Research Article - Der Pharma Chemica ( 2022) Volume 14, Issue 11
Synthesis of Biologically Active compound 1,4-dihydropyridine by using An Efficient and Versatile Silica supported MgO Catalyst
Dipak R Tope1, Shilpa L. Sangle2*, Jyoti A. Agashe1, Yogita R. Shelke1 and Ashok V. Borhade12Department of Chemistry, SSSM ASC College, Saikheda-422210, Nashik, India
Shilpa L. Sangle, Department of Chemistry, SSSM ASC College, Saikheda-422210, Nashik, India, Email: shilpasangle620@gmail.com
Received: 10-Nov-2022, Manuscript No. dpc-22-79479; Editor assigned: 12-Nov-2022, Pre QC No. dpc-22-79479; Reviewed: 26-Nov-2022, QC No. dpc-22-79479; Revised: 28-Nov-2022, Manuscript No. dpc-22-79479; Published: 05-Dec-2022, DOI: 10.4172/0975-413X.14.11.8-13
Abstract
A simple one pot synthesis has been developed for the synthesis 1,4-dihydropyridine using an efficient and reusable silica supported MgO solid catalyst by condensation of dimedone, ethyl acetoacetate, aldehyde and ammonium acetate in methanol as a solvent at room temperature. The reactions could be carried out under mild reaction conditions with very good yield of polyhydroquinoline, up to 92%. This catalyst could be recycled very easily, which makes this methodology environmentally benign.
Keywords
1,4-dihydropyridine; Multicomponent reaction; Silica supported MgO; Recyclable catalyst
INTRODUCTION
1,4-dihydropyridine (1,4-DHP) and their derivatives have attracted considerable interest in recent times because of their promising biological activities such as vasodilators, bronchodilators, Anti-atherosclerotic, anti-tumor, neuroprotective drugs, hepatoprotective and antidiabetic agents [1]. Moreover, 1,4-DHPs exhibits several medicinal applications which include neuroprotectant [2] and platelet anti-aggregatory activity [3]. Also they have been reported for their applications in treatment of Alzheimer’s diseases [4] due to their cerebral anti-schematic activity and chemo-sensitizer in tumor-therapy [5]. These examples clearly demonstrate the remarkable potential of 1,4-DHPs as a source of valuable drug candidate.
Dihydropyridines are particularly well known in pharmacology as L-type calcium channel blockers, used in the treatment of hypertension. Compared with certain other L-type calcium channel blockers (for example those of the phenylalkylamine class such as Verapamil) which have significant action at the heart, they are relatively vascular selective in their mechanism of action in lowering blood pressure.
In recent years, much attention has been focused on the synthesis of 1,4-dihydropyridyl compounds (1,4-DHPs), due to their significant biological activity. Cardiovascular agents such as nifedipine, nicardipine, amlodipine and other related derivatives are dihydropyridyl compounds, which are effective for the treatment of hypertension. 4-Aryl-1,4- dihydropyridines are analogs of NADH co-enzymes, which have been explored for their calcium channel activity and the heterocyclic rings are found in a variety of bioactive compounds such as bronchodilators, geroprotective and hepatoprotective agents. Extensive studies indicate that these compounds exhibit different medical functions, acting as neuroprotectants, antiplatelet aggregators, cerebral antiischemic agents and chemosensitizers. For these reasons, polyhydroquinoline compounds not only have attracted the attention of chemists to synthesize but also represent an interesting research challenge.
