Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 11
Synthesis of Some New 6-Chloro-3-substituted 5,6-Dihydro–[1,2,4] Triazolo [3,4a] Phthalazine Derivatives
Smita Singh* and Nitin Kumar
IFTM University, School of Pharmaceutical Sciences, Muradabad, UttarPradesh, India
- *Corresponding Author:
- Smita Singh
IFTM University
School of Pharmaceutical Sciences
Muradabad, UttarPradesh, India
Abstract
In this research a new series of 6-chloro-3-substituted 5,6-dihydro–[1,2,4] triazolo [3,4-a] phthalazine derivatives were synthesized by using phthalic anhydride as a starting material. All the compounds (4a-4h) characterized by Infrared (IR), Nuclear Magentic Resonance (NMR), MASS. All the newly synthesized compounds were screened for their anticonvulsant activity against MES –Induced seizure model, and the results from the evaluation that compound (4b) showed the best potential anticonvulsant activity compare to standard drug (carbamezapine).
Keywords
Anticonvulsant activity, Phthalazine, Phthalic anhydride, Carbamezapine, Triazole.
Introduction
Phthalazine are unique well known class of nitrogen- containing heterocyclic compounds. The discovery of first naturally accuring pyridazine derivative meant a milestone in the recognition of the potential of the 1,2-diazine core as a valuable unit in medicinal chemistry [1]. Hydralazine is also a hydrazine derivative used pharmacologically as an anti-hypertensive and vasodialating agent [2]. More recently, several 4-substituted phthalazine-1-one have been reported as pharmacologically active scaffold as anticonvulsants agent.
On the other hand, nitrogen containing heterocyclic molecules constitutes the largest portion of chemical entities, which are part of many natural product, fine chemicals and biologically active pharmaceuticals. Phthalazine is important building blocks in the construction of new molecular system for biologically active molecule [3-5]. The development of new and efficient methodologies for the potentially bioactive 4-hydrazinophthalazine-1-one derivative is important.
4-hydrazinophthalazine-1-one is of considerable interest due to their versitile pharmacological activity like anti inflammtory [1], PDE4 inhibitors [3,6] cytotoxic [4], anticancer [5], Vasorelaxant [7] antibacterial [8,9], antifungal [10], antidiabetic [11,12], and anticonvulsant activity [13-16]. A number of established drug molecule like budralaine [17] and Azelastine [6,9] are prepared from the corresponding phthalazine-1-one. Research to find the more effective and safer antiepileptic drugs are, therefore, imperative and challenging in medicinal chemistry [18-22].
Material Methods
Melting point was determined by melting point appratus and was uncorrected. Infrared (IR) spectra were recorded on Fourier Transform Infrared (FTIR) spectrophotometer 8400 (v in cm-1). Proton Nuclear Magnetic Resonance (1H-NMR) spectra were recorded on Brucer advanced 11400 NMR Spectroscopy in DMSO –d6 or CDCl3 using TMS as internal standard, chemical shift in ppm were experesed in delta unit, Saif Punjab Chandigarh, Reaction times were determined by using Thin Layer Chromatography (TLC) on silica gel plates that was purchages from merk. All the materials that are used in synthesis procedure obtained from Sigma Aldrich Chemical Corporation (sigma- Aldrich, St. Louis, MO, USA), Rankem, and Qualigens.
All other chemical were of analytical grade. All melting points are uncorrected and were measured on an elecrtothermal melting point appratus. Thin layer chromatography (TLC) was performed on Merk TLC aluminium sheets silica gel with detecting by using UV at 254 nm. FTIR Spectra of compounds were recorded using potassium bromide pellets (Perkin Elmer spectrum, RXI FT-IR System). Sample and potassium bromide (IR grade) was mixed in 1: 100 ratios and scanning was done between 4000-400 cm-1. 1H-NMR spectra were run in DMSO-d6 with TMS at the internal standard, on a Bruker advanced II 400 instrument (saif punjab technical university, punjab).
Synthesis
Synthesis of 2, 3-dihydrophthalazine-1, 4 dione (1)
The starting compound phthalic anhydride (50 g) was dissolved in ethanol (500 ml) in 30 min, and then to this mixture was added hydrazine hydrate (20.3 g) drop wise in an ice bath. The reaction mixture was stirred at room temperature for 1 h then made cool in ice bath. The white solid was collected through filtration and dried to give the compound (1) 2,3-dihydrophthalazine-1, 4-dione (13.064 g). Rf value-0.63, M.P-154ºC-156ºC, Yield-82.32%.
