Research Article - Der Pharma Chemica ( 2017) Volume 9, Issue 1
Synthesis of Quinoline Based Dihydroimidazo Pyridone Derivatives and Evaluation of Insect Antifeedant Activity
Udaya Sree K* and Ramana Reddy ALVUdaya Sree K, Department of Chemistry, Government Degree College for Men, Kadapa 516 004, India,
Abstract
In view of generating new compounds for future drug development, we have synthesized some synthesis of 2-(nitromethylene) imidazolidine (5) reacted with α,β unsaturated Acetates (4a-o) achieved via Micheal addition to give Quinoline based Dihydroimidazo Pyridone derivatives (6a-o). All the synthesized compounds were fully characterized on the basis of their detailed spectral studies and were evaluated for their insect Antifeedant activity.
Keywords
Tryethyl amine, Baylis-Hillman acetated, Michael addition, Antifeedant activity
Introduction
Heteroaromatic compounds have attracted considerable attention in the design of biologically active molecules and advanced organic materials. Hence a practical method for the preparation of such compounds is of great interest in synthetic organic chemistry, Pyrazole is a five member heterocyclic compound containing two nitrogen atoms adjacent to each other. In 1883, Knorr et al. [1] gave the generic name pyrazole to the compounds, which is a five member unsaturated ring compound with two adjacent nitrogen atoms. Pyrazoles and its derivatives, a class of well-known nitrogen containing heterocyclic compounds, occupy an important position in pharmaceutical and agrochemical industry due to their antimicrobial [2], anti-inflammatory [3] and antitumor [4] activities, antibacterial [5], antifungal [6], antiviral [7], antitubercular [8], antioxidant [9], antiandrogenic [10] etc. On the other hand, sulfonamides and their different derivatives are extensively used in medicine due to their pharmacological properties such as antibacterial activity [11,12].
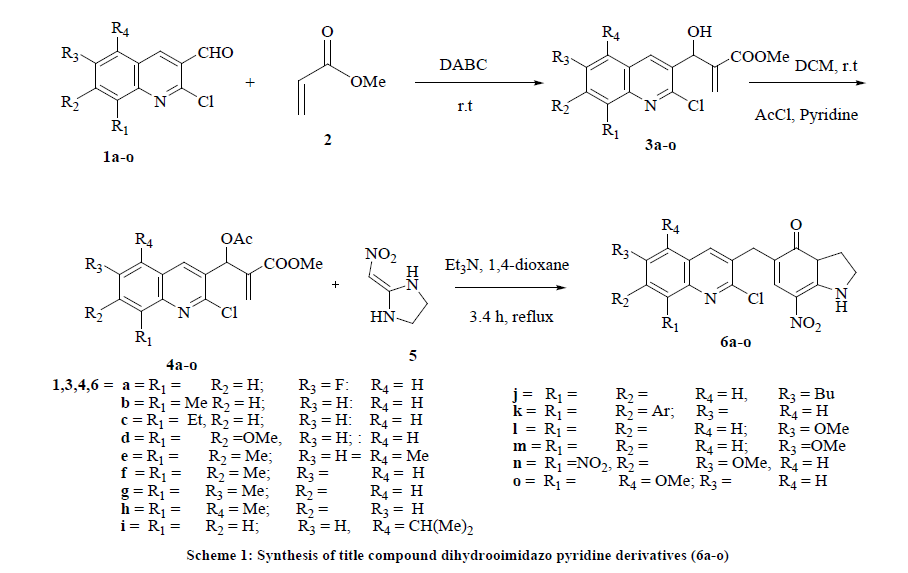
Pyridone represent an important class of scaffolds for the synthesis of several natural products and natural product like compounds. Synthesis of quinoline based Dihydroimidazo pyridine derivatives as potentially synthetic precursors for the preparation of various bio-active compounds. Our area of interest in research is design and synthesis of diverse biologically active heterocyclic compounds and screening their biological activities. In this direction we have developed a new method for the synthesis of several pyridine derivatives were synthesized by trating Baylis-Hillman acetates with 2-(nitroomethylene) imidazolidine and characterized well by spectroscopic techniques and screened their Insect Antifeedant activity.
Biological Activity
Insect antifeedant activity
Antifeedant activity of the compounds was assessed on tobacco caterpillar, Spdoptera litura (F). The experiments were conducted according to the classical no-choice leaf disk bio assay described earlier method [Akhtar and Isman Entomol]. To study the antifeedant activity of the test compounds, a small circular their upper surface with individual concentrations of the compounds, and one leaf disk each was transferred to each petri plate of 15 cm diameter containing moist filter paper. Control leaf discs were treated with the same volume of the acetone only. In each petri dish, prestarved healthy third instar larae S. litura were introduced to assess antifeedant activity.