Owing to the wide range of biological and medicinal activities, the synthesis of such compounds has become an important target in recent years. In 1882, Arthur Hantzsch [6] reported first synthesis of substituted 1,4-dihydropyridines by one-pot condensation of ethyl acetoacetate, aromatic aldehydes and ammonia. The reaction was carried out in acetic acid at reflux temperature in ethanol for long period provide low to moderate yields. Recently a number of methods have been reported for the synthesis of 1,4-dihydropyridine using alum, molecular iodine, [7] HClO4-SiO2 [8], TMSCl [9] ceric(IV)ammonium nitrate [10] L-proline [11] ionic liquids [12] expensive metal triflate Yb(OTf)3 [13] Sc(OTf)3 [14] scolecite [15], ZnO-beta zeolite [16], Baker’s yeast [17] and solid phase organic synthesis technique [18] All reported methods has its own merits, while some of these methods suffers from one or more drawbacks such as poor yields, longer reaction time, difficult work-up procedure and effluent pollution.
Hence development of clean, efficient and high yielding catalyst under mild reaction conditions for environmentally benign protocols is highly desired. The possibility of performing multi-component reactions with a heterogeneous catalyst could enhance their efficiency from an economic as well as ecological point of view. In continuation to our work on the applications of heterogeneous catalysts in organic transformations [19,21] we report here a convenient and efficient method for the synthesis of 1,4-dihydropyridine derivatives using silica supported MgO catalyst (Scheme 1).
EXPERIMENTAL
All chemicals were employed are commercial products (Aldrich Chemical Co.) and were used without purification. All yields refer to isolated products after purification. 1H (300 MHz) NMR and 13C (75 MHz) NMR spectra were recorded on Varian mercury XL-300 and Bruker spectrometer instruments using TMS as internal standard. The solvent used for NMR spectra was CDCl3 and DMSO-d6. Infra-red spectra were taken on Shimadzu FTIR–408 on KBr pellet. The mass spectra were recorded on Shimadzu GC-MS QP trap 2010A mass spectrometer with an ionization potential of 70 eV. Column chromatography was performed on silica gel (230–400 mesh) supplied by Acme Chemical Co.
RESULT AND DISCUSSION
Characterization of MgO nanoparticles
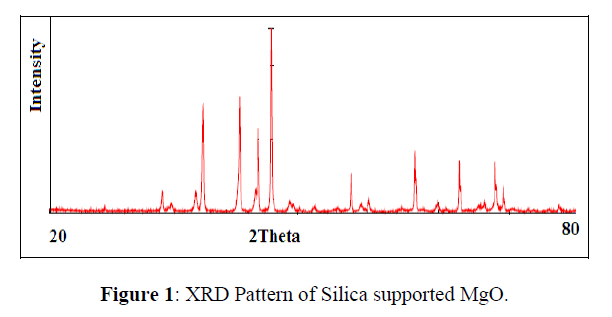
The XRD pattern of Silica supported MgO nanoparticles (figure 1) have fundamental peak due to diffraction of MgO on the plane 111, 200, 311, 222, 400, 331, 420 and 422. The XRD patterns of synthesized MgO nanoparticles show single phase system with average particle size of 47 nm (Figure 1).
The XRD pattern is in agreement with cubic structure of MgO nanoparticles (JCPDS card no. 02-1102). The SEM image (figure 2) decides the morphology of MgO nanoparticles. The SEM image confirms the cubic structure of MgO nanoparticles, uniform shape and size. The elemental analysis of MgO nanoparticles confirmed by EDAX spectrum , it shows the presence of Mg and O element in the synthesized sample material.
The TEM image (Figure 3a) reveals that nanomaterial is of cubic structure. The dark spot in the SAED micrograph (Figure 3b) can be alluded to synthesized MgO nanoparticles as the SAED pattern. Such a spot reveals the occurrence of cubic MgO in the total agreement with XRD data. The average size of MgO nanoparticles obtained by TEM was found around 47 nm (Figure 3).