Synthesis of 1, 4 dichlorophthalazine (2)
Compound (1) was taken and add in (45 ml) of phosphorus oxychloride and stirred under reflux for 4 h. Then the solvent was removed by fiteration with the help of vaccum pump after that the residue was dissolved in (200 ml) dichloromethane and stirred rapidly with the help of magenetic stirrer and the solution was neutralized by the addition of solid and aqueous sodium hydrogen carbonate. When effervescence had stopped then the organic layer was separated and the aqueous layer was extracted with dichloromethane (2 × 200 ml). The combined organic layers were dried over MgSO4 in desicator. The residue was collected compound (2) was 1, 4 dichlorophthalazine 8.5 g. Rf value-0.7, M.P.-162ºC-164ºC, Yield-60.2%.
Synthesis of 4-hydrazine 1-chlorophthalazine (3)
2 g of compound (2) was taken in 30 ml THF. This solution was added drop wise to a solution of hydrazine hydrate (3.28 g) in THF (10 ml) at room temperature. The mixture was stirred and heated at 60ºC for 1 h, then half of the solvent was removed and solution was poured into petroleum ether. The precipitate was filtered and washed with petroleum ether and collected. This product should be kept below 0ºC. Compound (3) 1, 4 dichlorophthalazine 8.5 g. Rf value- 0.7, M.P.-171ºC-173ºC, Yield-82.42%.
Synthesis of 6-chloro-3-(4-methoxyphenyl)-5, 6-dihydro-[1,2,4] triazolo [3,4-a] phthalazine by using 4-hydrazine 1-chlorophthalazine (4a)
4-Hydrazinophthalazin- 1-chloro phthalazine (3 g, 0.17 mol) was suspended in 1,4 dioxan (45 ml) with triethylamine (2.6 ml), and 4-methoxybenzoyl chloride (3.2 g) in dioxan (10 ml) was added dropwise over 10 min at room temperature. The reaction mixture was stirred at room temperature for 30 min, and then the dioxan was removed by rotary evaporation. The residue was dissolved in DMF (50 ml), and the mixture was then heated under reflux for 4 h, after which time water was added dropwise until the solution became cloudy. After the mixture was allowed to cool, a solid was collected by filtration, washed with water, and vacuum-dried (4a, 6-chloro-3- (4-methoxyphenyl)-5, 6-di hydro- [1, 2, 4] triazolo [3, 4-a] phthalazin-6(5H)-one). Rf value- 0.83, M.P.-185ºC-187ºC, Yield-63%.
Synthesis of 6-chloro-3-phenyl-5,6-dihydro-[1,2,4]triazolo[3,4-a] phthalazineby using 4-hydrazine 1-chlorophthalazine (4b)
4-Hydrazinophthalazin-1-chloro phthalazine (3 g, 0.17 mol) was suspended in 1,4 dioxan (45 ml) with triethylamine (2.6 ml), and benzoyl chloride (3.2 g) in dioxan (10 ml) was added dropwise over 10 min at room temperature. The reaction mixture was stirred at room temperature for 30 min, and then the dioxan was removed by rotary evaporation. The residue was dissolved in DMF (50 ml), and the mixture was then heated under reflux for 4 h, after which time water was added dropwise until the solution became cloudy. After the mixture was allowed to cool, a solid was collected by filtration, washed with water, and vacuum-dried yield 3-phenyl-5,6-dihydro-[1,2,4] triazolo [3,4-a] phthalazin-6(5H)-one (4b). Rf value-0.7, M. P- 190ºC-192ºC, Yield-72%.
Synthesis of 6-chloro-3-(4-fluorophenyl)-5,6-dihydro-[1,2,4]triazolo[3,4-a] phthalazineby using 4-hydrazine 1-chlorophthalazine (4c)
4-Hydrazinophthalazin- 1-chloro phthalazine (3 g, 0.17 mol) was suspended in 1,4 dioxan (45 ml) with triethylamine (2.6 ml), and 4-fluoro benzoyl chloride (3.2 g) in dioxan (10 ml) was added dropwise over 10 min at room temperature. The reaction mixture was stirred at room temperature for 30 min, and then the dioxan was removed by rotary evaporation. The residue was dissolved in DMF (50 ml), and the mixture was then heated under reflux for 4 h, after which time water was added dropwise until the solution became cloudy. After the mixture was allowed to cool, a solid was collected by filtration, washed with water, and vacuum-dried yield 6-chloro-3-(4-fluorophenyl)-5,6-dihydro-[1,2,4]triazolo[3,4-a] phthalazin-6(5H)-one (4c). Rf value-0.72, M. P- 190ºC-192ºC, Yield-54%.
