Research Article - Der Pharma Chemica ( 2023) Volume 15, Issue 1
Synthesis, Characterization of Diethylamino Benzylidene-5-Methyl-2-Phenyl-Pyrazol-3- One Substituted 1,2,3-Triazoles and Evaluation of their Anti-Microbial Activity
Dharmasothu Veeranna1, Lakavath Ramdas1, Guguloth Ravi1, Anil Kumar Bojja2 and Ramchander Jadhav1*2Assistant Professor of Chemistry, Government Degree College, Maheshwaram (Badangpet) Ranga Reddy, Telangana-500112, India
Ramchander Jadhav, Department of Chemistry, University College of Science, Osmania University, Hyderabad, Telangana-500 007, India, Email: ramorgchemou@gmail.com
Received: 07-Jan-2023, Manuscript No. dpc-23-86103; Editor assigned: 09-Jan-2023, Pre QC No. dpc-23-86103; Reviewed: 23-Jan-2023, QC No. dpc-23-86103; Revised: 24-Jan-2023, Manuscript No. dpc-23-86103; Published: 31-Jan-2023, DOI: 10.4172/0975-413X.15.1.13-20
Abstract
A new series of (Z)-4-(4-(diethylamino)-2-((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy)benzylidene)-3-methyl-1-phenyl-1H-pyrazol-5(4H)-one (5a-l)ha been synthesized by the reaction of (Z)-4-(4-(diethylamino)-2-(prop-2-yn-1-yloxy)benzylidene)-3-methyl-1-phenyl-1H-pyrazol-5(4H)-one with different aromatic azides. Chemical structures of all the new compounds were established by IR, 1H-NMR, 13C NMR, MS and elemental data. The Compounds 5a-l were evaluated for their antibacterial activity against Gram-positive bacteria viz Bacillus subtilis(MTCC 441), Bacillus sphaericus (MTCC 11),Staphylococcus aureus (MTCC 96), and Gram-negative bacteria viz. Pseudomonas aeruginosa (MTCC 741), Klebsiella aeroge(MTCC 39), Chromobacterium violaceum (MTCC 2656). Amongst them, compounds containing 5b[1-(4-bromophenyl)-1H-1,2,3-triazol-4-yl)], 5d[(1-(4-chlorophenyl)-1H-1,2,3-triazol-4-yl)], 5j [(1-(3-acetylphenyl)], 5k [-((1-(4-acetylphenyl)-1H-1,2,3-triazol-4-yl)] and 5l [(4-(diethylamino)-2-((1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)] were showed notable antibacterial activity, almost equal /more than the activity of the standard drug Streptomycin. Further, the compounds 5a-l were also shelter for their antifungal activity opposed to Candida albicans (ATCC 10231), Aspergillus fumigatus (HIC 6094), Trichophyton rubrum (IFO 9185), and Trichophyton mentagrophytes (IFO 40996). Most of these new compounds showed substantial activity opposed to test bacteria and fungi and appear as prospective molecules for further evolution.
Keywords
Synthesis (Z)-4-(4-(diethylamino)-2-((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy)benzylidene)-3-methyl-1-phenyl-1H-pyrazol-5(4H)-one; Antibacterial activity; Antifungal activity
INTRODUCTION
Heterocyclic compounds constitute the largest and most varied family of organic compounds [1]. It is well known that heterocyclic compounds are widely distributed in nature and are essential for life activities of plants and animals. They play a vital role in the metabolism of all living cells, for example the vitamins and co-enzyme precursors thiamine, riboflavin etc. Some of these are natural products, for example antibiotics such as penicillin and cephalosporin etc [2]. In particular, they are described as inhibitors of protein glycerin, antibacterial, antifungal, anticancer, antidepressant, anti-inflammatory, antituberculosis, antioxidant as well as antiviral agents [3].
Pyrazoles have illustrious history; in 1883, a German chemist Ludwig Knorr was the first to discover antipyretic action of pyrazole derivative in men, he named the compound antipyrine.
When he attempted to synthesize quinoline derivatives with antipyretic activity, accidentally obtained antipyrine (2,3-dimethyl-1-phenyl-3-pyrazolin-5- one) which has analgesic, antipyretic and antirheumatic activity; which stimulated interest in pyrazole chemistry [4].
Pyrazolones are five membered nitrogen containing heterocyclic compounds, where pyrazolone ring system represents an important class of compounds not only for their theoretical interest but also for their bio-activity. The 3-pyrazolone and 5- pyrazolone are most dominant classes having importance in pharmaceutical industry [5].
In our current research study, the 3-Methyl-1-PhenylPyrazol-5-one derivatives have been the subject of many research studies due to varieties of potential biological activities such as antimicrobial antiviral biological activities such as antimicrobial antiviral such as antimicrobial antiviral, antitumor, antihistaminic, antidepressant, antiinflammatory, anticancer, antioxidant, anticonvulsant, antidiabetic activities, and cytotoxic activities. The pyrazolone derivatives constitute an important moiety of numerous pharmaceuticals, agrochemicals, dyesand pigments, chelating and extracting agent. Pyrazolone can be considered as intermediate compound for the synthesis of various cyclic compounds of high biological activity [6-9].
The present examination distribute with the synthesis of some new (Z)-4-(4-(diethylamino)-2-((1-phenyl-1H-1,2,3-triazol-4- yl)methoxy)benzylidene)-3-methyl-1-phenyl-1H-pyrazol-5(4H)-one 5 a-l in good yields, from (Z)-4-(4-(diethylamino)-2-(prop-2-yn-1- yloxy)benzylidene)-3-methyl-1-phenyl-1H-pyrazol-5(4H)-one 4. The antibacterial and antifungal activities of the compounds 5a-lhave also been estimated.
RESULTS AND DISCUSSION
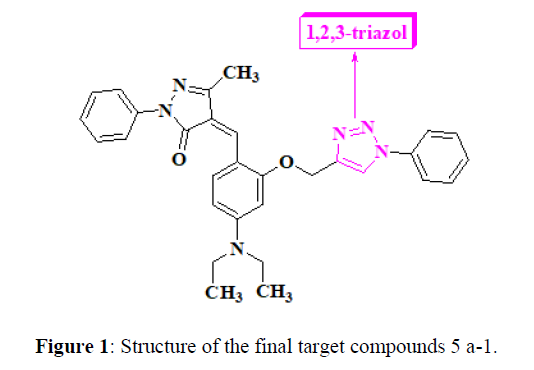
Inspired by the growing importance and the wide range biological applications of (Z)-4-(4-(diethylamino)-2-((1-phenyl-1H-1,2,3-triazol-4- yl)methoxy)benzylidene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one containing frameworks, we have developed an efficient easy synthesis of hybrid molecules containing Diethyl amino benzylidene-5-methyl-2-phenyl-pyrazol-3-one substituted 1,2,3-triazoles frameworks. (Figure 1)
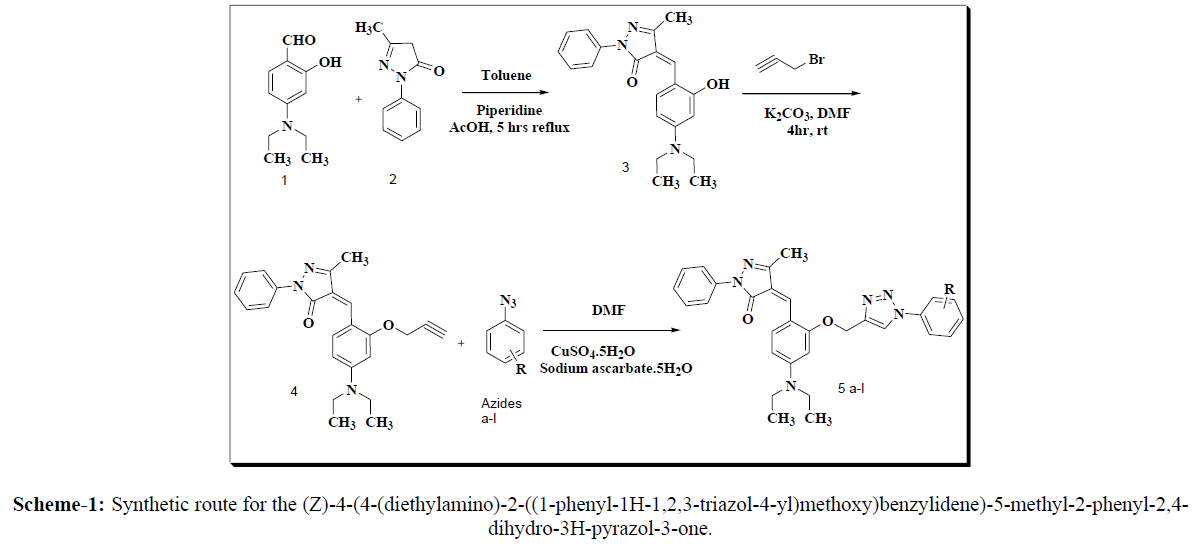
Synthetic route for the (Z)-4-(4-(diethylamino)-2-((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy)benzylidene)-5-methyl-2-phenyl-2,4-dihydro-3Hpyrazol- 3-one (5a-l) were summarized in scheme 1. The 1,2,3-triazole tethered pyrazol-3-one core nucleus was constructed from commercially available compounds. The synthesis was started in between cyclocondensation with the 4-(diethylamino)-2-hydroxybenzaldehyde (1) and 5-methyl- 2-phenyl-2,4-dihydro-3H-pyrazol-3-one (2) In presence of Piperidine few drops AcOH in toluene solvent for 5r reflux to afford (Z)-4-(4- (diethylamino)-2-hydroxybenzylidene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (3) and confirms by NMR data of at 9.8ppm signal disappear in compound 3. propargylation of 2-hydroxybenzylidene using propargyl bromidedry DMF and dry K2CO3 rt for 3-4 hrs to yield (Z)-4-(4- (diethylamino)-2-(prop-2-yn-1-yloxy)benzylidene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (4)confirms by disappears signal of -OH group in NMR and IR respectively which on further click reaction of substituted aryl azides at the terminal alkyne position to provide (Z)-4-(4- (diethylamino)-2-((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy)benzylidene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (5a-l). The structures of all the latest synthesized products were confirmed IR, 1H-NMR, 13C-NMR, MS and elemental analysis, obtained products in high yields (80- 90%).