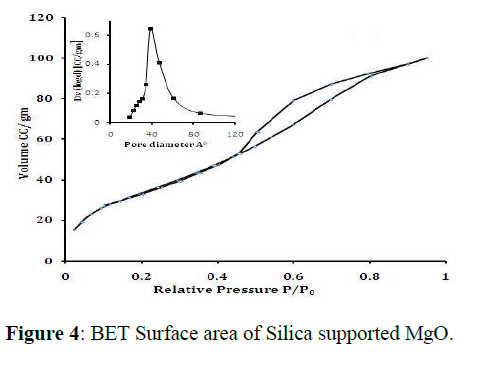
The N2 adsorption-desorption isotherms and BJH pore size distribution of MgO nanoparticles (figure 4) reveals that the samples have typical IV N2 adsorption-desorption isotherms with H1 hysteresis. The BJH pore size distribution demonstrates that all the samples have a narrow pore diameter range. Based on the N2 adsorption-desorption isotherms, the specific surface area (SBET) of CdO nanoparticles obtained from BET method is 29.71 m2/g, the average pore volume (VP) and pore diameter (dp) were 0.04630 cc/g and 24.87 Aº (Figure 4).
Catalytic results
In present work, we wish to report the one-pot multicomponent synthesis of substituted 1,4-dihydropyridines catalyzed by MgO nanoparticles as a heterogeneous solid catalyst. In a typical run, dimedone (1.1 mol), aryl aldehyde (1.3 mol), Ethyl acetoacetate (1.0 mol) and silica supported MgO (0.8 mol) nanoparticles were allowed to react in methanol at 80ºC for 25 min. The reaction mixture was directly filtered and washed with methanol. The recovered catalyst was dried and reused further in successive reactions. Filtrate was collected and evaporated under reduced pressure to afford the product. The isolated products were characterized by IR, NMR and Mass spectrometry.
Spectral data of representative compounds
Ethyl 1,4,5,6,7,8-hexahydro-2,7,7-trimethyl-5-oxo-4-phenylquinoline-3-carboxylate (5a): 1H NMR (300 MHz, CDCl3): δ 0.94 (s, 3H), 1.09 (s, 3H), 1.14 (t, J = 7.3 Hz, 3H), 2.13-2.34 (m, 4H), 2.37 (s, 3H), 4.05 (q, J = 7.3 Hz, 2H), 5.02 (s, 1H), 5.74 (s, 1H),7.03-7.34 (m, 5H); 13C NMR (75 MHz, DMSO-d6) δ 14.2,19.1, 21.3, 27.6, 36.5, 37.3, 59.8, 106.0, 113.7, 126.3, 127.8, 128.0, 143.3, 147.1, 149.2, 167.3, 194.8; IR (KBr cm-1): 3233, 3210, 3080, 1696, 1602, 1059, 692; m/z = 340 (M+H)+.
Ethyl 1,4,5,6,7,8-hexahydro-2,7,7-trimethyl-4-(3-nitrophenyl)-5-oxoquinoline-3-carboxylate (5b): 1H NMR (300 MHz, CDCl3): δ 0.96 (s, 3H), 1.04 (s, 3H), 1.22 (t, J = 7.3 Hz, 3H), 2.10-2.34 (m, 4H), 2.38 (s, 3H), 4.01 (q, J = 7.3 Hz, 2H), 4.96 (s, 1H), 6.32 (s, 1H), 6.74-7.38 (m, 4H); 13C NMR (75 MHz, DMSO-d6): δ 14.18, 19.32, 21.1, 27.3, 33.1, 33.90, 59.5, 105.4, 112.3, 121.2, 122.8, 128.6, 134.8, 144.6, 148.3, 149.5,151.0, 166.9, 196.0; IR (KBr in cm-1): 3303, 2954, 1683,1610, 1167, 759; m/z = 385 (M+H)+.
