Research Article - Der Pharma Chemica ( 2022) Volume 14, Issue 12
Synthesis of 1-(5-chloro-2-hydroxyphenyl)-3-(2-chloro phenyl)-1-oxo-2,3-epoxy propane derivatives as Antimicrobial agents
Awinash S. Chavan1* and Seema P. Rathod22School of Pharmacy Nanded, Swami Ramanand Teerth Marathwada University Nanded, India
Awinash S. Chavan, Department of chemistry, Raosaheb Patil Danve College of Pharmacy Badnapur, Dr. Babasaheb Ambedkar Technological University Lonere, Raigad, India, Email: avichavan4741@gmail.com
Received: 05-Dec-2022, Manuscript No. dpc-22-82303; Editor assigned: 07-Dec-2022, Pre QC No. dpc-22-82303; Reviewed: 21-Dec-2022, QC No. dpc-22-82303; Revised: 23-Dec-2022, Manuscript No. dpc-22-82303; Published: 30-Dec-2022, DOI: 10.4172/0975-413X.14.12.25-28
Abstract
Epoxides are the natural phytoconstituents with several biological significance such as Anti-inflammatory, Antioxidant, Anticancer and Antimicrobial activity. The Series 1-(5-chloro-2-hydroxyphenyl)-3-(2-chloro phenyl)-1-oxo-2,3-epoxy propane derivatives was designed and synthesized from 1-(5-chloro-2-hydroxyphenyl)-3-(2-chlorophenyl)-prop-2-en-1-one.
Current research work screened forIv-vitroanti-bacterial activity against Escherichia coli, Staphylococcus aureus (Pathogens obtained from animal), Xanthomonas citri and Xanthomonas malvacearum (Pathogens obtained from plant). The compound SAC-3 and SAC-6 shows good antibacterial activity while other shows moderate to good anti-microbial activity.
Keywords
Epoxide; Chalcone; Escherichia coli; Anti-bacterial Activity
INTRODUCTION
Chalcones are the natural phytoconstituents widely distributed in plants originate in fruits, vegetables, grains, bark, roots, stems and flowers. The different derivatives of chalcones were synthesized from chalcones with marked biological significance such as Antibacterial, Antifungal, Antimalaria, Anti-inflammatory, Anticancer and Antitubercular activity. Hence, Chalcones are considered as an indispensable component in a variety of nutraceutical, pharmaceutical, medicinal and cosmetic applications with versatile health benefits [1-3].
In organic chemistry epoxides are valuable building blocks in the synthesis of many important reactions for derivatization. Basically for the preparation of surfactants, corrosion protection agents, additives to laundry detergents, lubricating oils, textiles and cosmetics epoxides are play important role in industry.
Present research a novel 1-(5-chloro-2-hydroxyphenyl)-3-(2-chloro phenyl)-1-oxo-2,3-epoxy propane (SAC: 2 to 6) series of synthetic epoxide have been synthesized. Synthesis of novel derivatives is carried out using 1-(5-chloro-2-hydroxyphenyl)-3-(2-chlorophenyl) -1-oxo-2-propene as starting material and followed by to evaluate antimicrobial activity [4,5].
MATERIALS AND METHODS
Melting points were determined in an open capillary tube and are uncorrected. IR spectra were recorded in KBr on a Perkin-Elmer spectrometer. 1H NMR spectra were recorded on a Gemini 300-MHz instrument in Dimethyl Sulfoxide (DMSO) as solvent and TMS as an internal standard. The purity of products was checked by Thin Layer Chromatography (TLC) on silica gel [6-10].
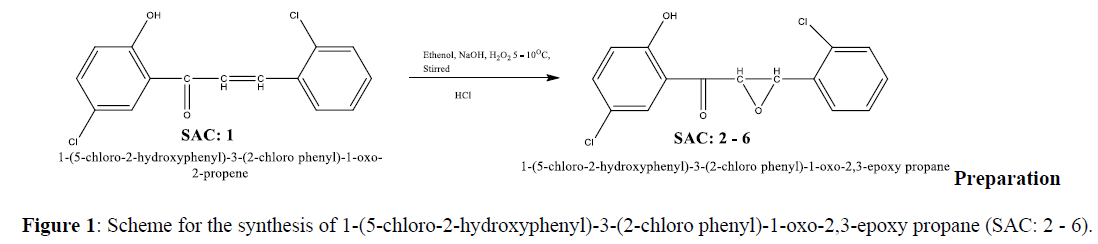
Synthesis Scheme (Figure 1)
Synthesis of 1-(5-chloro-2-hydroxyphenyl)-3-(2-chloro phenyl)-1-oxo-2,3-epoxy propane (SAC: 2 - 6)
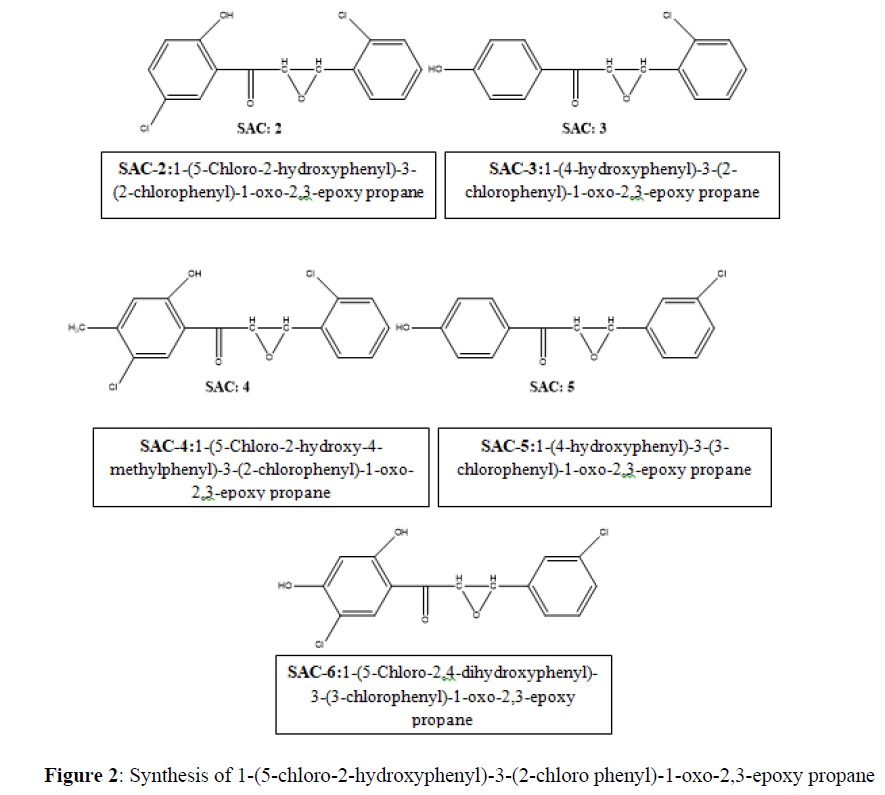
Take 0.01 ml (0.293 g) of 1-(5-chloro-2-hydroxyphenyl)-3-(2-chlorophenyl) -1-oxo-2-propene (SAC:1) and 25 ml of ethanol in a dry conical flask followed by 10 ml of 5% NaOH. Stir reaction mixture until chalcone get completely dissolved. Add 5 ml of 30% hydrogen peroxide in above reaction mixture and stir the reaction mixture for 2 Hrs. Collect the separated solid, filtered, washed with cold water and recrystallized from ethanol. All SAC: 2-6 were synthesized by same reaction mechanism (Figure 2) [11-17].
RESULTS AND DISCUSSION
Spectral Data of 1-(5-Chloro-2-hydroxyphenyl)-3-(2-chlorophenyl)-1-oxo-2,3-epoxy propane (SAC-2):
IR: ѵ max (cm-1): 3120 (OH), 1640 (C=O), 1682, 1585 (C=C aromatic).
1H NMR: δ 4.3 (d, 2H, CH), 4.45 (d, 2H, CH), 12.3 (s, 1H, OH), 7.56 (m, 3H, ArH), 7.95 (m, 4H, ArH).
Spectral Data of 1-(5-Chloro-2-hydroxy-4-methylphenyl)-3-(2-chlorophenyl)-1-oxo-2,3-epoxy propane (SAC-4):
IR: ѵ max (cm-1): 3125 (OH), 1640 (C=O), 1680, 1595 (C=C aromatic).
1H NMR:δ 4.43 (d, 2H, CH), 4.40 (d, 2H, CH), 12.35 (s, 1H, OH), 2.33 (s, 3H, CH3), 7.61 (m, 2H, ArH), 7.20 (m, 4H, ArH) (Table 1,2).
| Sr. No. | Code | Molecular Formula | Mol. Weight (g/mol) | Yield % | Melting Point (ºC) | Rf-Value | Appearance |
|---|---|---|---|---|---|---|---|
| 1 | SAC-2 | C15H10O3Cl2 | 308 | 78 | 156 | 0.6 | Yellow |
| 2 | SAC-3 | C15H11O3Cl | 274 | 75 | 152 | 0.82 | Yellow |
| 3 | SAC-4 | C16H12O3Cl2 | 287 | 79 | 155 | 0.7 | Yellow |
| 4 | SAC-5 | C15H11O2Cl | 258 | 64 | 180 | 0.81 | Brown |
| 5 | SAC-6 | C15H10O4Cl2 | 324 | 72 | 162 | 0.8 | Yellow |
| Sr. No. | CODE | IR (CM-1) | 1H NMR |
|---|---|---|---|
| 1 | SAC-2 | 3120 (OH), 1640 (C=O), 1682, 1585 (C=C aromatic) | δ 4.3 (d, 2H, CH), 4.45 (d, 2H, CH), 12.3 (s, 1H, OH), 7.56 (m, 3H, ArH), 7.95 (m, 4H, ArH). |
| 2 | SAC-4 | 3125 (OH), 1640 (C=O), 1680, 1595 (C=C aromatic) | δ 4.43 (d, 2H, CH), 4.40 (d, 2H, CH), 12.35 (s, 1H, OH), 2.33 (s, 3H, CH3), 7.61 (m, 2H, ArH), 7.20 (m, 4H, ArH). |
BIOLOGICAL SCREENING
Antibacterial Activity: The newly synthesized 1-(5-chloro-2-hydroxyphenyl)-3-(2-chloro phenyl)-1-oxo-2,3-epoxy propane (SAC-2 to SAC-6)were screened for antibacterial activity against Escherichia coli, Staphylococcus aureus (Pathogens obtained from animal), Xanthomonas citri and Xanthomonas malvacearum (Pathogens obtained from plant) using Disc Diffusion Method11. The Filter paper discs were soaked in solution of different compounds at concentration of 100 ppm [18-25]. The solvent aqueous DMF (5% 1ml) used for preparing solution of the compounds. The disc soaked in solution of compound placed at the center of bacteria seeded nutrient agar plates (Petri dishes). The Petri dishes were incubated at 26± 1°C for 24 hrs. The strength is reported by measuring the diameter of zone of inhibition in mm and results were standardized against tetracycline. The zone of inhibition was measured and reported in table 3.
| Sr. No. | CODE | Zone Inhibition in mm | |||
|---|---|---|---|---|---|
| Escherichia coli | Staphylococcus aureus | Xanthomonas malvacearum | X. Citri. | ||
| 1 | SAC-2 | 14 | 9 | 14 | 16 |
| 2 | SAC-3 | 12 | 7 | 11 | 12 |
| 3 | SAC-4 | 18 | 11 | 24 | 26 |
| 4 | SAC-5 | 15 | 14 | 17 | 21 |
| 5 | SAC-6 | 21 | 16 | 19 | 22 |
| 6 | Standard(Tetracycline) | 22 | 26 | 23 | 24 |
CONCLUSION
The series of 1-(5-chloro-2-hydroxyphenyl)-3-(2-chloro phenyl)-1-oxo-2,3-epoxy propane (SAC-2 to SAC-6) and derivatives have synthesized by conventional method and further screened for biological activity. All the compounds showed potent antibacterial activity [26-31].
ACKNOWLEDGMENT
Authors gratefully acknowledge to Raosaheb Patil Danve College of Pharmacy Badnapur, Dist. Jalna, Dr. Babasaheb Ambedkar Technological University Lonere, Raigad for providing laboratory facilities. Authors are also thankful to Principal, Teaching and Non-Teaching Members of Raosaheb Patil Danve College of Pharmacy Badnapurfor supporting in all work.
Conflict of interest statement
The authors report no conflict of interest.
REFERENCES
- Graham L Patrick. Oxford University press. 2006, p. 161-162.
- Akama T, Shida Y, Sugaya T, et al., J MedChem. 1996, 39: p. 3461-3469.
- Lili Ma, Bonfield K, Amato E, et al., Bioorg Med Chem. 2012, 20: p.2603-2613.
- Chulia AJ, Pouget C, Fagnere C, et al., Tetrahedron. 2000, 56: p. 6047-6052.
- Brueggemeier RW, Hackett JC, Kim YW, et al., BioorgMed Chem. 2005, 13: p. 4063-4070.
- Tran TD, YeonSook C, Jeong K, et al., Bioorganic Med Chem Lett. 2004, 14: p. 1165-1167.
- Cotelle N, Bernier J, Catteau J, et al., Free RadBioMed. 1996, 20: p. 35- 43.
- Khan MSY, Hasan SM. IJC. 2003, p. 1970-1974.
- Patil SG, Utale PS, Gholse SB, et al., J Chem Pharm Res. 2012, 4: p. 501-507.
- Nitin GG, Rajput PR. IntJ Pharm Bio Sci. 2102, 3: p. 389-395.
- HakanGoker, David W Boykin, SulhiyeYildiz. Bioorg Med Chem. 2005, 13: p. 1704-1714.
- Kim YW, Hackett JC, Brueggemeier RW. JMed Chem. 2004, 47: p. 4032- 4040.
- Yadav MR, Sabale PM, Geridhar R. Steroids. 2011, 76: p. 464- 470.
- Kumar S, Pandey AK. ScientificWorldJournal. 2013, p. 1-2.
- Priya k, Neha B, Robinka K. J Pharm SciPharmacol. 2014, 3: p. 2188-2216.
- Serafini M, Peluso I, Raguzzini A. ProcNutr Soc. 2010, 69: p. 273-278.
- Shashank K, Pandey K. ScientificWorldJournal. 2013, 16: p.162-750.
- Agrawal D. Int J Pharm Sci nanotech. 2011, 4: p. 7-11.
- Partha PR, Kunal R. J Pharm SciPharmacol. 2010, 62: p. 1717-1728.
- Virapong P, Naravut S, Chanin N, et al., Molecules. 2011, 16: p. 3597-3617.
- Yujie D, Qiang W, Xiuli Z, et al., Eur J Med Chem. 2010, 45: p. 5612-5620.
- Cavalli A, Bisi A, Bertucci C, et al., J Med Chem. 2005, 48: p. 7282-7289.
- Indu AG, Punnagai M, Vasavi CS, et al., Int J Pharm Sci Res. 2014, 6: p. 141-148.
- Tang G, Xiangping D, Wang Z, et al., RSC advances. 2017, p. 38171-38178.
- Patel S, Shah U. Asian J Pharm Clin Res. 2017, 10: p. 403-406.
- Leonor PR, Cardenas M, Marder M, et al., Bioorg Med Chem. 2006, 14: p. 2966-2971.
- Ahmed K, Murty JN, Viswanath A, et al., Bioorg Med ChemLett. 2012, 22: p. 4891-4895.
- Koneni VS, Manoj K, Abdhesh K. Tetrahedron Letters. 2012, 53: p. 2355-2359.
- Cutler SJ, Kabbani FM, KeaneC, et al., Eur J Med Chem. 1993, 28: p. 407-414.
- Bano S, Javed K, Ahmed S, et al., Eur J Med Chem. 2013, 65: p. 51-59.
- Patil SP. IJPBS. 2013, p.1-10.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref