Research Article - Der Pharma Chemica ( 2021) Volume 13, Issue 4
Synthesis, Characterization and Biological studies on 3d-metal complexes of 5-[(1, 5-dimethyl-3-oxo-2-phenyl-2, 3-dihydro-1H-pyrazol-4-yl) diazenyl]-1-ethyl-6-hydroxy-4-methyl-2-oxo-1, 2-dihydropyridine-3-carbonitrile
Shridhar P Melkeri, Parameshwara Naik*, G. Krishnamurthy, Prabhaker Walmik and Priyarani RS2Department of Chemistry, Kuvempu University, Shankaraghatta, Karnataka-577451, India
Parameshwara Naik, Department of Chemistry, Sahyadri Science College, Kuvempu University, Shivamogga, Karnataka-577201, India, Email: parashchem@gmail.com
Abstract
N-hetorocyclic ligand 5-[(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl) diazenyl]-1-ethyl-6-hydroxy-4-methyl-2-oxo-1,2Dihydropyridine-3-carbonitrile (L) and a few of their transition metal complexes have been synthesised and characterized by different physico-chemical techniques. The electronic and magnetic susceptibility measurements suggest an octahedral geometry for all the complexes excepting Zn(II) complex which possess tetrahedral geometry. The results of thermo-gravimetric analysis showed that the synthesized complexes have thermal stability and confirms the absence of coordinated or lattice water molecules. The bio-potency of all these compounds was investigated by using antimicrobial, antimycobacterial and cytotoxic activities. All the transition metal complexes were noticeably exhibited inhibition properties against the tested microbes.Keywords
Antipyrine, transition metal complexes, antimicrobial studies cytotoxic activities
Introduction
The development of multicurative drugs by the use of coordination compounds especially the complexes containing heterocyclic systems is an advanced research in the field of bio-inorganic and medicinal chemistry [1-5]. The use of metal based drugs for the treatment of various diseases not only cure the particular disease but also suppress the high toxicity and directs to identify the various mechanistic paths to overcome the clinical problems [6-9]. In order to achieve the above impending challenges, researchers across the globe trying to design metal based drugs having heterocyclic ligand system [10-12]. Among the general ligating systems known, the azo linked heterocyclic molecules are largely used in the preparation of transition metal chelates due to their versatile properties [13]. They readily form stable complexes with different coordination numbers and exhibit brilliant shades of colour due to the presence of azo linkage along with the metal ions [14]. The presence of heteroatoms, pi electrons and extended conjugation still enhances the various physical, optical, biological and electrochemical properties of the molecules [15-17].
The azo N-heterocycles exhibited versatile behaviour towards biological, electrochemical, optical and other industrial applications [18-21]. Furthermore, these can also be helpful as better chelating agents and can be able to form stable complexes with coordination number 4 and 6. Recent reports revealed that the metal chelates having pyridone nucleus proved to be efficient antimicrobial, antitubercular, anticancer, and anti-inflammatory agents [22-23]. Thus, in this paper we made an attempt to synthesize transition metal complexes containing pyridone moiety and studied their biochemical properties.
In continuation of our previous research work we have synthesised some heterocyclic compounds and characterized them by various spectral techniques. The biological activities of the ligand and their metal complexes have been evaluated. We observed that, the synthesised complexes exhibited excellent biological activity.
Experimental
Materials and methods
The chemicals, reagents and starting materials used for the synthesis of ligand and its metal complexes were obtained commercially from Sigma Aldrich Chemical Company and Spectrochem and they were used without further purification. The thin layer chromatography was used to monitor the progress of the reaction and also to check the purity of the final compounds. The melting point of the newly prepared compounds was measured on an electro-thermal apparatus by using open capillary tubes and is uncorrected.
The analytical techniques are essential in order to understand the structural features of the newly obtained molecules. The percentage of elements present in the molecules were analysed by Vario EL III CHN analyser. The important functional groups of the synthesized molecules were identified by Thermo fisher-FTIR spectrometer in the region 4000-400 cm-1. The electronic spectra were obtained from the Elico-SL 164 double beam spectrophotometer in pure DMSO (10-6 M) in the wavelength range of 200-800 nm. The 1HNMR spectrum of the synthesis ligand (L) was obtained at room temperature by using Avance III Instrument in DMSO-d6 solvent within the range of 0-16 δ or ppm. The liquid-chromatographic mass spectra of the compounds were recorded in LCMS 2010, SHIMADZU mass analyser. The thermal analysis of the metal complexes was studied in an inert atmosphere on a Perkin Elmer STA 6000 thermal analyser at the heating rate of 10oC min-1 from 30-800oC.
Synthesis of ligand (L)
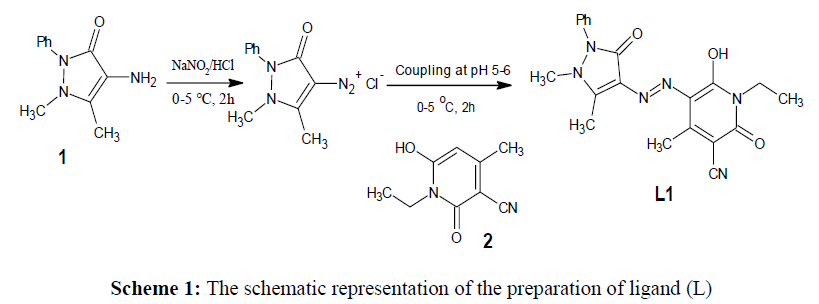
We have synthesized a ligand derived from 4-amino antipyrine by simple diazo-coupling method in an acidic medium at lower temperature. It is a simple, convenient and economical method of electrophilic substitution reaction between an aromatic amine with a strong nucleophile in acidic medium at 0-5oC as described in earlier reports [24-31]. An accurately weighed 0.002 mol solution of 4-aminoantipyrine (1) in dilute HCl was added to the cold solution of the NaNO2 in 2 mL H2SO4 with constant stirring at 0-5oC for 2 h. Then, the formed diazonium salt solution was slowly added to the previously cooled solution of a coupling component ethylpyridone (2) (0.002 mol) in 5 mL aqueous KOH and stirred for another 2 h at the same temperature. The pH of the reaction mixture was adjusted to 5-6 by adding required volume of carbonate solution and the obtained orangered coloured precipitate was filtered off, washed with water and dried in a vacuum desiccator. The final compound was recrystallized from ethanol. The overall reaction pathway represented in the scheme 1.
Synthesis of metal complexes
In a 500 mL round bottomed flask, ethanolic solution of the L (0.0003 g) was taken and refluxed on water bath, to this the ethanoilic solution copper acetate was added. The resulting solution L was refluxed for 6-7 h to afford the corresponding metal complex. The obtained coloured complex was filtered, washed with water and dried in a vacuum desiccator. Similarly, same procedure was followed for the preparation of different metal complexes. The melting point of the prepared complexes was determined and compared with the ligand.
Biological investigation
Antimicrobial activity
The metal chelates derived from heterocyclic azo dyes have potential inhibiting properties against various microbial strains. The azo-metal chelates can be more important bio-molecules in controlling the diseases caused by the microbes and it is also proven by the earlier reports [32]. Therefore, attempt has been made to check the antimicrobial properties of the synthesized azo dye ligand and its metal complexes by modified tube dilution method against three bacterial strains Escherichia Coli, Staphylococcus aureus, Klebsiella pneumoniae, Candida albicans, Aspergillus flavus, Aspergillus fumigatusas. The streptomycin and fluconazole were being the positive control and DMSO is the negative control for this activity. The final results of the study were recorded as the minimum inhibitory concentration (MIC) and are interpreted in the results and discussion section
Anti-mycobacterial activity
The anti-tubercular assay for the newly prepared compounds was carried out by microplate blue Almar assay [34]. It’s a non-toxic protocol, uses thermally stable reagent and have good correlation with the proportional and BACTEC radiometric assay. The Mycobacterium tuberculosis (Vaccine strain, H37 RV strain) with ATCC No- 27294 was used for the anti-TB studies of the newly synthesized molecules. The previously sterilized 96-well plate was taken and 200 L of sterile deionized water was added to the outer perimeter wells of the plate to minimize the evaporation of the media and in this case the Middlebrook 7H9 broth was used as a medium for the bacterial growth. In an experimental protocol, 100 L Middlebrook 7H9 broth was added to each well of the plate and the solutions of the test compounds in DMSO was added to the well. The serial dilution of the target compounds was directly made on to the plate and the final drug concentrations are in the range 100 to 0.2 μg/mL. Then, the plate was covered with and incubated for five days at 37oC. After completion of the incubation, the each well of the plate was treated with 25 L of a mixture of 1:1 Almar Blue reagent and 10% tween 80 and again incubated for 24 h at the same temperature. The result of the study was indicated by change in the colour of the well, if the colour change from pink to blue it is inferred that the bacterial growth suppression and pink colour suggests the growth of the bacteria. These results are tabulated in the form of MIC (minimum inhibitory concentration) and compared with the standard drugs (Isoniazid–1.6 μg/mL, Ethambutol–3.2 μg/mL, Pyrazinamide–3.125μg/mL, Rifampicin–0.8 μg/mL and Streptomycin– 0.8μg/mL).
Anticancer study
The anticancerousbehaviour of the newly synthesized ligand and its complexes was explored by the well-known spectrophotometric method MTT assay against different cell lines like K562, A549 and MDA-MB-231. The 3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide (MTT) assay is an simple colorimetric method to measure the cytotoxicity of the infected cells and the detailed experimental protocol adopted for this activity has been referred from the literature [33]. The results of the study was recorded in terms of IC50 (M) with varying concentrations of the target compounds in DMSO solvent and here we used Leibovitz’s L-15, Ham’s F-12K, and RPMI 1640 medium for subculture of the cell lines which are initially procured from National Centre for Cell Science (NCCS), Pune, India.