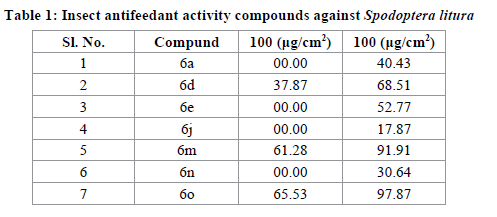
Progress of the consumption of the leaf area was measured at 6,12 and 24 hrs in both treated and control leafdisks. Areas of control and treated leaf discs consumed were measured after 6 hrs using a leaf areameter (AM-300, ADC, Biosceintifc Limited, England). The antifeedant index was then calculated as (C-T)/(C+T)×100, where C is the consumption of control leaf discs, and T is consumption of treated leaf discs (Isman et al.). For each concentration, 10 experimental sets were assayed. All tests were replicated three times. The mean of the 10 sets was taken for eah compound. Means were subjected to probit analysis [Finney D.J.]. The antifeedant activity was given as percentage for the compounds 6a-o is included in Table 1 for comparison. All the synthesized compounds were screened insect Antifeedant activity against spodptera litura, 7 compunds are Active. In this series of compounds 7m with dimethoxy at C-6 C-7, position.
Experimental Section
General
All commercially available chemicals were used without further purification. Melting points were determined on a Mel-Temp apparatus and are uncorrected. The NMR spectra were recorded on Bruckner Advance 300 magnetic resonance spectrometer at 300 MHz in CDCl3 and 13C NMR 500 MHz in CDCl3 TMS as internal standard (chemical shifts and ppm). ESI-MS were obtained on Thermo-Finning an MAT-1020B instrument. Elemental analysis was carried out with a Perkin Elmer 2400 Series II elemental analyzer (Scheme 1).
General procedure for the synthesis of quinoline based dihydroimidazo pyridone derivatives
2-(scetoxy(2-chloroquinoline-3-yl)acrylate (4a-o) (2.5 mmol) was taken in round bottom flask added to 2-(nitromethylene) imidazoline (5) (2.5 mmol) in 1,4-dioxane solvent (20 mL) and to this Et3N (3mL) was added. The mixture was refluxed for 3-4 hrs at 110ºC and cooled to room temperature and poured into water. The reaction mixture was filtered off and the residue was washed with H2O to give a crude product that was recrystallized by ethanol to form the final product (6a-o).
6-((2-chlroquinolin-3-yl) methyl)-8-nitro-2,3-dihydroimidazo[1,2]pyridine-5(1H)-one
Yellow solid; Yiels: 56%; mp: 272-274 ºC. 1H NMR (300 MHz, CDCl3): δ 8.16 (s, 1H), 8.00 (d, 1H, J=8.39 Hz), 7.92 (s, H), 7.80 (d, 1H, J=8.39 Hz), 7.70 (t, 1H J=8.08 Hz), 7.55 (t, 1H J= 8.08 Hz), 4.33 (t 2H, J=9.61 Hz), 4.07 (t, 2H, J=9.61 Hz), 4.03 (s, 2H); 13C NMR (300 MHz, CDCl3): δ 166.1, 159.09, 140.65, 136.16, 134.33, 130.95, 130.66, 129.97, 128.73, 127.52, 96.92, 52.70, 48.48, 42.22; IR (neat, cm-1): 3398, 2920, 2358, 2330, 1734, 1716, 1602, 1388, 1338, 1199, 1029, 754; MS (LCMS) m/z (%): 365 [M+H]+ (6a).
6-((2-chlro-8-methylquinolin-3-yl)methyl)-8-nitro-2,3-dihydroimidazo[1,2]pyridine-5(1H)-one
Yellow solid; Yiels: 60%; mp: 268-270 ºC. 1H NMR (300 MHz, CDCl3): δ 8.10 (s, 1H), 7.7900 (d, 1H, J=8.39 Hz), 7.63 (s, H), 7.54 (d, 1H, J=7.74 Hz), 7.42 (t, 1H J=8.08 Hz), 4.06 (t 2H, J=10.00 Hz), 4.03 (s, 3H); 13C NMR (300 MHz, CDCl3): δ 159.9, 159.0,157.5, 156.1, 152.4, 150.6, 147.4138.5, 135.2132.7130.0,126.7,126.2,124.743.542.8,32.128.8; IR (neat, cm-1):3365,2918, 2848, 2358, 2330, 1734, 1668, 1604, 1394, 1338, 1201, 1010,921, 761,; MS (LCMS) m/z (%): 371 [M+H]+ (6b).