In the IR spectra of synthesized final compound 5a-l, the alkyne C-H stretching frequency converted alkene C-H frequency in triazole ring those are 3380cm-1 and 3336cm-1 respectively, confirms the alkyne converted to triazole ring by click reaction it’s also supported by 1H-NMR data signal appears at 8.55ppm confirms triazole ring formation (Scheme-1).
Antibacterial Activity
All the synthesized compounds 5a-l were assayed for their antibacterial activity in opposition to Gram-positive bacteria viz Bacillus subtilis(MTCC 441), Bacillus sphaericus (MTCC 11), Staphylococcus aureus (MTCC 96), and Gram-negative bacteria viz. Pseudomonas aeruginosa (MTCC 741), Klebsiella aeroge(MTCC 39), Chromobacterium violaceum (MTCC 2656) by disc diffusion method[10], and the data of mean inhibition zone reported in Table-1. All assays incorporate the solvent and reference controls. Standard drug used as streptomycin.
| Compound | Mean zone inhibition (MZI)a in 100 μg/mL | |||||
|---|---|---|---|---|---|---|
| B. subtilis | B. sphaericus | S. aureus | P. aeruginosa | K. aerogenes | C. violaceum | |
| 6.3a | 19 | 16 | 17 | 20 | 25 | 23 |
| 6.3b | 29 | 26 | 25 | 21 | 25 | 23 |
| 6.3c | 12 | 14 | 13 | 16 | 17 | 16 |
| 6.3d | 32 | 29 | 32 | 23 | 29 | 26 |
| 6.3e | 21 | 22 | 20 | 18 | 13 | 14 |
| 6.3f | 20 | 10 | 11 | 14 | 16 | 17 |
| 6.3g | 18 | 20 | 16 | 16 | 20 | 21 |
| 6.3 h | 16 | 22 | 21 | 20 | 23 | 18 |
| 6.3i | 19 | 20 | 23 | 20 | 26 | 23 |
| 6.3j | 32 | 26 | 27 | 20 | 28 | 23 |
| 6.3k | 33 | 26 | 28 | 22 | 26 | 22 |
| 6.3l | 30 | 26 | 28 | 20 | 26 | 23 |
| Streptomycin | 31 | 27 | 26 | 19 | 27 | 24 |
| aValues are mean (n = 3). | ||||||
The exploration of screening data antibacterial activity discloses that almost all synthesized compounds 5a-l are potent and exhibit tolerable to marvelous antibacterial activity. Amidst them, compounds containing 5b[1-(4-bromophenyl)-1H-1,2,3-triazol-4-yl)], 5d [(1-(4-chlorophenyl)-1H-1,2,3-triazol-4-yl)], 5j [(1-(3-acetylphenyl-1,2,3-triazol-4-yl))], 5k [-((1-(4-acetylphenyl)-1H-1,2,3-triazol-4-yl)] and 5l [(4-(diethylamino)-2-((1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)] moiety exhibit noteworthy antibacterial activity, almost equal/greater than the activity compare to standard drug streptomycin.
Antifungal Activity
All the synthesized compounds 5a-l were assayed for their antifungal activity in opposition to Gram-positive bacteria viz Candida albicans (ATCC 10231), Aspergillus fumigatus (HIC 6094), Trichophyton rubrum (IFO 9185), and Trichophyton mentagrophytes (IFO 40996) in dimethyl sulfoxide (DMSO) by disc diffusion method. Amphotericin B was used as a standard drug and the data of mean inhibition zone (MZI) reported in Table-2.MZI data measured and compared with controls, the MZI values of the compounds screened.
| Compound | Mean zone inhibition (MZI)a in 100 μg/mL | |||
|---|---|---|---|---|
| C. albicans | A. fumigatus | T. rubrum | T. mentagropytes | |
| 6.3a | 16 | 15 | 14 | 18 |
| 6.3b | 28 | 24 | 15 | 28 |
| 6.3c | 21 | 18 | 20 | 18 |
| 6.3d | 28 | 26 | 26 | 26 |
| 6.3e | 21 | 22 | 22 | 24 |
| 6.3f | 16 | 15 | 16 | 12 |
| 6.3g | 18 | 18 | 18 | 20 |
| 6.3h | 21 | 20 | 22 | 23 |
| 6.3i | 20 | 19 | 10 | 25 |
| 6.3j | 28 | 24 | 23 | 25 |
| 6.3k | 28 | 24 | 23 | 26 |
| 6.3l | 30 | 26 | 25 | 26 |
| Amphotericin B | 29 | 25 | 24 | 27 |
| aValues are mean (n = 3) | ||||
The exploration of screening data antifungal activity discloses that almost all synthesized compounds 5a-l are potent and exhibit tolerable to marvelous antifungal activity. Amidst them, compounds containing 5b[1-(4-bromophenyl)-1H-1,2,3-triazol-4-yl)], 5d [(1-(4-chlorophenyl)-1H-1,2,3-triazol-4-yl)], 5j [(1-(3-acetylphenyl-1,2,3-triazol-4-yl))], 5k [-((1-(4-acetylphenyl)-1H-1,2,3-triazol-4-yl)] and 5l [(4-(diethylamino)-2-((1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)] moiety exhibit noteworthy antifungal activity, almost equal/greater than the activity compare to standard drug streptomycin.
CONCLUSIONS
A new sequence of (Z)-4-(4-(diethylamino)-2-((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy)benzylidene)-3-methyl-1-phenyl-1H-pyrazol-5(4H)-one 5a-l has been synthesized and appraise for their antimicrobial activity against Gram-Positive, Gram-negative bacteria and fungi. Most of the compounds exhibit a average degree of antimicrobial activity. Amidist them, compounds consists 5b[1-(4-bromophenyl)-1H-1,2,3-triazol-4-yl)], 5d [(1-(4-chlorophenyl)-1H-1,2,3-triazol-4-yl)], 5j [(1-(3-acetylphenyl-1,2,3-triazol-4-yl))], 5k [-((1-(4-acetylphenyl)-1H-1,2,3-triazol-4-yl)] and 5l [(4-(diethylamino)-2-((1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)] moiety showed average or more activity against standard drug streptomycin, Amphotericin respectively and emerged as potential molecules for further evaluation.
EXPERIMENTAL
Reagents were of commercial grade and were used as supplied or were prepared according to procedures described in literature. Reactions were monitored by thin-layer chromatography (TLC) on pre-coated silica gel F254 plates from Merck, and compounds visualized either by exposure to UV light. Chromatographic columns 70–230 mesh silica gel for separations were used. Melting points were determined on a Fisher–Johns apparatus and are uncorrected. IR spectra were recorded using KBr disk on a Perkin–Elmer FTIR spectrometer. The 1H NMR, 13C NMR spectra were recorded on a Varian Gemini spectrometer (300 MHz for 1H and 75 MHz for 13C). Chemical shifts are reported in δ ppm units with respect to TMS as internal standard and coupling constants (J) are reported in Hz units. Mass spectra were recorded on a VG micro mass 7070H spectrometer. Elemental analyses (C, H, N) determined by means of a Perkin–Elmer 240 CHN elemental analyzer, were within ± 0.4% of theory.
4.1.(Z)-4-(4-(diethylamino)-2-((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy)benzylidene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (4.9a):
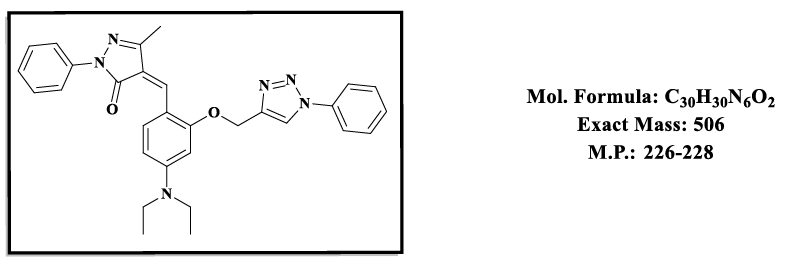
1H NMR (500 MHz, DMSO-d6) δ 8.55 (s, 1H), 7.87 – 7.81 (m, 2H), 7.79 (s, 1H), 7.74 (d, 1H), 7.59 – 7.35 (m, 8H), 6.83 (d, J = 2.2 Hz, 1H), 6.50 (s, 1H), 5.36 (s, 2H), 3.47 (q, J = 6.9 Hz, 4H), 2.30 (s, 3H), 1.12 (t, J = 7.0 Hz, 6H); 13C NMR (125 MHz, DMSO-d6) δ 163.60, 157.88, 152.53, 152.37, 146.65, 137.34, 135.73, 132.42, 129.38, 128.26, 126.92, 126.02, 120.14, 119.16, 118.62, 118.52, 118.51, 116.72, 107.55, 98.12, 58.52, 43.84, 13.23, 11.67.
4.2.(Z)-4-(2-((1-(4-bromophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-4-(diethylamino) benzylidene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol- 3-one (4.9b):
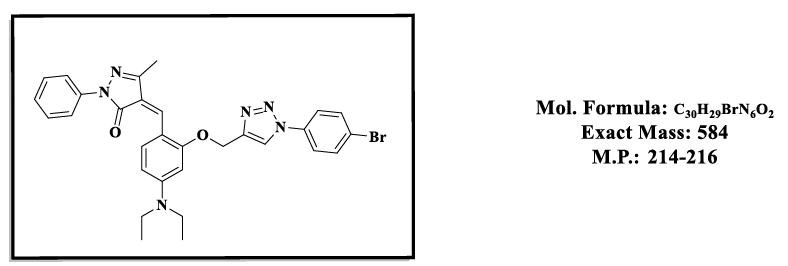
1H NMR (500 MHz, DMSO-d6) δ 8.55 (s, 1H), 7.87 – 7.81 (m, 2H), 7.79 (s, 1H), 7.74 (d, J = 8.1 Hz, 1H), 7.70 – 7.58 (m, 4H), 7.55 – 7.48 (m, 2H), 7.46 – 7.39 (m, 1H), 6.83 (d, J = 2.2 Hz, 1H), 6.50 (s, 1H), 5.36 (s, 2H), 3.47 (q, J = 6.9 Hz, 4H), 2.30 (s, 3H), 1.12 (t, J = 7.0 Hz, 6H); 13C NMR (125 MHz, Common NMR Solvents) δ 163.55, 157.83, 152.48, 152.32, 146.60, 137.29, 134.45, 132.97, 132.37, 128.21, 125.97, 120.83, 119.14, 118.98, 118.57, 118.47, 118.46, 116.67, 107.50, 98.07, 58.47, 43.79, 13.18, 11.62.