Results and Discussion
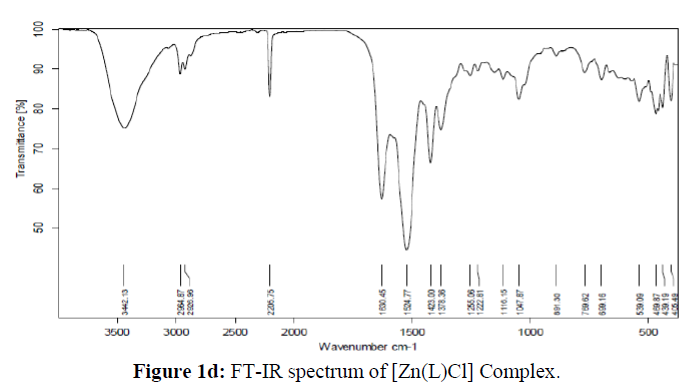
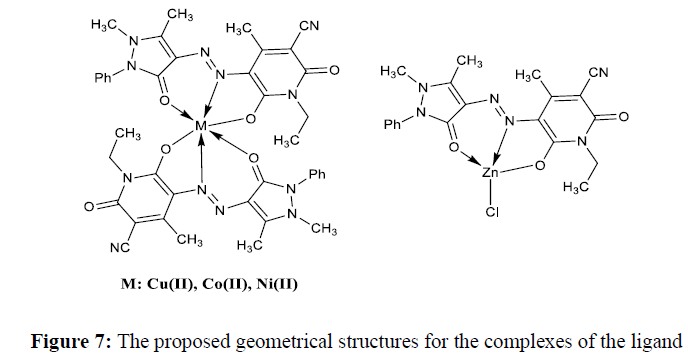
The main objective of the present work is to focus on the structural diversity and its impact on the pharmacological behaviour of the transition metal complexes in comparison with the azo dye ligand. As from the preliminary observation, all the synthesized complexes are exhibited the brilliant colour different from that of the ligand and are stable, non-hygroscopic also soluble in common organic solvents. The spectral, analytical data and preliminary observations indicated that the complexes formed in 1:2. metal: ligand ratio with general formula [M(L)2] for all the complexes except the zinc complex which has [Zn(L)Cl1], The general analytical data of all the compounds is given in the Table 1 and spectra are showed in (Figures 1a-1d)
| Comp.No. | Mol. Formula | Mol. Wt. | Colour | M. P. (oC) | Elemental analysis | |||
|---|---|---|---|---|---|---|---|---|
| C | H | N | M | |||||
| L | C20H20N6O3 | 392.41 | Brown | 142 | 61.21 (61.16) | 5.14 (5.02) | 21.42 (21.38) | - |
| (Cu(L)2) | C40H38CuN12O6 | 846.35 | Orange | >300 | 56.76 (56.66) | 4.53 (4.46) | 19.86 (19.78) | 7.51 (7.44) |
| [Co(L)2] | C40H38CoN12O6 | 841.73 | Black | >300 | 57.08 (56.98) | 4.55 (4.22) | 19.97 (19.82) | 7.00 (6.88) |
| [Ni(L)2] | C40H38NiN12O6 | 841.49 | Brick red | >300 | 57.09 (56.95) | 4.55 (4.49) | 19.97 (19.82) | 6.97 (6.91) |
| [Zn(L)cl] | C20H19ClN6O3Zn | 492.26 | Light yellow | >300 | 48.80 (4.77) | 3.89 (3.82) | 17.07 (16.99) | 13.29 (13.22) |
IR spectral studies
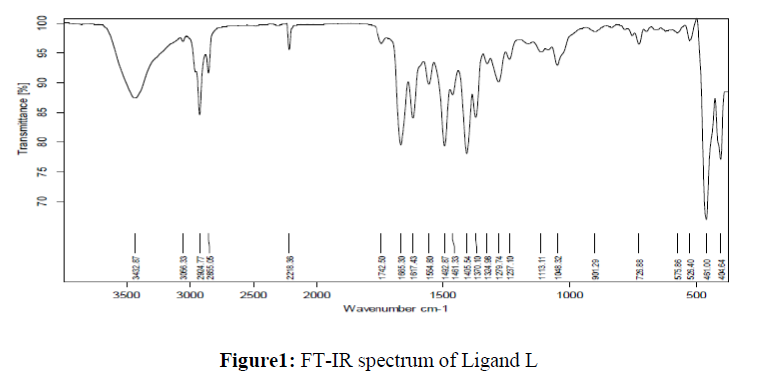
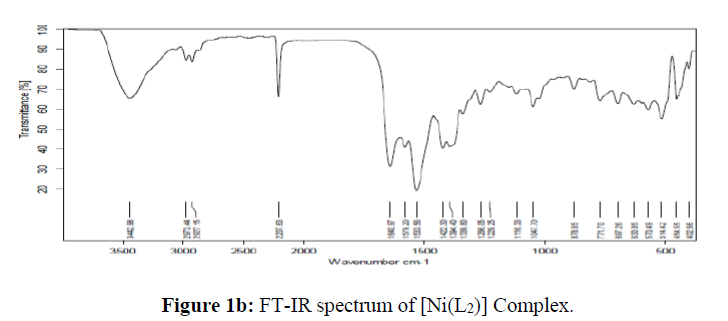
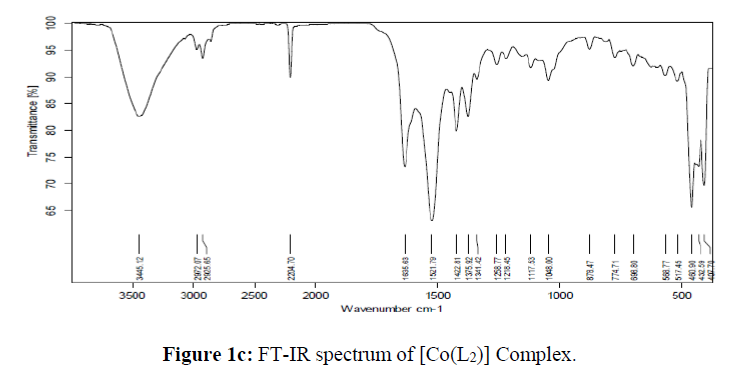
The molecular studies structure of the synthesized L and its complexes was accomplished by recording their FT-IR spectra in the range of 4000-400 cm-1 as KBr pellets and the obtained spectral data are summarized in the Table 2. The results clearly suggested that the peak corresponding to the phenolic OH in the micro-coordinated ligand appeared at 3056 cm-1 which is disappeared in all its metal complexes indicating its involvement in coordination with the metal ions viadeprotonation [35]. The peak appeared in the region 2218-2204 cm-1assigned to the C=N group in all the synthesized compounds [36]. The absorption bands appeared in the region 1742 and 1492 cm-1in the IR spectra of the ligand are assigned to the carbonyl and azo group respectively [37]. Figure 1. The absorption frequencies of these groups were shifted to the lower frequency side confirming their participation in coordination with the metal ions and appeared in the region 1642-1630 cm-1 and 1422-1417 cm-1 in all the complexes respectively. Further, the non-ligand bands appeared in the region 699-627 cm-1 and 774-760 cm-1in all the metal complexes corresponding to the M-Oand M-N bands respectively, which further confirms the coordination of the ligand with the respective metal ions [38]. From the above IR spectral data, it is evident that the L behaves as tridentate with the ONO donor sites.