Ethyl 1,4,5,6,7,8-hexahydro-4-(4-methoxyphenyl)-2,7,7-trimethyl-5-oxoquinoline-3-carboxylate (5g): 1H NMR (300 MHz, CDCl3): δ 0.96 (s, 3H), 1.06 (s, 3H), 1.22 (t, J = 7.2 Hz, 3H), 2.10-2.26 (m, 3H), 2.34-2.40 (m, 4H), 3.77(s, 3H), 4.02 (q, J =7.2 Hz, 2H), 5.08 (s, 1H), 5.85 (s, 1H), 6.71-7.24 (m, 4H); 13CNMR (75 MHz, DMSO-d6) δ 14.3, 17.9, 26.3, 28.8, 32.4,35.0, 50.1, 50.4, 55.1, 59.2, 102.7, 109.5, 113.4, 128.3, 128.5,140.0, 144.9, 149.1, 156.8, 168.2, 193.8; IR (KBr in cm-1):3281, 3199, 3080, 1708, 1607, 1224, 837; m/z = 370 (M+H)+.
RESULTS AND DISCUSSION
In order to determine the most appropriate reaction conditions and evaluate the catalytic efficiency of silica supported MgO catalyst for the synthesis of 1,4-dihydropyridine, initially a model study was carried out on the synthesis of 1,4-dihydropyridine (1,4-DHP) (Scheme 1) using p-chloro benzaldehyde as a model substrate. The reaction was monitored by TLC technique using ethyl acetate-hexane (3:7 v/v) as a solvent system.
To evaluate and optimize the effectiveness of silica supported MgO with different catalyst, we tried MgO, SiO2, 25%, 50% and 75% Silica supported MgO for the cyclization reaction of dimedone, ethyl acetoacetae and p-chlorobenzaldehyde. MgO, SiO2 gave poor yield while 25%, 50%, 75% silica supported MgO gave good yield but required more time as compared to silica supported MgO (Table 1).
| Entry | Catalyst | Time (min) | Yield* |
|---|---|---|---|
| 1 | MgO | 90 | 34 |
| 2 | SiO2 | 65 | 79 |
| 3 | SiO2/MgO | 25 | 94 |
| 4 | 75% SiO2/MgO | 30 | 89 |
| 5 | 50% SiO2/MgO | 50 | 81 |
| 6 | 25% SiO2/MgO | 40 | 79 |
With increasing silica loading from 25% to 100%, cyclization substantially increases and it reaches a maximum (94%) at the silica content of 100%. Therefore, it shows that 100 % silica supported MgO results in higher catalytic activity. Thus, it is obvious from our studies that silica supported MgO was superior in the cyclization reaction with good yield in short reaction time. To optimize catalyst required for the cyclization, we carried out reaction using various mole equivalents of the catalyst with respective dimedone (Table 2).
| Entry | Amount of SiO2/MgO (mol) | Time (min) | Yield* |
|---|---|---|---|
| 1. | 0.1 | 80 | 78 |
| 2. | 0.4 | 40 | 89 |
| 3. | 0.8 | 26 | 94 |
| 4. | 1.2 | 25 | 87 |
It was found that when reaction was carried out with 0.8 mol, equivalent catalyst gives 94% yield. Different solvents were studied such as DMF, methanol, ethanol, acetonitrile, dichloromethane and among the methanol were found to be the best choice (Table 3).
| Entry | Substituent (-X) | Time | Yield |
|---|---|---|---|
| a. | -H | 25 | 95 |
| b. | 0 | 35 | 92 |
| c. | 0 | 35 | 93 |
| d. | 2,3-OCH3 | 30 | 91 |
| e. | -OH | 20 | 90 |
| f. | -Cl | 20 | 92 |
| g. | -Br | 25 | 94 |
| h. | -F | 25 | 92 |
| i. | 0 | 40 | 89 |
| j. | -CH=CH-Ph | 40 | 87 |
| k. | -N(CH3)2 | 50 | 86 |
In order to evaluate the generality of the process, several diversified examples illustrating the present method for the synthesis of 1,4-dihydropyridine was studied (Table 4).