4.3.(Z)-4-(2-((1-(2-chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-4-(diethylamino) benzylidene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3- one (4.9c):
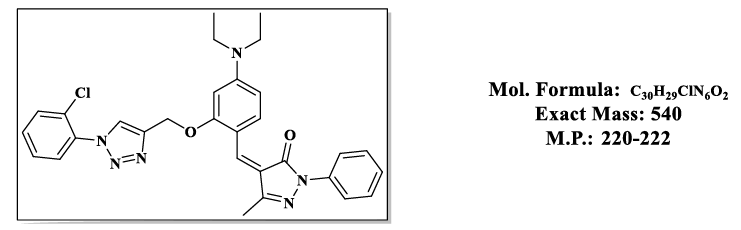
1H NMR (500 MHz, DMSO-d6) δ 8.55 (s, 1H), 7.87 – 7.81 (m, 2H), 7.79 (s, 1H), 7.74 (d, J = 8.1 Hz, 1H), 7.70 – 7.64 (m, 2H), 7.64 – 7.58 (m, 2H), 7.55 – 7.48 (m, 2H), 7.43 (dd, 1H), 6.83 (dd, J = 8.1, 2.2 Hz, 1H), 6.50 (s, 1H), 5.36 (s, 2H), 3.47 (q, J = 6.9 Hz, 4H), 2.30 (s, 3H), 1.12 (t, J = 7.0 Hz, 6H); 13C NMR (125 MHz, DMSO-d6) δ 163.59, 157.87, 152.52, 152.36, 145.53, 137.33, 134.99, 132.41, 130.93, 128.25, 128.16, 127.50, 127.10, 126.01, 120.39, 119.19, 118.61, 118.51, 118.50, 116.71, 107.54, 98.11, 58.68, 43.83, 13.22, 11.66.
4.4.(Z)-4-(2-((1-(4-chlorophenyl)-1H-1,2,3-triazol-4-yl)methoxy)-4-(diethylamino) benzylidene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3- one (4.9d):
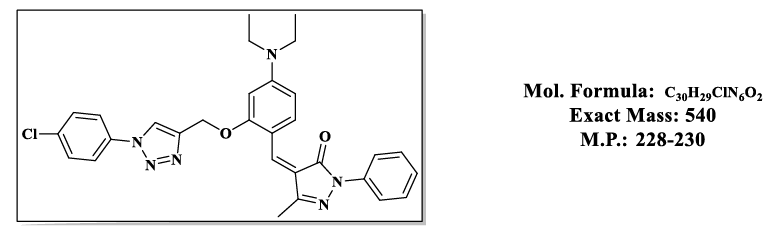
1H NMR (500 MHz, DMSO-d6) δ 8.55 (s, 1H), 7.87 – 7.81 (m, 2H), 7.79 (s, 1H), 7.76 – 7.70 (m, 3H), 7.55 – 7.48 (m, 2H), 7.48 – 7.39 (m, 3H), 6.83 (d, 1H), 6.50 (s, 1H), 5.36 (s, 2H), 3.47 (q, J = 6.9 Hz, 4H), 2.30 (s, 3H), 1.12 (t, J = 7.0 Hz, 6H);13C NMR (125 MHz, DMSO-d6) δ 163.60, 157.88, 152.53, 152.37, 146.65, 137.34, 134.18, 134.07, 132.42, 129.66, 128.26, 126.02, 121.53, 119.19, 118.62, 118.52, 118.51, 116.72, 107.55, 98.12, 58.52, 43.84, 13.23, 11.67.
4.5.(Z)-4-(4-(diethylamino)-2-((1-(4-hydroxyphenyl)-1H-1,2,3-triazol-4-yl)methoxy) benzylidene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol- 3-one (4.9e):
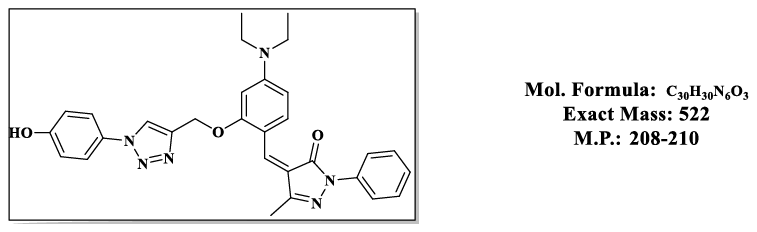
1H NMR (500 MHz, DMSO-d6) δ 9.12 (s, 1H), 8.54 (s, 1H), 7.87 – 7.81 (m, 2H), 7.79 (s, 1H), 7.76 – 7.66 (m, 3H), 7.55 – 7.48 (m, 2H), 7.46 – 7.39 (m, 1H), 6.97 – 6.90 (m, 2H), 6.83 (d, J = 2.2 Hz, 1H), 6.50 (s, 1H), 5.36 (s, 2H), 3.47 (q, J = 6.9 Hz, 4H), 2.30 (s, 3H), 1.12 (t, J = 7.0 Hz, 6H);13C NMR (125 MHz, DMSO-d6) δ 163.57, 157.85, 156.08, 152.50, 152.34, 146.62, 137.31, 132.39, 128.94, 128.23, 125.99, 121.35, 119.49, 118.59, 118.49, 118.48, 116.71, 116.69, 107.52, 98.09, 58.49, 43.81, 13.20, 11.64.