Synthesis of 6-chloro-3-(4-chlorophenyl)-5,6-dihydro-[1,2,4]triazolo[3,4-a] phthalazineby using 4-hydrazine 1-chlorophthalazine (4d)
4-Hydrazinophthalazin- 1-chloro phthalazine (3 g, 0.17 mol) was suspended in 1,4 dioxan (45 ml) with triethylamine (2.6 ml), and 4-chloro benzoyl chloride (3.2 g) in dioxan (10 ml) was added dropwise over 10 min at room temperature. The reaction mixture was stirred at room temperature for 30 min, and then the dioxan was removed by rotary evaporation. The residue was dissolved in DMF (50 ml), and the mixture was then heated under reflux for 4 h, after which time water was added dropwise until the solution became cloudy. After the mixture was allowed to cool, a solid was collected by filtration, washed with water, and vacuum-dried yield 6-chloro-3-(4-chlorophenyl)-5,6-dihydro-[1,2,4] triazolo [3,4-a] phthalazin-6-(5H)-one (4d). Rf value-0.54, M. P- 130ºC-132ºC, Yield-62%.
Synthesis of 3-(3-(trifluoromethyl) phenyl)-[1,2,4]triazolo[3,4-a] phthalazine- 6(5H)-one by using 4-hydrazine 1-chlorophthalazine (4e)
4-Hydrazinophthalazin- 1-chloro phthalazine (3 g, 0.17 mol) was suspended in 1,4 dioxan (45 ml) with triethylamine (2.6 ml), and 3-(tri fluoro methyl) benzoyl chloride (3.2 g) in dioxan (10 ml) was added dropwise over 10 min at room temperature. The reaction mixture was stirred at room temperature for 30 min, and then the dioxan was removed by rotary evaporation. The residue was dissolved in DMF (50 ml), and the mixture was then heated under reflux for 4 h, after which time water was added dropwise until the solution became cloudy. After the mixture was allowed to cool, a solid was collected by filtration, washed with water, and vacuum-dried yield 6-chloro-3-(4-chlorophenyl)-5,6-dihydro-[1,2,4]triazolo[3,4-a] phthalazin-6(5H)-one (4e). Rf value-0.53, M. P- 130ºC-132ºC, Yield-62%.
Synthesis of 3-(4-(dimethyl amino) phenyl)-[1,2,4] triazolo [3,4-a] phthalazin- 6 (5H)- one By Using 4-Hydrazine 1-Chlorophthalazine (4f)
4-Hydrazinophthalazin- 1-chloro phthalazine (3 g, 0.17 mol) was suspended in 1,4 dioxan (45 ml) with triethylamine (2.6 ml), and 4-(dimethyl-amino) benzoyl chloride (3.2 g) in dioxan (10 ml) was added dropwise over 10 min at room temperature. The reaction mixture was stirred at room temperature for 30 min, and then the dioxan was removed by rotary evaporation. The residue was dissolved in DMF (50 ml), and the mixture was then heated under reflux for 4 h, after which time water was added dropwise until the solution became cloudy. After the mixture was allowed to cool, a solid was collected by filtration, washed with water, and vacuum-dried yield 6-chloro-3-(4-chlorophenyl)-5,6-dihydro-[1,2,4]triazolo[3,4-a] phthalazin-6(5H)-one (4f). Rf value-0.56, M. P- 130ºC-132ºC, Yield-62%.
Synthesis of 3-(4-(chloromethyl) pheny-[1,2,4]triazolo[3,4-a] phthalazin-6(5H) one by using 4-hydrazine 1-chlorophthalazine (4g)
4-Hydrazinophthalazin- 1-chloro phthalazine (3 g, 0.17 mol) was suspended in 1,4 dioxan (45 ml) with triethylamine (2.6 ml), and 4- (chloromethyl) benzoyl chloride (3.2 g) in dioxan (10 ml) was added dropwise over 10 min at room temperature. The reaction mixture was stirred at room temperature for 30 min, and then the dioxan was removed by rotary evaporation. The residue was dissolved in DMF (50 ml), and the mixture was then heated under reflux for 4 h, after which time water was added dropwise until the solution became cloudy. After the mixture was allowed to cool, a solid was collected by filtration, washed with water, and vacuum-dried yield 6-chloro-3-(4-chlorophenyl)-5,6-dihydro- [1,2,4]triazolo[3,4-a] phthalazin-6(5H)-one (4g). Rf value-0.62, M. P- 130ºC-132ºC, Yield-62%.
