Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 8
Synthesis, Characterization, Anthelmintic and Insilico Evaluation of 4,6-Disubstituted Pyrimidine-2-One Derivatives
Sudha Rani K*, Lakshmi Durga J, Srilatha M, Sravani M, Sunand V, Vinod B
Department of pharmaceutical Chemistry, Sri Siddhartha Pharmacy College, Nuzvid, Andhra Pradesh, India-521201
- *Corresponding Author:
- Sudha Rani K
Department of pharmaceutical Chemistry
Sri Siddhartha Pharmacy College
Nuzvid, Andhra Pradesh, India-521201
Abstract
A Series of Bioactive compounds 4,6–Di Phenyl Pyrimidine-2-ol (4a), 4-(3- nitro phenyl)-6-Phenyl-2(1H)-Pyrimidinone (4b), 4-(2-hydroxy phenyl)-6-phenyl pyrimidin-2(1H)-one (4c), 4,[4-(Dimethyl amino) phenyl]-6-Phenyl Pyrimidine-2(1H)-one (4d), 4-(2-chlorophenyl)-6-Phenyl Pyrimidine-2(1H)-one (4e), were Synthesized according to the Literature methods. The Synthesized compounds were characterized by NMR, IR and Mass Spectroscopy. Anthelmintic Activity and Insilico evaluations were carried out for synthesized compounds.
Keywords
4,6-Disubstituted Pyrimidine-2- one, Anti-Helmintic, Insilico Evaluation, Molinspiration, O-Chem.
Introduction
Out of heterocyclic compounds, pyrimidines derivatives have been very well known for their therapeutic applications in medicinal chemistry. The presence of a pyrimidines base in thymine, cytosine and uracil, which are the essential Binding blocks of nucleic acids, DNA and RNA is one possible reason for their activity [1]. The name Pyrimidine (combination of words pyridine and amidine) was first applied by Pinner. The first Pyrimidine derivative to be isolated was alloxan in 1818 by Brugnatelli, oxidizing uric acid with nitric acid [2]. 2,4- Disubstituted Pyrimidines are a novel class of KDR kinase Inhibitors [3].
In 1967, Hunziker reported Pyrantel Pamoate as a Neuromuscular Blocking Agent that causes spastic paralysis in helminthes when employed in the treatment of infestations with pinworms and roundworms. This report suggests the possible use of pyrimidine derivatives as anthelmintic agents [4]. Helminthes infections are among the most widespread infections in humans, distressing a huge population of the world. Although the Majority of infections due to Helminthes are generally restricted to Tropical regions and cause enormous hazard to health and contribute to the prevalence of undernourishment, anaemia, Eosinophilia and pneumonia [5]. In endemic areas, Parasitic Disease cause ruthless morbidity affecting principally population [6]. The gastro-intestinal Helminthes becomes resistant to currently available anthelmintic drugs therefore there is a foremost problem in treatment of Helminthes Diseases [7]. Treatment with an anthelmintic Drug kills worms whose genotype renders them susceptible to the drug. Resistant worms are survived and pass on their Resistance genes. Accumulation of resistant worms results in treatment failure. In general intestinal worm infections are more easily treated than those in other locations in the body [8]. Because the worms need not be killed by the drugs and the drug need not be absorbed when given by mouth; there is usually a wider origin of safety then with drugs for worm infections in other site. Indiscriminate use of synthetic Anthelmintics can lead to resistance of parasites [9]. As reported Previously, Evaluation of Anthelmintic activity of any drug when carried out in artificial laboratory conditions by using the Earth worms (Lumbricus terrestris), isolated intestinal worms (from animals) and isolated (Ascaris lumbricoides) from stools (human) cannot be adaptable with artificial laboratory conditions [10]. Food supplements like Papaya (Carica Papaya), Cinnamon (Cinnamomum camphora, Cinnamomum zeylanicum), Turmeric (Curcuma longa), Asafoetida (Ferrula foetida), Long pepper (Piper longum), Black pepper (Piper nigrum), Carrot (Daucus carota), Saffron (Crocus sativus), Moringa (Moringaptery gosperma), Bitter guard (Momordica charantia), and fresh juices of pine apple have antihelminthic property [11].