4.6.(Z)-4-(4-(diethylamino)-2-((1-(2-methoxyphenyl)-1H-1,2,3-triazol-4-yl)methoxy)benzylidene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol- 3-one (4.9f):
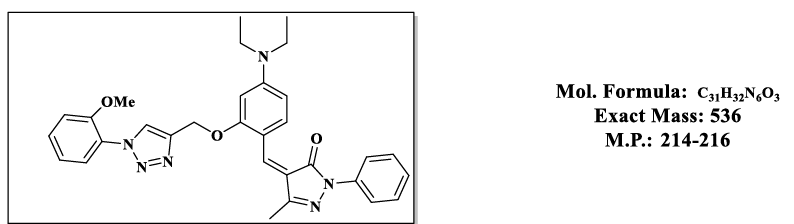
1H NMR (500 MHz, DMSO-d6) δ 8.44 (s, 1H), 7.87 – 7.81 (m, 2H), 7.79 (s, 1H), 7.76 – 7.69 (m, 2H), 7.55 – 7.48 (m, 2H), 7.46 – 7.37 (m, 2H), 7.18 (td, J = 7.2, 1.4 Hz, 1H), 7.05 (d, J = 1.2 Hz, 1H), 6.83 (d, J = 2.2 Hz, 1H), 6.50 (d, J = 2.2 Hz, 1H), 5.36 (s, 2H), 3.84 (s, 3H), 3.47 (q, J = 6.9 Hz, 6H), 2.30 (s, 3H), 1.12 (t, J = 7.0 Hz, 5H).; 13C NMR (125 MHz, DMSO-d6) 163.59, 157.87, 153.17, 152.52, 152.36, 145.29, 137.33, 132.41, 128.25, 127.95, 126.18, 126.01, 124.19, 119.23, 119.04, 118.61, 118.51, 118.50, 116.71, 113.36, 107.54, 98.11, 58.68, 55.04, 43.83, 13.22, 11.66.
4.7.(Z)-4-(4-(diethylamino)-2-((1-(4-methoxyphenyl)-1H-1,2,3-triazol-4-yl)methoxy)+benzylidene)-5-methyl-2-phenyl-2,4-dihydro-3Hpyrazol- 3-one (4.9g):
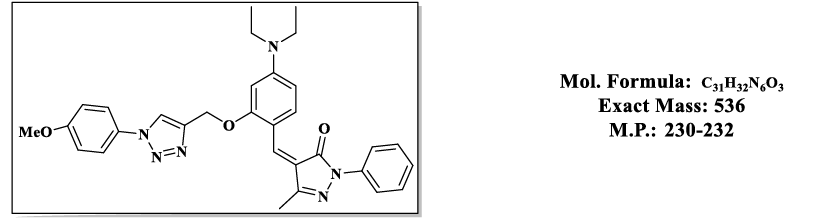
1H NMR (500 MHz, DMSO-d6) δ 8.53 (s, 1H), 7.87 – 7.81 (m, 2H), 7.79 (s, 1H), 7.74 (d, J = 8.1 Hz, 1H), 7.66 – 7.59 (m, 2H), 7.55 – 7.48 (m, 2H), 7.46 – 7.39 (m, 1H), 7.06 – 6.99 (m, 2H), 6.83 (d, J = 2.2 Hz, 1H), 6.50 (s, 1H), 5.36 (s, 2H), 3.76 (s, 3H), 3.47 (q, J = 6.9 Hz, 4H), 2.30 (s, 3H), 1.12 (t, J = 7.0 Hz, 6H); 13C NMR (125 MHz, DMSO-d6) δ 163.57, 159.17, 157.85, 152.50, 152.34, 146.62, 137.31, 132.39, 130.07, 128.23, 125.99, 121.41, 119.49, 118.59, 118.49, 118.48, 116.69, 114.64, 107.52, 98.09, 58.49, 54.64, 43.81, 13.20, 11.64.
4.8.(Z)-4-(4-(diethylamino)-2-((1-(o-tolyl)-1H-1,2,3-triazol-4-yl)methoxy)benzylidene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (4.9h):
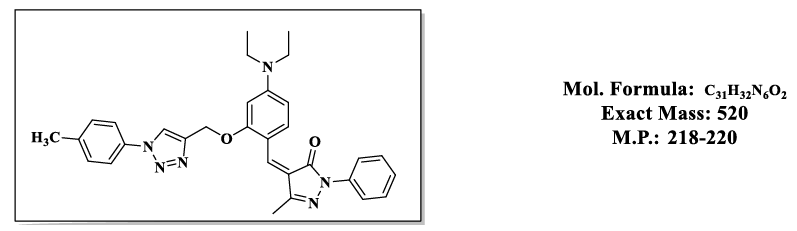
1H NMR (500 MHz, DMSO-d6) δ 8.57 (t, J = 1.0 Hz, 1H), 7.87 – 7.81 (m, 2H), 7.79 (s, 1H), 7.76 – 7.66 (m, 2H), 7.55 – 7.48 (m, 2H), 7.46 – 7.38 (m, 2H), 7.29 – 7.19 (m, 2H), 6.83 (d, J = 2.2 Hz, 1H), 6.50 (s, 1H), 5.36 (s, 2H), 3.47 (q, J = 6.9 Hz, 6H), 2.31 (s, 5H), 1.12 (t, J = 7.0 Hz, 5H);13C NMR (125 MHz, DMSO-d6) δ 163.60, 157.88, 152.53, 152.37, 146.04, 137.34, 135.88, 132.42, 132.09, 130.33, 128.26, 127.91, 126.78, 126.02, 119.96, 119.07, 118.62, 118.52, 118.51, 116.72, 107.55, 98.12, 58.69, 43.84, 16.83, 13.23, 11.67.
4.9.(Z)-4-(4-(diethylamino)-2-((1-(p-tolyl)-1H-1,2,3-triazol-4-yl)methoxy)benzylidene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (4.9i):
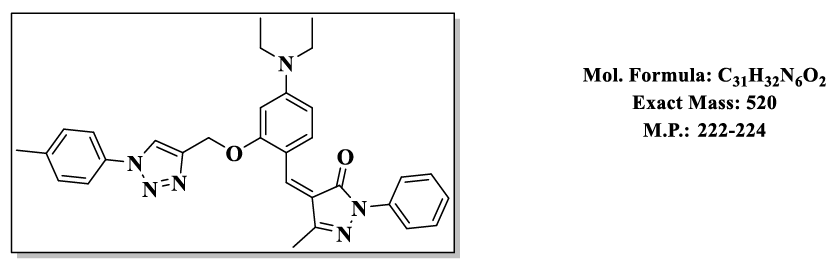
1H NMR (500 MHz, DMSO-d6) δ 8.57 (s, 1H), 7.87 – 7.81 (m, 2H), 7.79 (s, 1H), 7.76 – 7.66 (m, 2H), 7.55 – 7.48 (m, 2H), 7.46 – 7.38 (m, 2H), 7.29 – 7.19 (m, 2H), 6.83 (d, J = 2.2 Hz, 1H), 6.50 (s, 1H), 5.36 (s, 2H), 3.47 (q, J = 6.9 Hz, 4H), 2.31 (s, 6H), 1.12 (t, J = 7.0 Hz, 6H); 13C NMR (125 MHz, DMSO-d6) δ 163.76, 158.04, 152.69, 152.53, 146.81, 137.50, 136.66, 134.11, 132.58, 130.02, 128.42, 126.18, 119.80, 119.31, 118.78, 118.68, 118.67, 116.88, 107.71, 98.28, 58.68, 44.00, 20.56, 13.39, 11.83.
4.10.(Z)-4-(2-((1-(3-acetylphenyl)-1H-1,2,3-triazol-4-yl)methoxy)-4-(diethylamino) benzylidene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol- 3-one (4.9j):
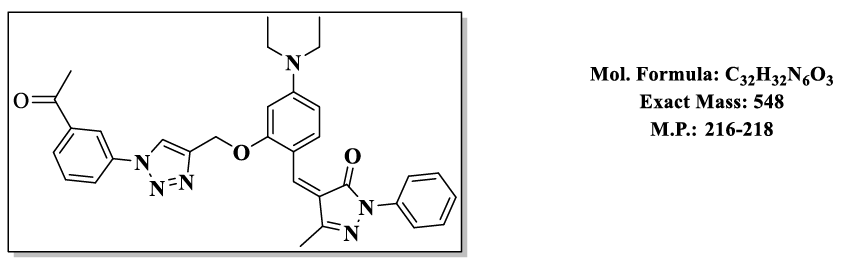
1H NMR (500 MHz, DMSO-d6) δ 8.64 (s, 1H), 8.15 (s, 1H), 7.87 – 7.77 (m, 4H), 7.76 – 7.65 (m, 2H), 7.59 (t, J = 7.4 Hz, 1H), 7.55 – 7.48 (m, 2H), 7.46 – 7.39 (m, 1H), 6.83 (d, J = 2.2 Hz, 1H), 6.50 (s, 1H), 5.36 (s, 2H), 3.47 (q, J = 6.9 Hz, 4H), 2.62 (s, 3H), 2.30 (s, 3H), 1.12 (t, J = 7.0 Hz, 6H); 13C NMR (125 MHz, DMSO-d6) δ 196.82, 163.60, 157.88, 152.53, 152.37, 146.66, 137.55, 137.34, 137.02, 132.42, 129.17, 128.26, 126.02, 125.51, 121.46, 118.99, 118.77, 118.62, 118.52, 118.51, 116.72, 107.55, 98.12, 58.52, 43.84, 26.03, 13.23, 11.67.