Synthesis of methyl 4-(6-oxo-5,6-dihydro-[1,2,4]triazolo [3,4-a] phthalazin-3-yl) benzoate by using 4-hydrazine 1-chlorophthalazine (4h)
4-Hydrazinophthalazin-1-chloro phthalazine (4.3 g, 0.17 mol) was suspended in 1,4 dioxan (45 ml) with triethylamine (2.6 ml), and methyl 4- (chlorocarbonyl) benzoate (3.2 g) in dioxan (10 ml) was added dropwise over 10 min at room temperature. The reaction mixture was stirred at room temperature for 30 min, and then the dioxan was removed by rotary evaporation. The residue was dissolved in DMF (50 ml), and the mixture was then heated under reflux for 4 h, after which time water was added dropwise until the solution became cloudy. After the mixture was allowed to cool, a solid was collected by filtration, washed with water, and vacuum-dried yield 6-chloro-3-(4-chlorophenyl)-5,6-dihydro- [1,2,4]triazolo[3,4-a] phthalazin-6-(5H)-one (4h). Rf value-0.61, M. P- 130ºC-132ºC, Yield-62%.
Results and Discussion
All the derivatives were prepared according to the Scheme 1. From the litrature review we have found out many method for the preparation and synthesis of target molecule as prescribed in the given Scheme 1, we were using phthalic anhydride (1) as a starting material for the for the synthesis of intermediate compound (3) according to X.-Y. Compound (3) further reacted with DMF and triethyl amine, dioxane, benzoyl chloride and treated under reflux condition target compounds were finally obtained, the target compounds (4a-4h) (Figure 1) were synthesized according to the R, carling et al.
R: a: benzoyl chloride, b: 4-chloro benzoyl chloride, c: 4-methoxy benzoyl chloride, d: 4-flouro benzoyl chloride, e: 3-(tri fluoro methyl) benzoyl chloride, f: 4-(dimethyl amino) benzoyl chloride g: 4-(chloromethyl) benzoyl chloride h: methyl 4-(chlorocarbonyl) benzoate
Figure 1: Synthesis of the compounds (4a-4h)
Pharmacological evaluation
The preliminary screening of (4a-4h) is summerized in Table 1. From the Table 1 the results of pharmacological experiments indicated that the anti convulsants activity of the tittle compounds was shows the significant response compare to the existing derivatives and compare with the standard drug. As shown in (Scheme 1) 2,3 dihydro phthalazine-1,4 dione reacted with substituted benzoyl chloride in the presence of DMF and reflux condition yield (4a-4h) that is target compounds 6-chloro-3-substituted 5,6-dihydro–[1,2,4] triazolo [3,4-a] phthalazine derivatives. All the synthesized compounds were tested for MES test in mice. These compounds were evaluated for anticonvulsant activity through monitoring their ability to inhibit the convulsion and made compration with vehicle and standard drug (carbamezapine) as shown in Table 1. Most of the compunds exihibited anticonvulsant activity at the dose 30, 100 and 300 mg/kg administered intraperitonially (i.p.). Compound 4a, 4b, 4c shown the significant response as compared standard drug. The performance of compounds 4a-4h and the reference drug carbamezapine in the MES test assay are presented in Table 1.
| Compound | R | MES (mg/kg) | |||||
|---|---|---|---|---|---|---|---|
| 30 | 100 | 300 | 30 | 100 | 300 | ||
| 4a | 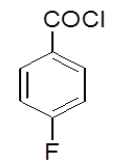 |
0/3 | 1/3 | 1/3 | 0/3 | 1/3 | 0/3 |
| 4b | 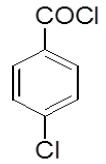 |
0/3 | 1/3 | 2/3 | 0/3 | 3/3 | 3-Jan |
| 4c | 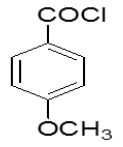 |
0/3 | 1/3 | 1/3 | 0/3 | 2/3 | 0/3 |
| 4d | 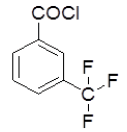 |
0/3 | 0/3 | 1/3 | 0/3 | 0/3 | 0/3 |
| 4e | 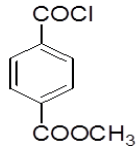 |
0/3 | 0/3 | 1/3 | 0/3 | 0/3 | 0/3 |
| 4f | 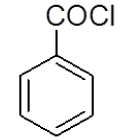 |
0/3 | 0/3 | 0/3 | 0/3 | 1/3 | 0/3 |
| 4g | 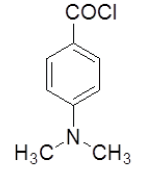 |
0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| 4h | 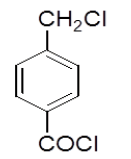 |
0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| Carbamazepine (standard drug) | 3-Feb | 2/3 | 1/3 | 3/3 | 2/3 | 1/3 | |
All the tested compounds were dissolved in Polyethylene glycol-400; Anticonvulsants activity was determined by maximal electroshock test (MES test)
Table 1: Anticonvulsant activity of the derivatives 4a-4h administerd I.P
Anticonvulsant activity
It was observed from the data given in Table 1 that the investigated compounds only some compounds showed the higher anticonvulsant activity, while other compounds showed moderate activities in compare with standard drug carbamezapine.
Conclusion
In the present study, the 6-oxo-[1,2,3]triazolo[3,4-a]phthalazine-3(3H)-one derivatives were synthesized, and evaluated them for the anticonvulsant activity by using MES test model on mice, from the evaluation they found that the compounds 4a, 4b and 4c shown significant anticonvulsant activity as compared to standard drug Carbamazepine.
Acknowledgement
The authors are grateful to the Prof. Ranjeet Singh,director of pharmacy department (shobhit university) and Prof. Vijay Kumar Sharma, director of pharmacy department (K.N. Modi institute) woh always motivate me and always support me for the complition of my research work.
References
- S.M. Mosaad, A. Goudah, N.A. Abotaleb, Eur. j. Med. chem., 2010, 45, 1267-1277.
- R. Bansal, D. Kumar, R. Carron, D.L. Calle, D.P. Jindal, Act Pharm., 2008, 58, 393-405.
- T. Hack, R. Fattori, F. Napoletano, Bioorg. Med. Chem., 2005, 13,4425.
- P. Gong, Y.B. Zhang, L. He, X. Zhai, Chinese Chem. Lett., 2008, 19, 29-32.
- P. Gong, X. Jing-Xion, Y. Fan, L. Jaun, Molecules., 2006, 19, 29-32.
- R.W. Carling, K.W. Moore, L.J. Street, D. Wild, C. Isted, P.D. Leeson, S. Thomas, J. Med. Chem., 2004, 47, 1807-1822.
- F.M. Awadallah, D.O. Saleh, W.E. Eraky, Eur. J. Med. Chem., 2012, 52, 419-420.
- A.M. Khalid, M.A. Berghot, M.A. Gouda, Eur. J. Med. Chem., 2009, 44(11), 4448-54.
- M. Napoletano, G. Norcini, F. Pellacini, F. Marchini, G. Morazzoni, P. Ferlenga, L. Pradella, Bioorg. Med. Chem. Lett., 2000, 10, 2235-2238.
- R. Sivakumar, G.S. Kishore, S. Ramachandran, Eur. J. Med. Chem.,2002, 37, 793-801.
- M. Bian, X. Qing Deng, J. Enzyme Inhi. Medicinal Chem., 2013, 28(4), 792-800.
- W. Loscher, D. Schmidt, Epilepsy Res., 1988, 2, 145-181.
- B. Abd El-Fattah, M.I. Al-Ashmawi, S. El-Feky, E. Roder, Egypt. J. Pharm. Sci., 1988, 29, 259-268.
- S. Ramaiya, K.G. Sundararaj, R. Somasundaram, T.L. Joseph, Eur. J. Med. Chem., 2002, 37, 793-801.
- R.L. Krall, J.K. Penry, B.G. White, H.J. Kupfer berg, E.A. Swinyard, Epilepsia., 1978, 19, 409-428.
- S. Singh, N. Kumar, world journal of pharmacy and pharmaceutical sciences., 2014, 3(3), 729-736.
- M.A. Khalil, S.M. El-Khawss, M.G. Kassem. Sci. Pharma., 1980, 48, 344-349.
- F. Nahed, A Mona, H.M. Zaki, J. Am. Sci., 2011, 7(4), 771-781.
- W.A. Bayoumi, M.A.E. Barghash, M.M. Gineinah, A. Mohamed, Der Pharma Chemica., 2014, 6(3), 89-102.
- X.Y. Sun, Y.Z. Jin, F.N. Li, G. Li, K.Y. Chai, Z.S. Quan, Arch. Pharm. Res., 2006, 29, 1080-1085.
- Z.S. Quan, J.M. Wang, J.R. Rho, K.C. Kwak, H.C. Kang, C.S. Chai, Bull Kor. Chem. Soc., 2005, 26, 1757-1760.
- S.K. Kulkarni, Handbook of Experimental Pharmacology, 2005,133.