The literature also indicated that compounds having Pyrimidine nucleus possess broad range of biological activities such as 5-fluorouracil as anticancer; idoxuridine and Trifluoridine as antiviral; zidovudine and stavudine as anti-HIV, trimethoprim, sulphamethiazine and sulphadiazine as antibacterial; sulphadoxin as antimalarial and antibacterial; minoxidil and prazosin as antihypertensive; barbiturates e.g. Phenobarbitone as sedative, hypnotics and anticonvulsant; propylthiouracil as anti-thyroid; thionzylamine as H1-antihistamine; and toxoflavin and fervennuline as antibiotics [12].
The aim of the present work is to investigate the antihelmintic activity of newly synthesized 4,6- Disubstituted Pyrimidine-2 -one derivatives and to conduct Insilico studies. 4,6-Disubstituted Pyrimidine-2-ones are very effective against various Helminthes in decades.
Materials and Methods
Melting points were determined in open glass capillaries using GallenKamp (MFB-600) melting point apparatus and were uncorrected. IR spectra (KBr discs) Bruker analyzers were confirmed by Shimadzu FT-IR Spectrophotometer using KBr pellets technique, Model No.8400S (Japan). 1H and 13C-NMR spectra were recorded on Bruker 400 MHz Nuclear Magnetic resonance (NMR) spectrometer (Switzerland) using DMSO as solvent. T.L.C. was run on silica gel G plates using ethyl acetate: n-hexane (7: 3) as developing solvent to assess the progress of reaction and purity of the compounds. All other chemicals used in the present study were of analytical grade.
Drugs and chemicals
Benzaldehyde-(MERCK-B. No:SC1S610109), O-Hydroxy Benzaldehyde-(MERCK-B. No: PC/201/16-2), 2-Chloro Benzaldehyde-(PALLAVB. No:PC/388-2/17-2), m-Nitro Benzaldehyde-(PALLAV- B. No: PC/2186/16-2), 4-Dimethyl Amino Benzaldehyde- (MERCK-B. No: QD5Q650881), Acetophenone- (FINAR-B. No: 5065602212BP), Ethanol-(CSS. B. No-110605), Potassium Hydroxide-(FISHER), Silica gel-G- (RSEARCH-LAB FINE CHEM industries-b. No: 1317310113), Ethyl acetate-(AVRA), Dimethyl Sulphoxide-(AVRA-B. No: N140122180), Sodium Chloride- (FINAR-B. No: 76274020).
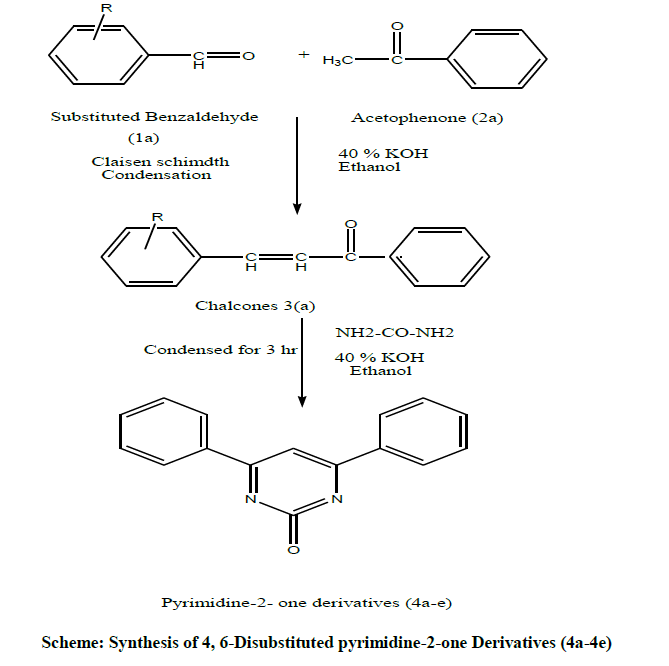
General procedure for synthesis of 4, 6-Disubstituted pyrimidine-2-one Derivatives (4a-4e)
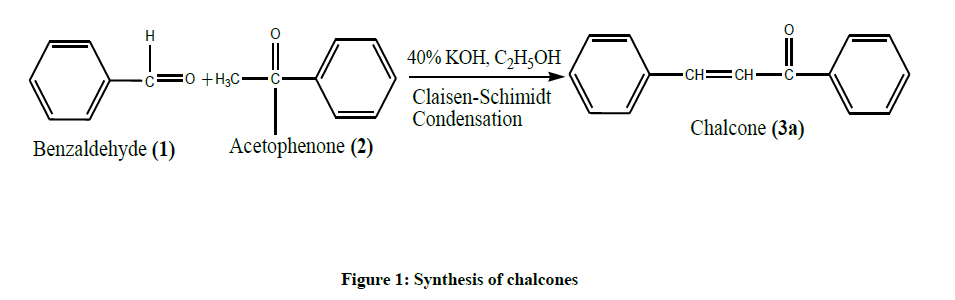
Step 1: Synthesis of Chalcones (Figure 1)
Acetophenone (0.01 mol, 1.2 g, Benzaldehyde (0.01 mol, 1.06 g) were mixed and dissolved in Ethanol (10 ml). To this aqueous potassium hydroxide solution (10 ml) was added slowly with constant stirring. The reaction mixture was stirred continuously for 3 h at room Temperature. The completion of reaction was confirmed by monitoring Thin Liquid Chromatography (TLC) using silica gel-G. After completion of the reaction, the reaction mixture was kept in refrigerator overnight. The product was filtered and washed with cold water till the washings were neutral to Litmus, if necessary acidified with dilute HCl. The product was dried and recrystallized from Rectified spirit to get pale yellow coloured solid chalcones.
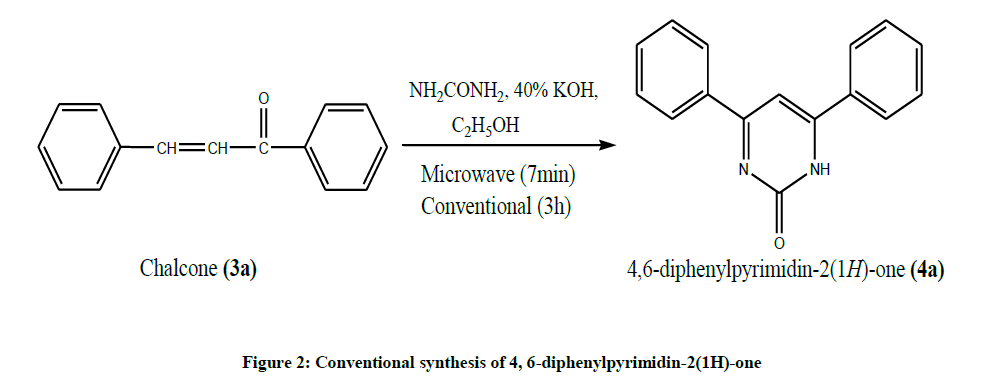
Step 2: synthesis of 4,6-diphenylpyrimidin-2(1H)-one (4a): Conventional Synthesis (Figure 2)
Chalcones (0.01 mol, 2.08 g), urea (0.01 mol, 0.6 g) were mixed and dissolved in ethanol (10 ml). To this 40% aqueous potassium hydroxide solution 10 ml was added slowly with constant stirring. The Reaction Mixer was refluxed on water bath for 3 h. In between TLC was monitored to check the completion of reaction. After completion of reaction, the reaction mixture was cooled to room temperature. And then poured in to ice cold water and neutralized by adding dilute HCl. The Precipitate obtained was filtered, washed with water and dried. The product was recrystallized from rectified spirit.
The procedure was illustrated under Scheme 1 and the synthesized compounds were tabulated in Table 1.
| Compound | 4a | 4b | 4c | 4d | 4e |
|---|---|---|---|---|---|
| R | -H | 0 | -OH | -N (CH3)2 | -Cl |
Table 1: Synthesized compounds
Compound [4a] :4,6-Diphenyl Pyrimidin-2(1H)-one
Yield- 80%; IR Data: FTIR(γmax,cm-1) 1651 (-C=Cstretch), 3032 (=C-H stretch), 1071 (=C-H bend), 1711 (-C=O), 1541 (-C=N); 1H-NMR (400 MHZ,CDCl3): δ (ppm)=7.14, 7.21, 7.3, 7.6 (C-H), δ (ppm)=4.9 (H), δ (ppm)=8.0 (N-H), δ (ppm)=10.238 (NH-C=O), δ (ppm)=7.019, 7.756 (Ar- H), δ (ppm)=0.67 (C=N); 13C-NMR (400 MHZ,CDCl3): δ (ppm)=106, 126, 128, 129, 131.1 (C-H), δ (ppm)=133, 134, 156, 163, 164 (C), δ (ppm)=163 (C=O), δ (ppm)=133 (C=C), δ (ppm)=164 (C=N), δ (ppm)=156 (O=C-NH).
Compound [4b]: 4-(3- Nitro phenyl)-6-Phenyl-Pyrimidin-2(1H)-one
Yield- 70%, IR Data: FTIR (γ max, Cm-1) 1651 (-C=Cstretch), 3032 (=C-H Stretch), 1071 (=C-H Bend), 1711 (-C=O), 1541 (-C=N), 1305(-C-N), 1510 (Asymmetric Stretch -NO2), 1338 (Symmetric Stretch R-NO2); 1H-NMR (400 MHZ,CDCl3): δ (ppm)=7.14, 7.21, 7.3, 7.6 (C-H), δ (ppm)=4.9 (H), δ (ppm)=8.0 (N-H), δ (ppm)=10.238 (NH-C=O), δ (ppm)=7.019, 7.756 (Ar-H), δ (ppm)=0.67 (C=N); 13C-NMR (400 MHZ,CDCl3): δ (ppm)=106, 126, 128, 129, 131.1 (C-H), δ (ppm)=133, 134, 156, 163, 164 (C), δ (ppm)=163 (C=O), δ (ppm)=133 (C=C), δ (ppm)=164 (C=N), δ (ppm)=156 (O=C-NH), δ (ppm)=70.8 (Ar-NO2-C).
Compound [4c]: 4-( 3- Hydroxy phenyl)-6-Phenyl-Pyrimidin-2(1H)-one
Yield- 75%, IR Data: FTIR (γmax,cm-1) 1651 (-C=Cstretch), 3032 (=C-H stretch), 1071 (=C-H bend), 1711 (-C=O), 1541 (-C=N) 3743 (O-H stretch); 1H-NMR (400 MHZ,CDCl3): δ 7.14, 7.21, 7.3, 7.6 (C-H), δ 4.9 (H), δ 8.0 ( N-H), δ 10.238 (NH-C=O), δ 7.019, 7.756 (Ar-H), δ 0.67 (C=N), δ 9.3 (-OH); 13C-NMR (400 MHZ,CDCl3): δ 106, 126, 128, 129, 131.1 (C-H), δ 133, 134, 156, 163, 164 (C), δ 163 (C=O), δ 133 (C=C), δ 164 (C=N), δ 156 (O=C-NH), δ 157 (-C-OH).
Compound [4d]: 4,[4-(Dimethylamino)phenyl]-6-phenyl pyrimidine-2(1H)-one
Yield- 60%, IR Data: FTIR (γmax,cm-1) 1651 (-C=Cstretch), 3032 (=C-H stretch), 1071 (=C-H bend), 1711(-C=O), 1541 (-C=N) 1159 (-C-N-); 1H-NMR (400 MHZ,CDCl3): δ (ppm)= 7.14, 7.21, 7.3, 7.6 (C-H), δ (ppm)=4.9 (H), δ 8.0 ( N-H), δ (ppm)=10.238 (NH-C=O), δ (ppm)=7.019, 7.756 (Ar-H), δ (ppm)=0.67 (C=N); 13C-NMR (400 MHZ,CDCl3): δ (ppm)=106, 126, 128, 129, 131.1 (C-H), δ (ppm)=133, 134, 156, 163, 164 (C), δ (ppm)=163 (C=O), δ (ppm)=133 (C=C), δ (ppm)=164 (C=N), δ (ppm)=156 (O=C-NH), δ (ppm)=47 (-N-CH3).
Compound [4e]: 4-(2-Chlorophenyl)-6-phenyl Pyrimidine -2(1H)-one
Yield- 80%, IR Data: FTIR (γmax,cm-1) 1651 (-C=Cstretch), 3032 (=C-H stretch), 1071 (=C-H bend), 1711 (-C=O), 1541 (-C=N) 775 (C-Cl); 1H-NMR (400 MHZ, CDCl3): δ (ppm)=7.14, 7.21, 7.3, 7.6 ( C-H), δ (ppm)=4.9 (H), δ (ppm)=8.0 ( N-H), δ (ppm)=10.238 (NH-C=O), δ (ppm)=7.019, 7.756 (Ar-H), δ (ppm)=0.67 (C=N); 13C-NMR (400 MHZ, CDCl3): δ (ppm)=106, 126, 128, 129, 131.1 (C-H), δ ( ppm)=133, 134, 156, 163, 164 (C), δ (ppm)=163 (C=O), δ 133 (C=C), δ (ppm)=164 (C=N), δ (ppm)=156 (O=C-NH), δ (ppm)=70 (-C-Cl).
Antihelmintic evaluation [13]
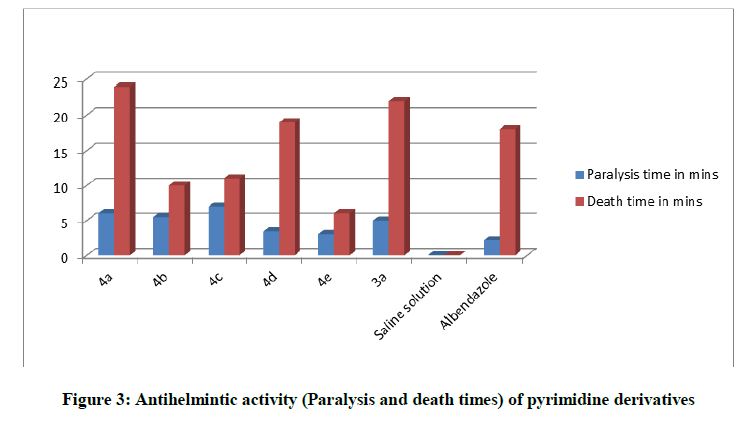
Indian adult earthworms of the genus and species, Pheritima posthuma (family: Megascolecidae), were used to study the antihelmintic activity. The earthworms were collected from the water logged areas of soils in Nuzvid, Andhra Pradesh, India were washed with normal saline to remove all the fecal matter and waste surrounding their body. The earth worms (Pheritima posthuma) 5-8 cm in length and 0.2-0.3 cm width weighing 0.8–3.04 g were used for all experiment protocols. The earthworms resembled the intestinal roundworm parasites of human beings both anatomically and physiologically and hence were used to study the antihelmintic activity Figure 3.
Procedure
Gum acacia solution (1%) was prepared in normal saline. Test solutions were prepared by using this solution. Sample were taken petriplates and adult healthy earth warms (n=6) were introduced in to them. Observations were made for the time taken to paralyze and time taken for death of the organism. Paralysis was said to occur when the worms do not review even in normal saline. Death was concluded when worms lost their motility followed by fading away of the body color, and the values are summarized in Table 2.
| S. No. | Compounds | Dose | Paralysis time in minutes | Death time in minutes |
|---|---|---|---|---|
| Mean ± s.e.m | Mean ± s.e.m | |||
| 1 | 4a | 100 mg/ml | 6 ± 0.5 | 24 ± 0.416 |
| 2 | 4b | 100 mg/ml | 5.48 ± 0.529 | 10 ± 0.36 |
| 3 | 4c | 100 mg/ml | 7 ± 0.763 | 11 ± 0.611 |
| 4 | 4d | 100 mg/ml | 3.45 ± 0.76 | 19 ± 0.8 |
| 5 | 4e | 100 mg/ml | 3.08 ± 0.36 | 6 ± 0.72 |
| 6 | 3a | 100 mg/ml | 5 ± 1.2 | 22 ± 0.781 |
| 7 | Control (Normal saline) | ------- | ------ | |
| 8 | Albendazole | 100 mg/ml | 2.51± 1.1 | 18 ± 2.1 |
Table 2: Antihelmintic activity of Pyrimidine derivatives
Insilico evalution
The Online Chemical Modelling Environment (OCHEM) a unique and a web-based platform which supports all the steps required to create a predictive model: one such model developed was cytochrome P450 with 5 subtypes the compounds were evaluated to assess their Inhibition on the subtypes of cytochrome P450.
Results and Discussion
Physicochemical and analytical data were tabulated for synthesized compounds 4a-4e as shown in Table 1. The Structures of the compounds were characterised through IR and 1H and 13C-NMR Spectral data, whereas results of antihelmintic studies tabulated in Table 2 reveals that compounds 4e, 4b and 4c were found to be the most potent Compounds in the series which when compared to the standard, compounds 4e, 4b and 4c showed high activity, other compounds 4a, 4d, and 3a showed significant antihelmintic activity.
The derivatives synthesised were evaluated by two online softwares- MOLINSPIRATION, OCHEM. (Tables 3-5) From the OCHEM results, all the synthesised compounds were found to inhibit the subtype CYP1A2 of cytochrome P450. Molinspiration results inferred that all the derivatives satisfy Lipinski rule of five so as to behave as a drug and found to have kinase and enzyme inhibition properties.
| Compound | IUPAC name | Log P | Polar Surface Area | H– Bond Acceptor | H– Bond Donor | Volume |
|---|---|---|---|---|---|---|
| 4a | 4,6 –Diphenyl Pyrimidine-2-ol | 3.22 | 45.75 | 3 | 1 | 226.67 |
| 4b | 4– (3-Nitrophenyl)–6-Phenyl 2 (1H)- Pyrimidinone | 3.15 | 91.58 | 6 | 1 | 250.01 |
| 4c | 4- (2-Hydoxyphenyl)-6-phenyl- pyrimidin-2 (1H)-one | 2.95 | 65.98 | 4 | 2 | 234.69 |
| 4d | 4,[4- (Dimethylamino)phenyl]-6- phenyl pyrimidin-2 (1H)-one | 3.32 | 48.99 | 4 | 1 | 272.58 |
| 4e | 4- (2-Chloophenyl) -6- phenyl Pyrimidin -2 (1H)-one | 3.85 | 45.75 | 3 | 1 | 240.21 |
Table 3: Molinspiration: Physical properties
| Compound | GPCR Ligand | Ion Channel Modulator | Kinase | Nuclear Receptor | Protease | Enzyme Inhibitor |
|---|---|---|---|---|---|---|
| Inhibitor | Ligand | Inhibitor | ||||
| 4a | -0.13 | -0.36 | 0.2 | -0.27 | -0.58 | -0.01 |
| 4b | -0.2 | -0.38 | 0.13 | -0.24 | -0.56 | -0.1 |
| 4c | -0.07 | -0.36 | 0.26 | -0.08 | -0.54 | 0.02 |
| 4d | -0.025 | -0.35 | 0.3 | -0.12 | -0.42 | -0.01 |
| 4e | -0.05 | -0.34 | 0.27 | -0.19 | -0.52 | -0.03 |
Table 4: Molinspiration: Bioactive Scores
| Compound | Aqueous solubility | LogIGC50 | AMES | CYP3A4 | CYP2D6 | CYP2C19 | CYP2C9 | CYP1A2 |
|---|---|---|---|---|---|---|---|---|
| 4a | 0.64 | 1.22 | Active | - | - | - | + | + |
| 4b | 0.78 | 1.14 | Active | - | - | - | + | + |
| 4c | 0.57 | 1.22 | Inactive | - | - | - | - | + |
| 4d | 0.75 | 1.25 | Active | - | - | + | - | + |
| 4e | 0.74 | 1.15 | Inactive | - | - | + | + | + |
+ Inhibitor, - Non inhibitor, AQ-aqueous, IGC 50-Environmental toxicity
Table 5: O-CHEM (Online Chemical Modeling Environment)
Conclusion
A series of 4,6- Disubstituted Pyrimidine-2- one derivatives prepared by a novel method and their ability to paralyze and cause death of Indian earthworms. Though the mechanisms underlying this process remain to be fully elucidated detailed mechanistic studies and lead optimization of these 4,6- Disubstituted Pyrimidine-2- one derivatives are under investigation. It is intended that the results from these studies will assist in elucidating their precise mechanism of action and provide an approach to develop new potent antihelmintic prototypes for further optimization and development to get new leads in the treatment of helminth infestations.
Acknowledgement
The authors are thankful to the Sri Siddhartha Pharmacy College for providing necessary facilities to carry out this research work.
References
- I.L. Finar, Org. Chem., 6th (Edn.), 1, 864-864.
- I.L. Finar Org. Chem., 5th (Edn.), 2, Pearson (Edi.), 642-645.
- P.J. Manley, Bioorg. Med. Chem. Lett., 2003, 13(10), 1673-1677.
- F. Hunziker, Helv. Chim. Acta., 1967, 50, 1588.
- D.A. Bundi, Trans. Royal. Soc. Trop. Med. Hyg., 1994, 8, 259-261.
- S. Tagbota, S. Townson, Adv. Parasitol.,2001, 50, 199-205.
- S.M. Sondhi, R. Shahu, M. Archana, Indian Drugs., 1994, 31(7), 317-320.
- Department of party headquarters U.S. Army Survival Manual Fm 21-76, Barns & Noble Inc Encyclopaedia Britannica. Encyclopaedia Britannica Online, 22 Jan, 2009.
- D. Singh, C.P. Swarnkar, F.A. Khan, J. Vet. Parasit., 2002, 16, 115-130.
- S. Kosalge, R.A. Fursule, Asian J. Pharmaceuti. Res., 2009, 2(2), 69-71.
- K.M. Nadkarni, Indian Materia Medica, 2nd (Edn.), Bombay popular prakashan, Mumbai, 2002, 2, 237-239.
- D. Rupseh, S. Pramod Kumar, J. Adv. Sci. Res.,2011, 2(3), 10-17.
- K.N.V. Chenchu Lakshmi, D. Koti, B. Anupama, A. Chakravarthy, M. Priyanka, Int. J. Pharm. Life Sci., 2014, 5(8), 765-3773.