6-((2-chlro-8-ethylquinolin-3-yl) methyl)-8-nitro-2,3-dihydroimidazo[1,2]pyridine-5(1H)-one
Yellow solid; Yiels: 56%; mp: 268-270 ºC. 1H NMR (300 MHz, CDCl3): δ 8.10 (s, 1H), 7.79 (d, 1H, J=8.39 Hz), 7.63 (s, H), 7.54 (d, 1H, J=7.74 Hz), 7.42 (t, 1H J=8.08 Hz), 4.06 (t 2H, J=10.00 Hz), 4.03 (s, 3H); 13C NMR (300 MHz, CDCl3): δ 159.9, 159.0,157.5, 156.1, 152.4, 150.6, 147.4138.5, 135.2132.7130.0,126.7,126.2,124.743.542.8,32.128.8; IR (neat, cm-1):3365,2918, 2848, 2358, 2330, 1734, 1668, 1604, 1394, 1338, 1201, 1010,921, 761,; MS (LCMS) m/z (%): 371 [M+H]+ (6c).
6-((2-chlro-7-fluoroquinolin-3-yl) methyl)-8-nitro-2,3-dihydroimidazo[1,2]pyridine-5(1H)-one
Yellow solid; Yield: 65%; mp: 266-268 ºC. 1H NMR (300 MHz, CDCl3): δ 8.17 (s, 1H), 7.95 (d, 1H, J=8.39 Hz), 7.81 (s, H), 7.77 (d, 1H, J=7.74 Hz), 7.63 (t, 1H J=8.08 Hz), 7.33 )s,1H), 4.32 (t 2H, J=10.00 Hz), 4.06 (s, 3H) 4.01 (s, 2H); 13C NMR (300 MHz, CDCl3): δ 159.9, 159.0,157.5, 156.1, 152.4, 150.6, 147.4138.5, 135.2132.7130.0,126.7,126.2,124.743.542.8,32.128.8; IR (neat, cm-1):3365,2918, 2848, 2358, 2330, 1734, 1668, 1604, 1394, 1338, 1201, 1010,921, 761,; MS (LCMS) m/z (%): 375 [M+H]+ (6d).
6-((2-chlro-6-methylquinolin-3-yl) methyl)-8-nitro-2,3-dihydroimidazo[1,2]pyridine-5(1H)-one
Yellow solid; Yield: 61%; mp: 228-230 ºC. 1H NMR (300 MHz, CDCl3): δ 8.10 (s, 1H), 7.87 (d, 1H, J=8.39 Hz), 7.81 (s, H), 7.54 (d, 1H, J=7.74 Hz), 7.63 (t, 1H J=8.08 Hz), 7.54 (d,1H, J= 7.93 Hz), 7.43 (s, 1H), 4.33 (t 2H, J=10.00 Hz), 4.06 (s, 3H); 4.03, (s, 2H); 13C NMR (300 MHz, CDCl3): δ 160.49, 157.41, 151.32, 146.02, 139.42, 137.86,136.34, 133.34, 133.21, 130.09, 129.95, 126.78, 125.17, 118.18, 98.23, 44.42, 43.15, 33.00,29.67; IR (neat, cm-1):3365, 2970,2358, 2330, 1734, 1716, 1541, 1338, 1203, 1083, 1012, 763; MS (LCMS) m/z (%): 369 [M+H]+ (6e).
6-((2-chlro-7,8-dimethylquinolin-3-yl) methyl)-8-nitro-2,3-dihydroimidazo[1,2]pyridine-5(1H)-one
Yellow solid; Yield: 65%; mp: 208-210 ºC. 1H NMR (300 MHz, CDCl3): δ 8.04 (s, 1H), 7.84 (d, 1H, J=8.39 Hz), 7.79 (s, H), 7.54 (d, 1H, J=7.74 Hz), 7.53 (t, 1H J=8.08 Hz), 7.35, 4.31 (t 2H, J=10.00 Hz), 4.05 (s, 3H); 4.00, (s, 2H) 2.69 (s, 3H) 2.48 (s, 3H); 13C NMR (300 MHz, CDCl3): δ160.52, 151.37, 150.04, 146.02, 139.32, 137.94, 133.65, 133.10, 129.87, 128.71, 125.77, 124.12, 118.48 112.93, 44.45, 43.15, 32.87,20.66, 13.32; IR (neat, cm-1):3365, 2970, 2358, 2330, 1734, 1616, 1600, 1338, 1199, 1033, 927, 761; MS (LCMS) m/z (%): 383 [M+H]+ (6f).
6-((2-chlro-6,8-dimethylquinolin-3-yl) methyl)-8-nitro-2,3-dihydroimidazo[1,2]pyridine-5(1H)-one
Yellow solid; Yield: 66%; mp: 223-225 ºC. 1H NMR (300 MHz, CDCl3): δ 8.00 (s, 1H), 7.83 (s, H), 7.81 (brs, 1H), 7.38 (s, 1H), 7.37(s, 1H), 4.31 (t 2H, J=10.00 Hz), 2.71 (s, 3H); 13C NMR (300 MHz, CDCl3): 160.49, 151.34, 149.21, 144.64, 138.79, 136.64, 135.86, 133.11, 132.41,129.80, 127.51, 124.03, 118.36, 112.91, 44.43, 43.15, 32.97, 21.53, 17.66; IR (neat, cm-1): 3365, 2970, 2358, 2330, 1734, 1616, 1600, 1338, 1199, 1033, 927, 761; MS (LCMS) m/z (%): 383 [M+H]+ (6g).
6-((2-chlro-5,8-dimethylquinolin-3-yl) methyl)-8-nitro-2,3-dihydroimidazo[1,2]pyridine-5(1H)-one
Yellow solid; Yield: 66%; mp: 218-220 ºC. 1H NMR (300 MHz, CDCl3): δ 8.30 (s, 1H), 7.81 (s, H), 7.80 (brs, 1H), 7.32 (d, 1H J =7.17 Hz), 7.25(d, 1H J=7.17 Hz), 4.32 (t 2H, J=9.06 Hz), 4.06 (t, 2H, J=9.06), 4.04 (s, 2H0, 2.70 (s, 3H) 2.63 (s, 3H); 13C NMR (300 MHz, CDCl3): 160.49, 151.31, 149.73, 146.39, 136.49, 134.16, 132.89, 131.93, 129.83,129.19, 127.26, 126.82, 118.52, 112.89, 44.42, 43.13, 33.27, 18.61, 17.74; IR (neat, cm-1):3336, 2924, 2358, 2328, 1647, 1647, 1595, 1386, 1328, 1201, 1087, 759; MS (LCMS) m/z (%): 407 [M+H]+ (6h).
6-((2-chlro-6-isopropylquinolin-3-yl)methyl)-8-nitro-2,3-dihydroimidazo[1,2]pyridine-5(1H)-one
Yellow solid; Yield: 70%; mp: 268-270 ºC. 1H NMR (300 MHz, CDCl3): δ 8.10 (s, 1H), 7.90 (s, H), 7.79 (brs, 1H), 7.92 (d, 1H J =7.17 Hz), 7.60(d, 1H J=7.17 Hz), 7.59 (s, H), 4.32 (t 2H, J=9.06 Hz), 4.06 (t, 2H, J=9.06), 4.01 (s, 2H), 3.07 (sep, 1H) 1.34 (s, 3H) 1.32 (s, 3H); 13C NMR (300 MHz, CDCl3): 160.51, 151.33,150.25, 147.74, 145.56, 138.89, 133.33, 130.07, 130.04, 127.88, 127.39, 123.38, 117.93, 112.86, 44.40, 43.16, 34.03; IR (neat, cm-1):3392, 2956, 2920, 2358, 2343, 1716, 1658, 1602, 1506, 1328, 1197, 1029, 1006, 761; MS (LCMS) m/z (%): 397 [M+H]+ (6i).
6-((6-butyl-2-chlroquinolin-3-yl) methyl)-8-nitro-2,3-dihydroimidazo[1,2]pyridine-5(1H)-one
Yellow solid; Yield: 75%; mp: 229-231 ºC. 1H NMR (300 MHz, CDCl3): δ 8.07 (s, 1H), 7.89 (s, H), 7.92 (d, 1H J =7.17 Hz), 7.80 (brs, 1H), 7.34 (d, 1H J =7.17 Hz), 7.06(d, 1H J=7.17 Hz), 4.32 (t 2H, J=9.06 Hz), 4.06 (t, 2H, J=9.06), 4.0 (s, 2H), 3.91 (s, 3H); 13C NMR (300 MHz, CDCl3): 160.50, 151.41, 150.25, 145.51, 141.96, 138.75,133.34, 131.68, 130.07, 128.86, 127.41, 125.56, 118.15, 112.95, 44.46, 43.17, 35.56, 33.26, 33.10, 22.29, 13.92; IR (neat, cm-1): 3402, 2927, 2858, 2358, 2330, 1734, 1668, 1602, 1394, 1338, 1197, 1029, 761; MS (LCMS) m/z (%): 411 [M+H]+ (6j).
6-((2-chlro-6-methxyquinolin-3-yl) methyl)-8-nitro-2,3-dihydroimidazo[1,2]pyridine-5(1H)-one (6k)
Yellow solid; Yield: 75%; mp: 263-265 ºC. 1H NMR (300 MHz, CDCl3): δ 8.06 (s, 1H), 7.92 (s, H), 7.89 (d, 1H J =7.17 Hz), 7.80 (brs, 1H), 7.34 (d, 1H J =7.17 Hz), 7.06(d, 1H J=7.17 Hz), 4.32 (t 2H, J=9.06 Hz), 4.06 (t, 2H, J=9.06), 4.0 (s, 2H), 3.91 (s, 3H); 13C NMR (300 MHz, CDCl3):158.55, 156.08, 149.42, 146.28, 140.26, 135.70, 131.49, 129.44, 127.15, 126.67, 120.74, 114.38, 110.51, 103.45, 53.76, 42.31, 41.62, 30.75; IR (neat, cm-1):3419, 2918, 2848, 2358, 1734, 1660, 1392, 1338, 1197, 1037, 831, 763; MS (LCMS) m/z (%): 387 [M+H]+ (6k).
6-((2-chlro-6,7-dimethxyquinolin-3-yl) methyl)-8-nitro-2,3-dihydroimidazo[1,2]pyridine-5(1H)-one
Yellow solid; Yield: 77%; mp: 244-246 C. 1H NMR (300 MHz, CDCl3): δ 8.01 (s, 1H), 7.89 (s, H), 7.82 (brs, 1H), 7.32 (s,1H), 7.02(s,1H),7.43 (s,1H), 4.32 (t 2H, J=9.06 Hz), 4.05 (t, 2H, J=9.06), 4.00 (s, 2H), 3.99 (s, 3H) 3.97 (s, 2H); 13C NMR (300 MHz, CDCl3):160.10, 152.52, 151.01, 149.71, 142.82, 136.83, 132.66, 128.32, 122.65, 116.26, 106.30, 105.11, 95.58, 55.66, 55.58, 43.88, 43.21, 31.93,; IR (neat, cm-1):2970, 2358, 2341, 1716, 1651, 1606, 1506, 1327, 1234, 1217, 1143, 1035, 1006, 848, 759; MS (LCMS) m/z (%): 417 [M+H]+ (6l).
6-((2-chlro-6,7-dimethxy-8-nitroquinolin-3-yl)methyl)-8-nitro-2,3-dihydroimidazo[1,2]pyridine-5(1H)-one
Yellow solid; Yield: 75%; mp: 238-240 C. 1H NMR (300 MHz, CDCl3): δ 7.93 (s, 1H), 7.81 (s, H), 7.51 (s, 1H), 4.32 (t 2H, J=9.06 Hz), 4.08 (t, 2H, J=9.06), 4.06 (s, 2H), 4.04 (s, 2H) 3.98 (s, 2H); 13C NMR (300 MHz, CDCl3):160.31, 154.90, 151.97, 151.49, 143.36, 142.92, 139.99, 133.42, 132.64, 130.79, 117.26, 114.87, 112.87, 110.55, 62.60, 56.59, 44.46, 43.20, 33.16; IR (neat, cm- 1):2970, 2358, 2341, 1716, 1651, 1606, 1506, 1327, 1234, 1217, 1143, 1035, 1006, 848, 759; MS (LCMS) m/z (%): 484 [M+H]+ (6m).
6-((2-chlro-6,7-dimethxy-8-nitroquinolin-3-yl)methyl)-8-nitro-2,3-dihydroimidazo[1,2]pyridine-5(1H)-one (6n)
Yellow solid; Yield: 75%; mp: 268-270 C. 1H NMR (300 MHz, CDCl3): δ 8.48 (s, 1H), 7.79 (brs, 1H), 7.75 (s, H), 6.92 (d, J=8.54 Hz), 6.76 (d, 1H, J=8.54 Hz) 7.43 (s, 1H) 4.33 (t 2H, J=9.06 Hz), 4.05 (t, 2H, J=9.06), 4.03 (s, 2H), 4.01 (s, 3H) 3.95 (s, 3H); 13C NMR (300 MHz, CDCl3):158.29, 149.15, 148.20, 146.18, 133.61, 131.84, 130.79, 128.65, 118.19, 114.35, 111.58, 110.10, 107.06, 103.25, 54.26, 53. 98, 42.13, 41.46, 30.62; IR (neat, cm-1):2970, 2358, 2341, 1716, 1651, 1606, 1506, 1327, 1234, 1217, 1143, 1035, 1006, 848, 759; MS (LCMS) m/z (%): 484 [M+H]+.
6-((2-chlro-6,7-dimethxy-8-nitroquinolin-3-yl)methyl)-8-nitro-2,3-dihydroimidazo[1,2]pyridine-5(1H)-one (6n)
Yellow solid; Yield: 70%; mp: 220-222 ºC. 1H NMR (300 MHz, CDCl3): δ 7.96 (s, 1H), 7.88 (s, H), 7.82 (brs, 1H), 7.28 (s, 1H), 7.02 9s, 1H), 6.10 (s, 2H), 4.33 (t 2H, J=9.06 Hz), 4.06 3.95 (s, 2H); 13C NMR (300 MHz, CDCl3): 152.37, 148.24, 146.49, 137.84, 134.85, 133.26, 122.48, 122.33, 110.11, 108.55, 105.24, 104.90, 102.22, 101.90, 101.64, 44.44, 43.16, 29.67; IR (neat, cm-1):3749, 2970, 2358, 2330, 1716, 1647, 1541, 1506, 1338, 1217, 1033, 933, 848, 759; MS (LCMS) m/z (%): 401 [M+H]+.
Conclusion
A simple and highly efficient method developed was developed for the synthesis of new bicyclic pyridines. This method was straightforward and provides highly efficient, short reaction times, easy procedure and work up of the reactions and afford the expected products in good yields, make this procedure a suitable method for this type of heterocyles. Our approach for the construction of bicyclic Pyridines ring system is novel and opens a new avenue for generation of molecular diversity, employing economical. All the quinoline based dihydroimidazo pyridone derivatives were synthesized screened their insect antifeedant activity, the compounds possessing moderate to excellent activity.
References
[1] L. Knorr, B. Chem. Ges., 1883, 16, 2597.
[2] E.M. Robin, R.G. David, S. Vijayalekshmi, J. Phytochem., 2008, 69(3), 2704-2707.
[3] N. Gokhan-Kelekci, S. Yabanoglu, E. Kupeli, U. Salgin, O. Ozgen, G. Ucar, E. Yesilada, E. Kendi, A. Yesilada, A.A. Bilgin, J. Bioorg. Med. Chem., 2007, 5(17), 5775-5786.
[4] L. Ronghui, C. George, Y. Yang, J.C. Peter, L. Shengjian, L. Yanhua, A. Mary, A.R. Fuentes-Pesquera, L.E. Stuart, M.G. Lee, Bioorg. Med. Chem. Lett., 2007, 17(16), 4557-4561.
[5] S.G. Roelfvan, C. Arnold, K. Wellnga, J. Agric. Food. Chem., 1979, 84, 406.
[6] K. Rajendra, K. Wanare, J. Chem. Pharm. Res., 2011, 3(5), 136-144.
[7] N. Meghasham, N. Narule, J. Chem. Pharm. Res., 2011, 3(3), 38-47.
[8] H.Z. Katri, S.A, Vunii, J. Ind. Chem. Soc., 1981, 55, 168.
[9] S. Mohan; S. Ananthan, J. Chem. Pharm. Res., 2011, 3(1), 402-413.
[10] G. Amr Ael, N.A. Abdel-Lalif, M.M. Abdalla, Bioorg. Med. Chem., 2006, 14(2), 373.
[11] O. Zbek, N. Katirciog"lu, H. Karacan, T. Baykal, Bioorg. Med. Chem., 2007, 15, 5105-5109.
[12] B.K. Mahantesha, V.K. Manohar, P.R. Vijaykumar, P. Harishchandra, S.M. Sumit, A.M. Ashwini, Eur. J. Med. Chem., 2002, 45(3), 1151-1157.