| Compounds | OH cm-1 | C=N cm-1 | C=O cm-1 | N=N cm-1 | M-O cm-1 | M-N cm-1 |
|---|---|---|---|---|---|---|
| L | 3056 | 2218 | 1742 | 1492 | - | - |
| [Cu(L)2] | - | 2208 | 1642 | 1417 | 627 | 760 |
| [Co(L)2] | - | 2204 | 1635 | 1422 | 698 | 774 |
| [Ni(L)2] | - | 2207 | 1640 | 1422 | 630 | 771 |
| [Zn(L)Cl] | - | 2205 | 1630 | 1423 | 699 | 769 |
Electron absorption spectral studies
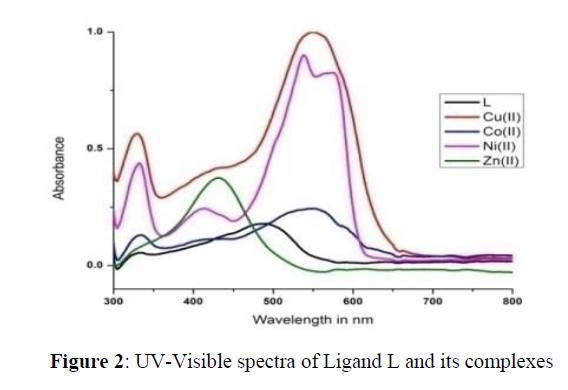
The UV-visible absorption spectroscopy is a most effective analytical tool to identify the type of geometry of the newly synthesized molecules. The electronic spectra of synthesized compounds were record in DMSO solvent between the wavelength 200-800 nm at room temperature. The spectroscopic data obtained from the above study was summarized in the following Table 3 and the spectrum of the ligand and their complexes presented in Figure 2.
| Compounds | Wavenumber (cm-1) | Assignments | meff (B.M.) | Proposed geometry |
|---|---|---|---|---|
| L | 27520 18900 |
n→π*, π →π* |
- | - |
| [Cu(L)2] | 16300 29569 |
2B1g → 2A1g 2B1g → 2B2g 2B1g → 2Eg |
1.75 | Octahedral |
| [Co(L)2] | 15200 23256 31200 |
4T1g (F) → 4T2g (F), 4T1g(F) →4A2g (F) 4T1g (F) →4T2g (P) |
4.32 | Octahedral |
| [Ni(L)2] | 17425 24725 33218 |
3A2g → 3T1g (F) 3A2g (F) → 3T1g (P) |
3.19 | Octahedral |
| [Zn(L)Cl] | 28800 | LMCT (M→N) | Diamagnetic | Tetrahedral |
The UV-visible spectrum of the ligand containing azochromophore exhibited two absorption bands in the region 27520 and 18900 cm-1 responsible for n→π* and π →π* transitions respectively. There is a shift in the absorption maxima in the electronic spectra of the metal complexes indicate the complexation of the metal ions with the ligating sites in the azo molecule.
The orange coloured copper(II) complex of the ligand showed a broad band over the range of 16300-29569 cm-1 responsible for the transitions 2B1g → 2A1g, 2B1g → 2B2g and 2B1g → 2Eg and these transitions are corresponding to the octahedral environment around the copper ion. The electronic spectrum of the cobalt(II) complex showed three bands at 15200, 23256 and 31200 cm-1 corresponding to 4T1g(F) → 4T2g(F),4T1g(F) →4A2g(F) and 4T1g(F) →4T2g(P) transitions respectively. These transitions suggest octahedral geometry for the Co(II) complex .The Ni(II) complex of the azo dye showed three bands each at 17425, 24725 and 33218 cm-1corresponds to the 3A2g → 3T1g(F) and 3A2g(F) → 3T1g(P) transitions respectively suggesting an octahedral geometry [42]. Similarly the electronic spectrum of the zinc complex showed a broad peak due to charge transfer transitions LMCT (M→N). Since, zinc is a d10 system and no unpaired electron present in the d-orbital exhibits only charge transfer transitions but not d-d transitions. The magnetic susceptibility of the above complexes were also recorded for further confirmation of the geometry by VSM technique at room temperature and the corresponding effective magnetic moments for all the complexes were given in the Table 3. The magnetic moment for Cu, Co and Ni complexes of the L was found to be 1.75, 4.32 and 3.19 B.M. respectively, which are in accordance with the reported values for the octahedral geometry [39-41]. Further, the zinc complex was found to be formed in tetrahedral structure and it is diamagnetic in nature.
1H NMR spectral data of the L
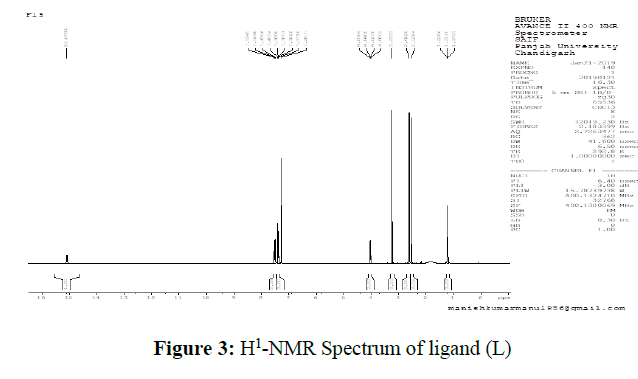
The molecular structure of the azo dye was confirmed by recording its 1H NMR spectrum in CDCl3 solvent between the range of 0-16 δ ppm in the spectrum of the ligand, a broad signal observed as singlet at 15.09 ppm corresponding to the phenolic-OH attached to the pyridone ring. The aromatic protons of the phenyl group which is attached to the pyrazole moiety have resonated as multiplet in the region 7.26-7.53 ppm. The N-methyl group of the pyrazole ring exhibited a singlet at 3.23 ppm and the remaining three methyl groups of both pyrazole and pyridone rings showed peaks in the region 2.61-1.19 ppm. The singlet’s appeared at 3.23, 2.61 and 2.52 ppm corresponds to the N-CH3 of pyrazole and methyl groups respectively. The ethyl group attached to the pyridone ring exhibited as quartet and triplet in the regions 4.05-4.00 and1.22-1.99 ppm respectively. (Figure 3)
Mass spectral studies
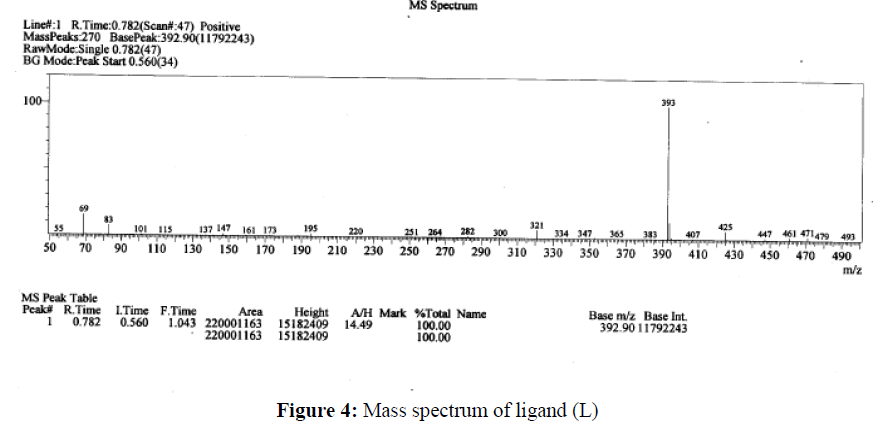
The mass spectral study is an important spectroscopic technique to get the molecular weight of the newly synthesized compounds. The LCMS of the ligand L, Cu(II), Co(II), Ni(II) and Zn(II) complexes were showed a molecular ion peak at m/z 393 (M+1); 845 (M-1); 840, 841 (M-1, M); 840 (M-1), 493 (M+1) respectively. These values obtained from the LC-MS are in close agreement with the calculated molecular weight of the synthesized compounds. The mass spectra of the Ligand and their compounds are in Figure 4.
Thermal evaluation of metal complexes
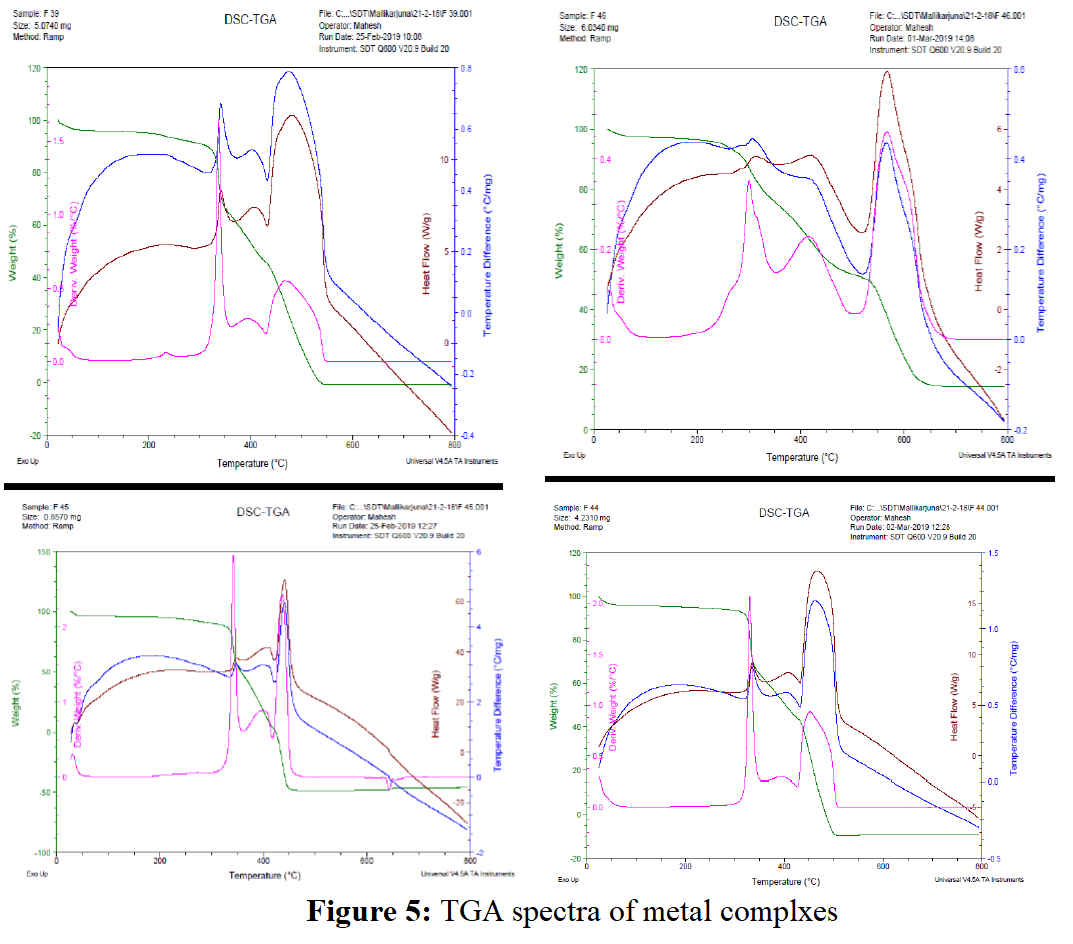
The thermal stability of the copper, cobalt, nickel and zinc complexes of the L was measured by recording their thermogram at room temperature to 800oC in an inert atmosphere with a heating rate of 10oC min-1 and is showed in Figure 5. The corresponding decomposition pattern for each metal complex at each decomposition temperature was calculated and compared with the theoretical values as depicted in Table 4. The thermogram of all the metal complexes exhibited first step of decomposition in the temperature range 220-303oC suggesting the quite appreciable thermal stability and confirms the absence of lattice/coordinated water molecules.
The thermogram of the copper complex showed 4 steps of degradation with the decomposition temperature 220, 404, 509 and 620oC with mass loss of 6.75% (calc. 6.62%), 31.5% (calc. 31.69%), 14.30% (calc. 14.30%) and 37.36% (calc. 37.48%) due to the elimination of C3H6N, C15H16N4O, C6H5N2O and C16H23N5O2 respectively and finally the CuO remained as residue. Similarly, the thermal decomposition pattern for of the complexes has been examined as well as the probable weight loss was estimated and presented in the following Table 4. The experimentally obtained values were almost equivalent to the theoretically assigned values.
| Complex | Decomposition temp. (oC) | % weight loss | % metal oxide | Tentative fragments lost | ||
|---|---|---|---|---|---|---|
| Obsd. | Cald. | Obsd. | Cald. | |||
| [Cu(L)2] | 220 | 6.75 | 6.62 | - | - | C3H6N |
| 404 | 31.5 | 31.69 | - | - | C15H16N4O | |
| 509 | 14.30 | 14.30 | - | - | C6H5N2O | |
| 620 | 37.36 | 37.48 | 10.09 | 9.39 | C16H23N5O2+ CuO | |
| [Co(L)2] | 314 | 7.90 | 7.73 | C4H3N | ||
| 353 | 20.29 | 20.34 | C11H11N2 | |||
| 448 | 24.09 | 24.61 | C9H11N4O2 | |||
| 501 | 38.94 | 39.23 | 8.78 | 8.90 | C16H20N5O3+ CoO | |
| [Ni(L)2] | 323 | 11.80 | 11.77 | C4H5NO2 | ||
| 360 | 23.93 | 23.91 | C11H11N3O | |||
| 441 | 22.68 | 22.71 | C9H11N4O | |||
| 511 | 33.89 | 34.14 | 7.70 | 8.87 | C16H23N4O+ NiO | |
| [Zn(L)Cl] | 303 | 14.49 | 14.43 | C3H5NO | ||
| 515 | 36.15 | 35.99 | C9H11N3O | |||
| 645 | 33.44 | 34.45 | 15.92 | 16.53 | C8H10N2Cl + ZnO | |
Bio-chemical studies
Antimicrobial activity
The novel N-heterocyclic ligand and their metal chelates were evaluated for their antimicrobial activity against three bacterial and three fungal strains [42] such as E. Coli, S. aureus and K. pneumoniae, C. albicans, A. flavus and A. fumigatus respectively by new tube dilution assay. The results obtained for these compounds in terms of MIC (mg/mL) are summarized in Table 5. By observing the results of table-4, it is concluded that the chelation has made larger contribution to exhibit more inhibitory activity against the microbes than that of the uncoordinated ligand. So that, the metal chelates exhibited higher antimicrobial activity against S. aureus, C. Albican sand A. Fumigates. Whereas, normal behaviour exhibited towards the control, other of the studied microbial strains. This can be illustrated that the chelation of the metal ion to the ligand decreases the lipophilic character of the cell wall of the microbe and thereby inhibit the protein synthesis by blocking the active site of the enzymes and finally result in the death of the cells [43-45]. Hence, metal complexes having heterocyclic systems can be a better candidate for the development of the novel drugs to cure the microbial infections.