4.11.(Z)-4-(2-((1-(4-acetylphenyl)-1H-1,2,3-triazol-4-yl)methoxy)-4-(diethylamino) benzylidene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol- 3-one (4.9k):
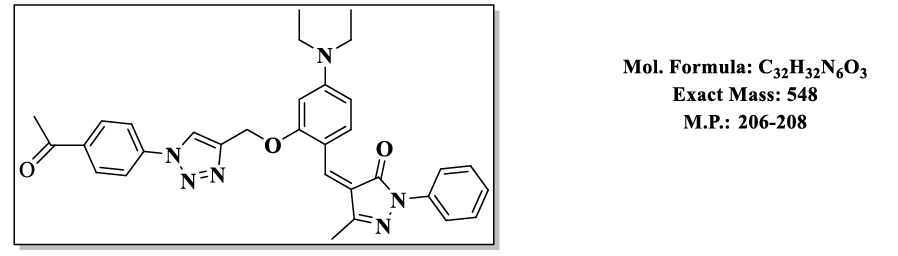
1H NMR (500 MHz, DMSO-d6) δ 8.59 (s, 1H), 7.95 – 7.89 (m, 2H), 7.87 – 7.81 (m, 2H), 7.79 (s, 1H), 7.76 – 7.72 (m, 3H), 7.55 – 7.48 (m, 2H), 7.46 – 7.39 (m, 1H), 6.83 (d, J = 2.2 Hz, 1H), 6.50 (s, 1H), 5.36 (s, 2H), 3.47 (q, J = 6.9 Hz, 4H), 2.56 (s, 3H), 2.30 (s, 3H), 1.12 (t, J = 7.0 Hz, 6H); 13C NMR (125 MHz, DMSO-d6) δ 196.11, 163.63, 157.91, 152.56, 152.40, 146.68, 137.57, 137.37, 134.77, 132.45, 129.96, 128.29, 126.05, 119.42, 119.22, 118.65, 118.55, 118.54, 116.75, 107.58, 98.15, 58.55, 43.87, 25.76, 13.26, 11.70.
4.12.(Z)-4-(4-(diethylamino)-2-((1-(4-nitrophenyl)-1H-1,2,3-triazol-4-yl)methoxy) benzylidene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3- one (4.9l):
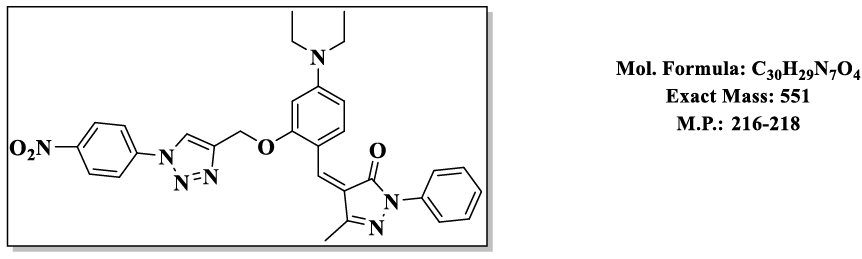
1H NMR (500 MHz, DMSO-d6) δ 8.57 (s, 1H), 8.37 – 8.30 (m, 2H), 7.97 – 7.90 (m, 2H), 7.87 – 7.81 (m, 2H), 7.79 (s, 1H), 7.74 (d, J = 8.1 Hz, 1H), 7.55 – 7.48 (m, 2H), 7.46 – 7.39 (m, 1H), 6.83 (d, J = 2.2 Hz, 1H), 6.50 (s, 1H), 5.36 (s, 2H), 3.47 (q, J = 6.9 Hz, 4H), 2.30 (s, 3H), 1.12 (t, J = 7.0 Hz, 6H);13C NMR (125 MHz, DMSO-d6) δ 163.57, 157.85, 152.50, 152.34, 148.92, 146.62, 138.87, 137.31, 132.39, 128.23, 125.99, 125.30, 120.50, 119.64, 118.59, 118.49, 118.48, 116.69, 107.52, 98.09, 58.49, 43.81, 13.20, 11.64.
Acknowledgements
The authors are grateful to the Head, Department of Chemistry, OU, HYD, for providing laboratory facility, Principal, Govt. Degree College, Bhadrachalam and author special acknowledge to DST-PURSE for providing funding.
REFERENCES
- Al-Mulla A. Der Pharma Chemica. 2017, 9(13): p. 141- 147.
- Hote SV, Bhoyar SP. J Applied Chemistry. 2014, p. 43-46.
- Nirwan N, Pareek C, Chohadia A. 2015.
- Rajpara JG. 2017.
- Vijesh A, Isloor AM, Isloor S, et al., Der Pharma Chemica. 2011, 3(4): p. 454-463.
- Wang Aq, Jin Ts, Cheng Z. Asian Journal of Chemistry. 2010, 22(3): p. 1973.
- Shaglof, Ahlam, Mohamad F Ali, et al., J Pharmaceutical and Applied Chemistry. 2021, 7: p. 8-22.
- Kolb HC, Finn MG, Sharpless KB. Angewandte Chemie International Edition. 2001, 40(11): p. 2004-2021.
- Sirion U, Kim HJ, Lee JH, et al.,Tetrahedron letters. 2007, 48(23): p. 3953-3957.
- Collins CH. Emerg Infect Dis. 2009, 15: p. 1171-1175
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref