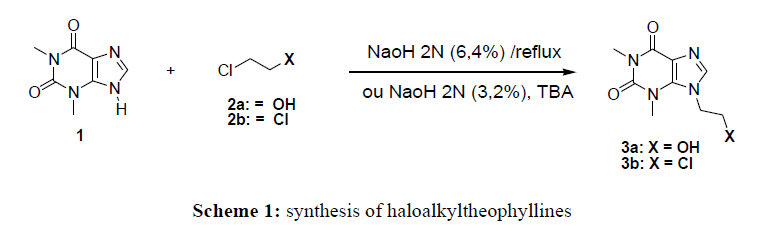Research Article - Der Pharma Chemica ( 2021) Volume 13, Issue 5
Synthesis of 7- (Ethylaryl) Theophylline DDérivatives from Acyclovir
Sagne Jacques Akpa*, GbeGondo Didier Diomande, Venance Martial Say and Ané AdjouSagne Jacques Akpa, Laboratoire de Constitution et de Recherche de la Matière – UFR SSMT – Université Félix Houphouët, Boigny 22 B.P. 582 Abidjan 22, Cote D'Ivoire, Email: sagnbest@yahoo.fr
Received: 28-Apr-2021 Accepted Date: Jul 15, 2021 ; Published: 22-Jul-2021
Abstract
In the search for new antiviral drugs, we decided to synthesize new acyclovir derivatives which are widely used in the treatment of Herpes. This work consisted of exploring the positon-7 of theophylline by alkyl halides, which led to compounds 3a-b. The introduction of chloroaryl and thioaryl groups on the latter allowed to obtain compounds 5a-c and 7a-b. The chemical structures of all synthesized compounds were determined by 1 H, 13 C NMR and electron impact mass spectrometry.
Keywords
Theophylline, Acylclovir, Alkoxylaryl, Akylsulfanylaryl
Introduction
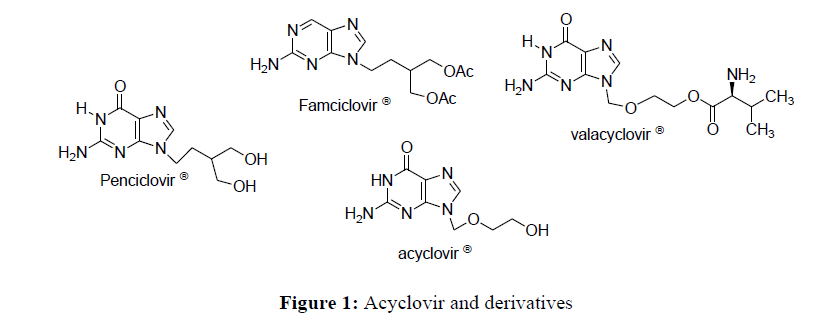
Theophylline is a heterocyclic compound, derived from purine [1]. It results from the combinationof a pyrimidine nucleus and an imidazole cycle. A little work has been done on this heterocycle. This could be explained by its low solubility [2,3] in organic solvents. Studies have been made to highlight a few main substitution sites at position 7, 8 and 9. The variations in these different positions allow to obtain theophylline derivatives [4]. Biologically, theophylline and its derivatives constitute a heterocyclic support for a few drugs such as Penciclovir ®, acyclovir®, Famciclovir® and Valacyclovir® (Figure 1), which are antivirals [5] for the treatment of genital herpes [6] and other viruses.
In this work we make a contribution to the synthesis of theophylline derivatives by introducing alkoxylaryl and akylsulfanylaryl groups at position - 7 of acyclovir [7-11].
Materials and Methods
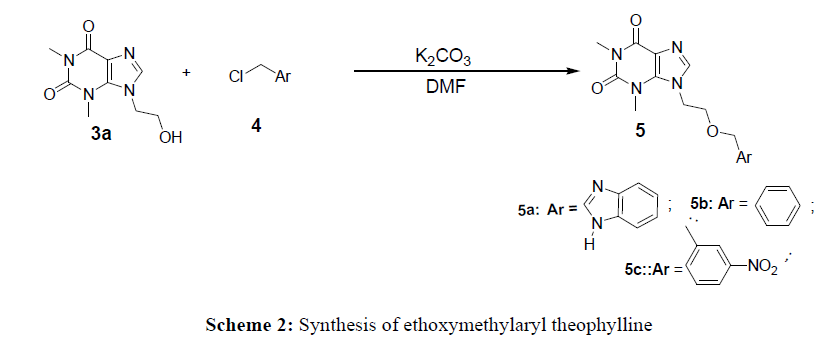
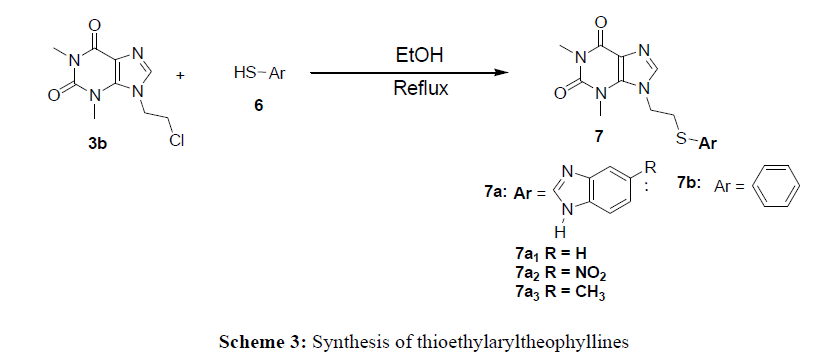
Due to its low solubility in organic solvents, the reactivity of theophylline vis-à-vis 2-chloroethanol and 1,2-dichloroethane has not been easy. This led us to do a correlation study with the solvent (NaOH) and the yield (%). For this purpose, only concentrated sodium hydroxide (2N) 6.4% allows to have a yield above 36%. In addition, the yield is increased around 80% by adding a catalyst such as tetrabutylammonium (TBA) in the 2N sodium hydroxide solution at 3.2% The method used for the synthesis of compounds 3a-b consisted of reacting theophylline with 1,2-chloroethanol (2a) and 1,2-dichloroethane (2b) in a 2N sodium hydroxide solution in the presence of 2% TBA at reflux. This allowed us to obtain 7- (2- chloroethyl) theophylline with a yield of 88% and 7- (2-chloroethanol) theophylline at 86%. This work consisted of synthesizing 7N-theophylline derivatives by using coupling ethylthioaryl and ethoxymethylaryl groups. Thus, condensation of chloromethylaryl and thioaryl with compounds 3a and 3b lead to the 7N-theophylline derivatives. To obtain compounds 5a-c, the synthesis provides for a nucleophilic substitution reaction which consisted of reacting the chloromethylaryl group with compound 3a. This reaction took place in DMF in the presence of K2CO3. As for molecules 7a-b, they were obtained by exploiting the nucleofuge characteristics of the chlorine atom of compound 3b and the nucleophile of the sulfur atom of compound 4. Thus the mixture of 1.5eq of thioaryl4 and of the compound 3b at reflux in ethanol led to derivatives 7a-b.
Experimental Section
The Nuclear Magnetic Resonance (NMR) spectra of the 1H proton (300–400 MHz) and those of the 13C carbon (75-100 MHz) were recorded on a Brüker advance 300 device. Tetramethylsilane (TMS) is used as the internal reference for chemical shifts expressed in ppm. Mass spectra (MS) were performed on an HP 5889A quadrupole spectrometer with electronic impact (IE) or chemical ionization (CI). Melting points were determined using a KÖFFLER temperature graded bench (40°C-260°C). Dichloromethane, toluene, ethanol, ethyl acetate and hexane are distilled under atmospheric pressure. The purifications by column chromatography were carried out on silica gel of Kieselgel 60 type (230-400 mesh-Merck).
General procedure for the synthesis of compounds 3
In a Bicol, 2 g (1.1 mmol) of theophylline are dissolved in 40 ml of a 2N sodium hydroxide solution (3.2%). The solution mixture is stirred for 15 minutes. Then, 5 ml of compound 2 and (0.2 eq) of tetrabutylammonium (TBA) are added. The reaction mixture is brought to reflux for 2 hours. At the end of the reaction, a 10% hydrochloric acid solution is added to the reaction medium. After extracting with ethyl acetate, the organic layer is dried over magnesium sulfate. The solvent is evaporated under reduced pressure and the crude product is purified by chromatography on silica gel (Ethyl acetate / Hexane: 4/1).
7- (2-Hydroxyethyl) theophylline 3a
Yield: 86%: mp = 127°C; -RMN 1H (400MHz, chloroform-d, δ ppm): 3.35 (s, 3H, CH3); 3.54 (s, 3H, CH3); 3.87 (t, 2H, CH2); 4.42 (t, 2H, CH2); 7.9 (s, 1H, CH3).-RMN13C (chloroform-d, δ ppm): 49.2 (N-CH3); 60.2 (CH2 - OH);-SDM: m / z = 225 (100%); 226 (15%); 224 (10%)
7- (2-Chloroethyl) theophylline 3b
Yield: 88%; mp = 160°C;-RMN 1H (400MHz, chloroform-d, δ ppm): 3.39 (s, 3H, CH3); 3.59 (s, 3H, CH3); 3.98-3.87 (m, 2H, N-CH2-); 4.57 (dd, 2H, Cl-CH2-); 7.63 (s, 1H, N = CH); 7.92 (s, 1H, OH).; -RMN13C (chloroform-d, δ ppm): 28.13 (CH3-); 29.98 (CH3); 43.38 (N-CH2-); 49.02 (-CH2-Cl); -SDM: m / z = 241.9 (10%); 243 (100%); 245 (40%).
7- (etoxymethylbenzimidazolyl) theophylline 5a
In a bicol, 0.2g (0.000893 mol) of 7- (2-hydroxyethyl) theophylline and 2.5 eq (0.00223 mol) of potassium carbonate are stirred indimethylformamide (DMF) for 30 minutes.. Then, 1.2 eq of chloromethylbenzimidazole is added to the mixture mixture, followed by refluxing for 3 hours. A 10% hydrochloric acid solution is added to the reaction medium after cooling to room temperature. The organic phase is extracted with ethyl acetate and washed with salt water. The solvent is evaporated under reduced pressure. The crude product is recrystallized from a mixture of solvent (ethyl acetate / Hexane 1/1). yield = 87%; mp = 232-234°C.
7- (Ethoxybenzyl) theophylline 5b
In a bicol, 0.2g (0.000893mol) of 7- (2-hydroxyethyl) theophylline and 2.5 eq (0.00223 mol) of potassium carbonate are stirred in dimethylformamide (DMF) for 30 minutes.. Then, 1.2 eq of benzyl chloride are added to the mixture mixture, followed by refluxing for 3 hours. A 10% hydrochloric acid solution is added to the reaction medium after cooling to room temperature. The organic phase is extracted with ethyl acetate and washed with salt water. The solvent is evaporated under reduced pressure. The crude product is recrystallized from a mixture of solvent (ethyl acetate / Hexane 1/1).
Yield = 75%; mp> 270°C.
7- (Ethoxy-3-nitrobenzyl) theophylline 5c
In a bicol 0.2 g (0.000893mol) of 7- (2-hydroxyethyl) theophylline and 2.5 eq(0.00223 mol) of potassium carbonate are stirred in DMF for 30 minutes. Then, 1.2 eq of 3-nitrobenzyl chloride are added to the mixture mixture, followed by refluxing for 3 hours.. A 10% hydrochloric acid solution is added to the reaction medium after cooling to room temperature. The organic phase is extracted with ethyl acetate and washed with salt water. The solvent is evaporated under reduced pressure. The crude product is recrystallized from a mixture of solvent (ethyl acetate / Hexane 1/1). Yield = 60%; mp> 270°C.
7- (Ethylthiobenzimidazolyl) theophylline 7a
In a Bicol, 0.322g (2mmol) of 7- (2-chloroethyl) theophylline are dissolved in 10ml of an ethanol solution. The solution mixture is stirred for 15 minutes. After adding 1.5 eq of 2-mercaptobenzimidazole the reaction mixture is brought to reflux for 4 hours. Cooling to room temperature, the reaction medium is neutralized by adding a 5% sodium hydrogen carbonate solution (NaHCO3). The organic phase is extracted with ethyl acetate and dried over magnesium sulfate. The solvent is evaporated off under reduced pressure and the crude product is purified by chromatography on silica gel (Ethyl acetate / Hexane: 4/1).
Yield = 72%; Mp = 155°C.
RMN 1H (400MHz, DMSO-d6, δ ppm) : 3,22 (s, 6H , -CH3- ) ; 3,74(dd , J=6.6,5.1 Hz, 2H, N-CH2-) ; 4,60 (dd, 2H, -CH2-S) ; 7,04 (dd, 2H, HAr) ; 7,32 (dd, 2H , HAr) 7,96 (s, 1H, N=CH-): RMN 13C ( DMSO, δ ppm ) : =27,56 (CH3-); 29,22 (CH3-) ;32,00 (N-CH2-) ;46,49 (-CH2-S) ;150( CAr) ; 149,34( CAr) ; 148,34( CAr).
7- (2-ethyltiophenyl) theophylline 7b
In a Bicol, 0.3 g (1.2397 mmol) of 7- (2-chloroethyl) theophylline are dissolved in 10 ml of an ethanol solution and stirred under magnetic for 15 minutes. After adding 1.5 eq of thiophenol the mixture is brought to reflux for 4 hours. The reaction medium is cooled and neutralized with a 5% sodium hydrogen carbonate solution (NaHCO3). The organic phase is extracted with ethyl acetate and dried with magnesium sulfate. The solvent is evaporated off under reduced pressure and the crude product is purified by chromatography on silica gel (Ethyl acetate / Hexane: 4/1).(Schemes 1- 3).
Yield = 77%; Mp = 120°C; 1H NMR (CDCl3, δ ppm): 3.40 (s, 3H, CH3-); 3.49 (t, 2H, N-CH2-); 3.50 (s, 3H, CH3-); 4.43 (t, 2H, -CH2-S); 7.25 (m, 5H, HAr); 7.48 (s, 1H, N = CH-); RMRN 13C (CDCl3, δ ppm): 29.73 (CH3-); 29.90 (CH3-); 34.65 (N-CH2-); 47.08 (-CH2-S); 126.75 (CAr); 127.50 (CAr); 128.89 (CAr); 129.05 (CAr); 130.17 (CAr); 133.95(CAr).
Results and Discussions
9 derivatives of 7- (ethylaryl) theophylline (3a-b, 5a-c and 7a-b) were synthesized. The reaction yields, the melting points, as well as the spectroscopic data (1 H and 13 C NMR, MS) were determined. All these new theophylline derivatives are carried at position-7 with a thioalkylaryl and ethoxymethylaryl chain. Analysis of the 1 H NMR spectra for compound 3arevealled the presence of two triplets δ = 3.9ppm and δ = 4.4ppm which are attributed to the protons of the alkoxyl group. The analysis also revealled the disappearance of the proton signal from pyrrol nitrogen which was around 12 ppm. However, we had no signal around 5ppm, which corresponded to the hydroxyl proton. This could be explained by the use of deuterated methanol (CD3OD) as solvent for the 1 H NMR. As for the 13C NMR spectrum, it showed two significant peaks at δ = 49.2ppm and δ = 60.2 ppm which indicated the presence of alkoxyl group (NCH2CH2OH). Also the mass spectrum M + 1 as base peak had the value of m / z = 225, confirmed the mass of our expected compound. As for compound 3b, its mass spectrum gave a basic peak at m / z = 241.66 which corresponded to the peak M + 1. This confirmed that the expected compound has been obtained. Compounds 5b-c had low solubility in organic solvents. Consequently, its 1 H NMR spectrum had poor resolution. However, between δ = 7ppm and δ = 8ppm, there was a multiplet corresponding to the aromatic protons. Compound 5a also had low solubility in most organic solvents. This made it difficult to produce the 1 H NMR spectrum, which affected the quality of the spectrum. However around δ = 11 ppm, we noted the presence of a signal corresponding to the pyrrol nitrogen proton of the benzimidazole nucleus. This major information confirmed the structure of the expected compound. As for compound 7a, Analysis of the 1 H NMR spectrum in DMSO revealled the presence of two signals at 3.74 ppm and 4.60 ppm which could be attributed to the two methylenes between nitrogen and sulfur (N-CH2-CH2-S). We also observed the presence of two important signals at 7.04 ppm and 7.32 ppm corresponding to the four protons of the benzene nucleus of benzimidazole. On the 13C NMR spectrum, we noted the presence of two peaks at 32 ppm and 46.49 ppm indicating the carbons of the ethylene group. This confirmed the formation of compound 7a. Compound 7b presented on its 1H NMR spectrum, two important signals at 3.5 ppm and 4.43 ppm corresponding to the protons of the two methylenes (N-CH2-CH2-S). We also observed the presence of a broad multiplet around 7.25 ppm corresponding to the protons of the benzene aromatic nucleus. On the 13C NMR spectrum, we noted the presence of two peaks at δ=29.73 ppm and δ=29.90 ppm which could be attributed to the carbons of the ethylene group. This confirmed the formation of compound 7b.
Conclusion
This work allowed us to access by chemical synthesis new derivatives of theophylline, carried at position-7 with a chain thioalkylaryl and alkoxylaryl. This could open investigation route towards the development of a chemical class of anti-infective.
Acknowledgements
Our sincere thanks to the laboratory of the University of Amiens for the spectroscopic analyzes.
References
- Alain MARTINE, universite des sciences et techniques de lille, 1974, p. 2-5.
- Georges Philippossian and Enslen Marc. 1980, p. 26-11.
- Georges Philippossian and Enslen Marc. 1985.
- Teresa H. Bacon, Barbara A. Howard and Lindsay C. J Antimicrob Chemot. 1996, 37, p. 303-313.
- Dannaoui E, Trepo C and F Zoulim. Antiviral Chemistry & Chemotherapy. 1997, 8(1): p. 38-46
- Georges Philippossian and Marc Enslen.1985, p. 1-5
- De Clercq, E. J Med Chem. 2005, 48, p. 1297.
- Balzarini J. Nucleosides Nucleotides. 1996, 15: p. 821.
- De Clercq, E. Biochem J. 1982, 205: p. 1.
- Reusser P. Exp. Opin Invest Drugs. 2001, 10: p. 1745.
- Nichols WG and Boeckh M. J Clin Virol. 2000, 16: p. 25.