| Comp. No. | 100 | 50 | 25 | 12.5 | 6.25 | 3.12 | 1.6 | 0.8 | 0.4 | 0.2 |
|---|---|---|---|---|---|---|---|---|---|---|
| E. Coli | ||||||||||
| L | S | S | S | S | S | S | S | R | R | R |
| [Cu(L)2] | S | S | S | S | R | R | R | R | R | R |
| [Co(L)2] | S | S | S | S | S | S | R | R | R | R |
| [Ni(L)2] | S | S | S | S | R | R | R | R | R | R |
| [Zn(L)Cl] | S | S | S | S | R | R | R | R | R | R |
| S. aureus | ||||||||||
| L | S | S | S | S | S | S | S | S | R | R |
| [Cu(L)2] | S | S | S | S | R | R | R | R | R | R |
| [Co(L)2] | S | S | R | R | R | R | R | R | R | R |
| [Ni(L)2] | S | S | R | R | R | R | R | R | R | R |
| [Zn(L)Cl] | S | S | S | R | R | R | R | R | R | R |
| K. Pneumoniae | ||||||||||
| L | S | S | S | S | S | S | S | S | R | R |
| [Cu(L)2] | S | S | S | S | S | S | S | S | R | R |
| [Co(L)2] | S | S | S | S | S | S | S | S | S | R |
| [Ni(L)2] | S | S | S | R | R | R | R | R | R | R |
| [Zn(L)Cl] | S | S | S | S | S | S | S | R | R | R |
| C. albicans | ||||||||||
| L | S | S | S | S | S | S | S | R | R | R |
| [Cu(L)2] | S | S | R | R | R | R | R | R | R | R |
| [Co(L)2] | S | S | S | R | R | R | R | R | R | R |
| [Ni(L)2] | S | S | R | R | R | R | R | R | R | R |
| Zn(L)Cl] | S | S | S | R | R | R | R | R | R | R |
| A. flavus | ||||||||||
| L | S | S | S | S | S | S | S | R | R | R |
| [Cu(1)2] | S | S | S | S | S | R | R | R | R | R |
| [Co(L)2] | S | S | S | S | S | S | S | R | R | R |
| [Ni(L)2] | S | S | S | S | S | R | R | R | R | R |
| Zn(L)Cl] | S | S | S | S | S | S | S | R | R | R |
| A. fumigates | ||||||||||
| L | S | S | S | S | S | S | S | S | R | R |
| [Cu(L)2] | S | S | R | R | R | R | R | R | R | R |
| [Co(L)2] | S | S | S | S | R | R | R | R | R | R |
| [Ni(L)2] | S | S | R | R | R | R | R | R | R | R |
| Zn(L)Cl] | S | S | S | R | R | R | R | R | R | R |
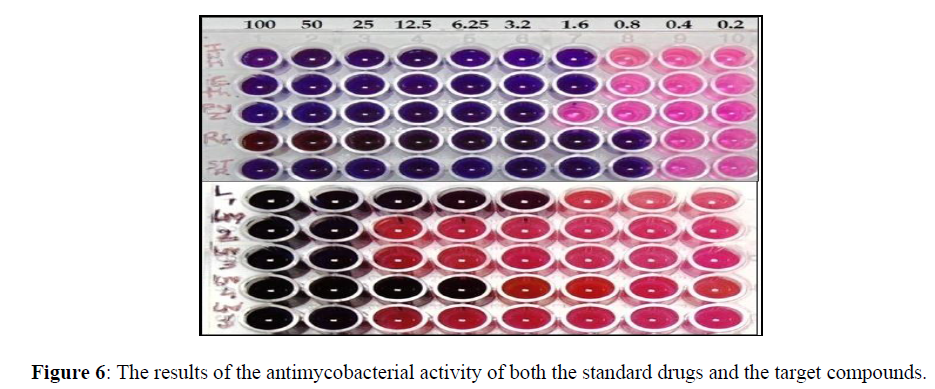
Anti-TB studies
The microbial infections are seriously studied due to their wide spread native and serious problems to the mankind. We know that the sufficient treatments through drugs can cure microbial infections but some of them failed in most of the cases due to enhanced toxicity and multidrug resistance strains. To overcome these peculiar effects by the microbes several researchers focused on the development of transition metal based drugs. Among the microbial diseases, tuberculosis is a very common disease caused by M. tuberculosis which directly impact on the respiratory systems [46]. So by observing the recent studies, we attempted to check the inhibitive ability of the newly synthesized ligand and its metal complexes containing nitrogen based heterocycles against M. tuberculosis by MABA method. The result of the analysis was appended in the Figure 6. It is clearly visible that, the ligand and the Ni(II) complex exhibited appreciable antitubercular activity with MIC value of 3.2 and 6.25 μg/mL respectively. Whereas, moderate activity shown by the rest of the compounds [47-48].
Anticancer activity
The new research findings suggested that the transition metal chelates having heterocyclic core showed excellent anticancer behaviour [45]. In continuation of this, we tested the target molecules for their preliminary anticancerous activity against three cell lines K562, A549 and MDA-MB-231 by MTT assay and the results were tabulated in the Table 6. From the results, it is observed that Co(II) and Ni(II) complexes of the ligand exhibited lowest IC50 value of about 19.311 and 5.324 μM against K562 and A549 cell lines respectively. Further, good to moderate values are reported for the rest of the compounds Table 6.
| K562 | A549 | MDA-MB- 231 | |
|---|---|---|---|
| Sample | IC50 µM | IC50 µM | IC50 µM |
| L1 | 44.384 | >50 | > 50 |
| [Cu(L)2] | 24.188 | >50 | > 50 |
| [Co(L)2] | 19.311 | 17.778 | > 50 |
| [Ni(L)2] | 25.874 | >50 | > 50 |
| [Zn(L)Cl] | 24.515 | 5.324 | > 50 |
Conclusion
The main objective of the present work is to understand the bio-potency of the 3d-metal complexes of the ethyl pyridone based ligand having azo linkage. The synthetic strategy of the azo dye and their metal chelates was so simple, convenient, eco-friendly and economic. The various spectroscopic methods were used to elucidate the geometry of the complexes and from these results, octahedral geometry was proposed for all the complexes except the Zn(II) has tetrahedral in nature. The general proposed structures for the synthesized complexes were displayed in Figure 7. Further, the metal chelates have noticeable antimicrobial, antitubercular and anticancer activities. From these biological investigations, it is come to know that, these metal chelates have higher inhibitive effects as compared to the parent ligand. From the present investigation, we observe that, these organic molecular structures having central metal atom play an important role in supressing the toxic nature of the microbes and may be used in designing metal based antibiotics.
Acknowledgements
We express our sincere thanks to the Chairman, Department of Chemistry, Sahyadri Science College, Kuvempu University for providing laboratory facilities. The authors are also thankful to the SAIF, Panjab University, Chandigarh for their spectral studies.
References
- Poojari S, Naik P and Krishnamurthy G, Tetrahedran Letters, 2014. 55: p.305-309
- Poojari S, Naik P and Krishnamurthy G, Tetrahedran Letters, 2012. 53: p. 4639-4643
- Badea M, Olar R, Cristurean E et al., J Therm Analysis Calorimetry. 2004. 77(3): p.815-824.
- Hassib HB,Abdel-Latif SA, SpectrochimActa. 2003, 59A: p.2425-2434.
- Aiwoo N, Kongchang C and Tian He, Dyes and pigments, 2001. 50(1): p.13-19.
- Metwally MA, Yaser A, Suleiman et al., Int J Mod Org Chem, 2012. 1(3): p.213-225.
- JunXu, Metal-Organic, and Nano-Metal Chemistry, 2013. 43(10): p.1329-1333.
- Harpstrite, Scott E, Silvia Collins D et al., Medicinal Chemistry, 2008. 4(4): p.392.
- Aboul-Fadl, Tarek AS, Fayzah et al., Eu J MedChem, 2010. 45(10): p.4578-4586.
- Rosenberg B, Van Camp L, J E Troskov, V H Monsour, Nature, 1969, 222: p.385.
- Abd-Alla, Mohamed A, Abdel-Hamid Ahmed N et al., 1992. 57(7): p.1547-1552.
- Muneera M, Sirajul, Joseph J, JPhotochemPhotobio Bio. 2016. 163: p.57-68.
- Venugopal N, Krishnamurthy G, Bhojyanaik HS, J Inorg Organomet Polymers Materials, 2019. 1191 p.85-94.
- Venugopal N, Krishnamurthy G and Bhojyanaik HS, J MolStruc, 2019. 1183: p 37-51.
- Rafique, Shazia, Idrees M et al., Biotech MolBiolRev, 2010. 5(2): p.38-45.
- Yan, Y Kai, M Melchart, A Habtemariam, J P Sadler, Chemical communications, 2005. 38: p.4764-4776.
- Metwally MA, Yaser Suleiman A, Gouda MA et al., Int J Med Org Chem, 2012, 1(3): p.213-225.
- Bin, Wei, Wu Yi-Qun et al., Chinese physics letters, 2003. 20(9): p. 596.
- Fu-Xin, Huang, Wu Yi-Qun et al, Chinese physics letters, 2003. 20(12): p.2259.
- Hamada, Emiko, Fujii T et al., Japanese journal of applied physics, 1997, 36(1): p.593.
- Chang, Dohoon, Yoon D et al., Japanese J appphys, 2003. 42(2): p.754.
- Sunil kumar N, Krishnamurthy G andYadavBodke, New J chem, 2018. 43(2): p.790-806.
- Naik S, ParamashwaraNaik P, Krishnamurthy G. Res J Chem Environ, 2020. 24(10): p.93-103.
- Shoair AF, El-Bindary AA, El-Sonbati AZ et al., J Mol Liq. 2016. 215: p.740-748.
- Moanta, Anca, Samide A et al., Int J Electrochem Sci, 2013. 8: p.780-91.
- Al-Juaid andSalih S, Portugaliae, ElectrochimicaActa, 2007. 25(3): p.363-373.
- Chen CC and Wang J, Dyes and Pigm, 1991. 15: p. 69-82.
- Venugopal N, Krishnamurthy G and Bhojyanaik HS, J Inorg and Organometallic polymer and materials. 2020. 30: p.2608–2625.
- Mallikarjuna NM, Keshavayya J, Maliyappa MR et al., J Mol Struc, 2018. 1165: p.28-36.
- Mallikarjuna NM, Keshavayya J, Ravi BN, J MolStruc, 2018. 1173: p.557-566.
- Mallikarjuna NM, Keshavayya J, J King Saud University-Science, 2020. 32(1): p. 251-259.
- Schwalve M and Goodwin AC, Antimicrobial Susceptibility Testing Protocals. 2007.
- Milaeva ER, Dmitry Shpakovsky B, Yulia Gracheva A et al., Dalton Transactions, 2013. 42(19): p.6817-6828.
- Mangalam, Sebastian MC, Selvan A et al., J MolStruc, 2017. 1129: p.305-312.
- Smolyaninov IV, Poddel’sky AI, SmolyaninovaSA et al., Molecules, 2020. 25(8): p. 1770.
- Ershov OV, Bardasov IN, Yu Alekseeva A et al., Russian J Org Chem, 2017. 53(7): p. 1025–1029
- LoaiAljerf, J EnvironManag, 2018. 225: p. 120-132
- Dianu ML, Kriza A, Stanica N et al., J Serb ChemSoc, 2010. 75(11): p. 1515–1531
- Doyle A and Griffiths JB, (Eds.), Cell and Tissue Culture for Medical Research, Wiley, 2000.
- Mallikarjuna MN, Keshavayya J, J Coordination Chem, 2019.72(12), p.1994-2014.
- Mallikarjuna NM, Keshavayya J, King Saud University Science, 2020. 32(1): p. 251-259
- R William. Jacobs, R Barry et al., Tuberculosis, 2014. P. 253-268.
- Chohan ZH, Arif M, Akhtar MA et al., BioinorgChemAppl, 2006 p.1-14.
- Wahab ZHA, Mashaly MM, Salman AA et al., Spectrachim. Acta, 2004. 60: p.2861-2873.
- Andrew M, Jacintha OS, Kennedy J et al., Therapeutic advances in medical oncology, 2016. 8(4): p.276-293.
- Tweedy BG, Phytopathology, 1964. 55: p. 910-914.
- Mahesh K, Ramasamy K, Mani V et al., Arabian J Chem, 2014. 7(4): p.396-408.
- Ratan BT, Anand B, Yogeeswari P et al., Bioorganic & medicinal chemistry letters, 2005. 15(20): p.4451-4455.