ± 1.46 | 62.0 ± 1.00 |
| 4 | 4d | 86.2 ± 1.06 | 84.1 ± 0.44 |
| 5 | 4e | 80.6 ± 1.40 | 83.6 ± 1.55 |
| 6 | 4f | 78.0 ± 1.35 | 76.0 ± 1.68 |
| 7 | 4g | 80.8 ± 1.20 | 85.2 ± 1.06 |
| 8 | 4h | 62.4 ± 1.60 | 55.0 ± 1.04 |
| 9 | 4i | 66.0 ± 1.01 | 58.1 ± 1.49 |
| 10 | 4j | 84.6 ± 1.26 | 85.0 ± 1.98 |
| 11 | Ascorbic Acid (standard) | 91.4 ± 0.021 | 89.5 ± 1.98 |
Table 1: Antioxidant activity of tested compounds (4a-4j).
OH radical scavenging assay
Hydroxyl radicals scavenging activity was measured with Fenton’s reaction. The reaction mixture contained 60 l of FeCl2 (1mM).2.4ml of phosphate buffer (pH 7.8), 150 μl of 0.17 M H2O2 and 1.5 ml of individual newly synthesized organic compounds (1 mM). The reaction mixture was kept at room temperature for 5 minutes incubation and absorbance was recorded at 560nm using UV-Visible spectrophotometer. Ascorbic acid (1mM) was used as the reference compound. The compound (4d, 4g and 4j) show good OH radical scavenging activity as compared with Ascorbic acid (89.5 ± 0.021) (Table 1).
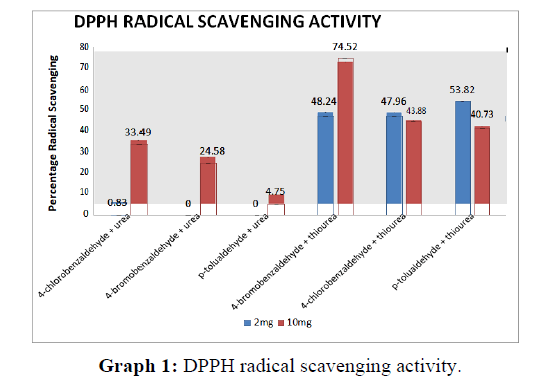
From the above graph, the compound synthesised from the reagents 4 - bromobenzaldehyde and thiourea has got highest scavenging activity for both 2 mg and 10 mg concentrations. The compound synthesised using urea does not show much scavenging activity. Among them 4- chlorobenzaldehyde shows the maximum activity (Graph 1).
Conclusion
Based on results obtained, we have developed a green, efficient and eco-friendly synthesis for the preparation of aryl substituted pyrimidinone (4a-j) derivatives by one pot three component condensation reactions of substituted aromatic aldehydes, ethyl acetoacetate and aliphatic amides in the presence of magnesium bromide. The product can be easily isolated by simple workup technique, short time, less expensive, ambient reaction condition and give excellent isolated yields. These synthesized compounds screened for antioxidant activity. Most of the synthesised compounds have potent antioxidant activity.
Acknowledgment
The authors are grateful to the St Aloysius College (Autonomous), Mangalore for financial assistance and Lab. facility and authors also thankful to Indian Institute of Science for spectral and analytical data.
References
- Suresh AS and Jagir S Sandhu. Discovery. 2012, 63: p. 133.
- Safari Javad AN and Zohre Zarnegar. RSC Adv. 2013.
- Patil A, Kumar NV, Kokke WC et al., J Org Chem. 1995, 60: p. 1182.
- Atwal KS, Rovnyak GC, O’Reilly et al., J Org Chem. 1989, 54: p. 5898.
- Suresh AS and Jagir S Sandhu. Discovery. 2012, 63: p. 133.
- Mizuno N and Misono M. Chem Rev. 1981, 98: p. 99.
- Yadav JS, Reddy BVS and Ramalingam TJ. Chem Res Synop. 2001, p. 1341-1345.
- Xue S, Shen YC Li, Shen Y L et al., Chin J Chem. 2002, p. 85.
- Gourhari M, Pradip K, Chandrani G. Tetrahedron Lett. 2003, 44: p. 2757.
- Kappe CO, Kumar D, Sharma R. Synthesis. 1999, p. 1799.
- Peng J and Deng Y. Tetrahedron Lett. 2000, 42: p. 917.
- Bigi F, Carloni S, Frullanti B et al., Tetrahedron Lett. 1993, 40: p. 465- 468.
- Y QianMa, WangCL, Yang M. J Org Chem. 2000, 65: p. 3864.
- Salehi, Hojatollah, Qing-Xiang et al., Chinese J Chem. 2005, 23: p. 91-97.
- Kashima C, Takahashi K, Fukusaka K. J Heterocycle Chem. 1995, 32: p. 1775.
- Jui WH, Chair DC and Man KL. Tetrahedron Lett. 1999, 40: p. 8647.
- Matsumoto A and Nakamura S. J Appl Polym Sci. 1999, 74: p. 290.
- Anastas PT and Warner JC. Green Chemistry. 1998, 1: p. 129.
- AlehiH and Guo QX. Synth Commun. 2004, 34: p. 171.