The cyclization reaction dimedone was checked by treating with wide range of substituted aldehydes bearing electron donating (such as hydroxy, methoxy, methyl, N,N-dimethyl) or electron withdrawing (nitro, halides) was carried out in the presence of Silica supported MgO catalyst. The reaction of aromatic aldehyde with electron donating groups and electron withdrawing groups reacted very well. Treatment of substituted aldehydes with dimedone and ethyl acetoacetate in methanol with Silica supported MgO (0.8 mol) at 80ºC temperature afforded 1,4-dihydropyridine with excellent yield. The results obtained are illustrated in table 4. All the products obtained were characterized by IR, 1H-NMR, 13C-NMR and Mass spectrometry.
| Sr. no. | Solvent | Time | Yield* |
|---|---|---|---|
| 1 | MeOH | 25 | 94 |
| 2 | EtOH | 25 | 91 |
| 3 | DMF | 45 | 86 |
| 4 | CH3CN | 40 | 89 |
| 5 | CH2Cl2 | 60 | 69 |
The reusability of the catalyst was tested in the synthesis of DHPs as shown in Table 5. The catalyst was recovered after each successive run, washed three times with acetone, dried in oven at 120ºC for 3 hrs. Prior to use and tested for its activity in the subsequent run. The catalyst was tested for 5 runs. It was observed that the catalyst found to very good reusability (Table 5).
| Sr. no | Reaction run | time | Yield* |
|---|---|---|---|
| 1 | 1 | 25 | 94 |
| 2 | 2 | 25 | 94 |
| 3 | 3 | 25 | 92 |
| 4 | 4 | 25 | 87 |
| 5 | 5 | 25 | 90 |
CONCLUSION
In conclusion, we developed an efficient and simple alternative method for the preparation of 1,4-dihydropyridine (1,4-DHP) using silica supported MgO catalyst at 80ºC. Prominent among the advantages of this new method are simple and easy workup procedure, short reaction time, good yield and use of cheap, nontoxic and easily synthesized silica supported MgO catalyst.
References
- Godfraind T, Miller R, Wibo M. Pharmacol Rev. 1986, 38: p. 321.
- Klusa V. Drugs Fut. 1995, 20: p. 135.
- Bretzel RG, Bollen CC, Maeser E, et al., Am J Kidney Dis. 1993, 21: p. 53.
- Bretzel RG, Bollen CC, Maeser E, et al., Drugs Fut. 1992, 17: p. 465.
- Boer R, Gekeler V. Drugs Fut. 1995, 20: p. 499.
- Hantzsch A. Ann Chem. 215: p. 1.
- Ko S, Sastry MNV, Lin C, et al., Tetrahedron Lett. 2005, 46: p. 5771.
- Maheswara M, Siddaiah V, Damu GLV, et al., Arkivoc. 2006, p. 201.
- Sabitha G, Reddy GSKK, Reddy CS, et al., Tetrahedron Lett. 2003, 44: p. 4129.
- S. Ko, C.F. Yao, Tetrahedron, 2006, 62, 7293.
- Karade NN, Budhewar VH, Shinde SV, et al., Lett Org Chem. 2007, 4: p. 16.
- Ji SJ, Jiang ZQ, Lu J, et al., Synlett. 2004, 831.
- Wang LM, Sheng J, Zhang L, et al., Tetrahedron. 2005, 61: p. 1539.
- Donelson JL, Gibbs RA, De SK. J Mol Catal A: Chem. 2006, 256, 309.
- Gadekar LS. Bull Korean Chem Soc. 2009, 30: p. 11.
- Katkar SS, Arbad BR, Lande MK. Arab J Sci and Eng. 2011, 36: p. 39-46.
- Kumar A, Maurya RA. Tetrahedron Lett. 2007, 48: p. 3837.
- Gordeev MF, Patel DV, Gordon PM. J Org Chem. 1996, 61: p. 924.
- Shinde SV, Jadhav WN, Lande MK, et al., Catal Lett. 2008, 125: p. 57.
- Gadekar LS, Katkar SS, Vidhate KN, et al., Bull Catal Soc Ind. 2008, 7: p. 79.
- Cid R, Pecci G. Appl Catal. 1985, 14: p. 15.