Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 7
Synthesis of Some Pyrimidine and Fused Pyrimidine Derivatives with Antimicrobial and Anticancer Activities
Mohamed A Shaaban1, Khalid O Mohamed1, Mohamed E Hegazi2, Montaser Sh A Shaykoon3 and Yaseen AMM Elshaier2*
1Pharmaceutical Organic Chemistry Department, Faculty of Pharmacy, Cairo University, Cairo, 11562, Egypt
2Pharmaceutical Organic Chemistry Department, Faculty of Pharmacy, Al-Azhar University, Assiut, 71524, Egypt
3Pharmaceutical Chemistry Department, Faculty of Pharmacy, Al-Azhar University, Assiut, 71524, Egypt
- *Corresponding Author:
- Yaseen AMM Elshaier
Pharmaceutical Organic Chemistry Department
Faculty of Pharmacy
Al-Azhar University
Assiut, 71524, Egypt
Abstract
A new series of pyrimidines and fused pyrimidines derivatives including differently substituted benzylidenes, heterocycles such as pyrazole ring and fused ring systems such as triazolo [4,3-a] pyrimidine, pyrido [2,3-d] pyrimidine and pyramido [4,5-d] pyrimidine systems is reported. The synthetic routes were adopted via formation of substituted pyrimidine core structure in simple and efficient procedures. The chemical structures of new compounds were fully characterized by using complete spectral analyses data. The new compounds were evaluated for their anti-cancer and antimicrobial activities. Among the tested compounds, compounds 7i, 7h and 11c exhibited the best activity against different cancer cell lines. Compounds 8c, 11c and 14b showed potent activity as anti-microbial agents compared to standard drugs.
Keywords
Pyrimidine, Triazolo [4,3-a] pyrimidine, pyrido [2,3-d] pyrimidine and pyrimido [4,5-d] pyrimidine.
Introduction
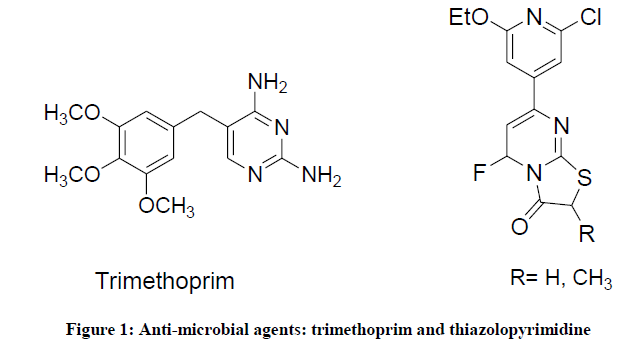
Pyrimidine derivatives are an important class of nitrogen heterocycles that have attracted more attention in the last decades. Due to their utilities as a precursor for the construction of condensed heterocyclic systems, they represent an interesting pharmacophore for pharmaceutical products [1-6]. Pyrimidine derivatives have diversified activities such as antiviral [7], antitumor [8], antifolate [9], antibacterial [10], antifungal [11], CNS active [12], diuretic [13], uricosuric [14], diabetogenic, analgesic [15], anti-inflammatory [16], antioxidant [17], bronchodilator [13], antihistaminic and cardiac agents [18]. Currently, bacterial and fungal resistance is a major challenge problem in treatment of infectious diseases [19]. New strains of microbes have been reported to threaten millions of people around the world every year [20]. Several pyrimidine derivatives have long ago been identified as potent bactericidal and fungicidal agents, among which trimethoprim (Proloprim®) has been successfully used in the treatment of urinary tract infections (Figure 1) [21].
In addition, thiazolo [3,2-a] pyrimidin-3 (5H)-one derivatives, as a fused pyrimidine system, were reported to possess potent antibacterial activity against S. typhi and fair sensitivity towards Escherichia coli and Staphylococcus aureus (Figure 1) [22,23].
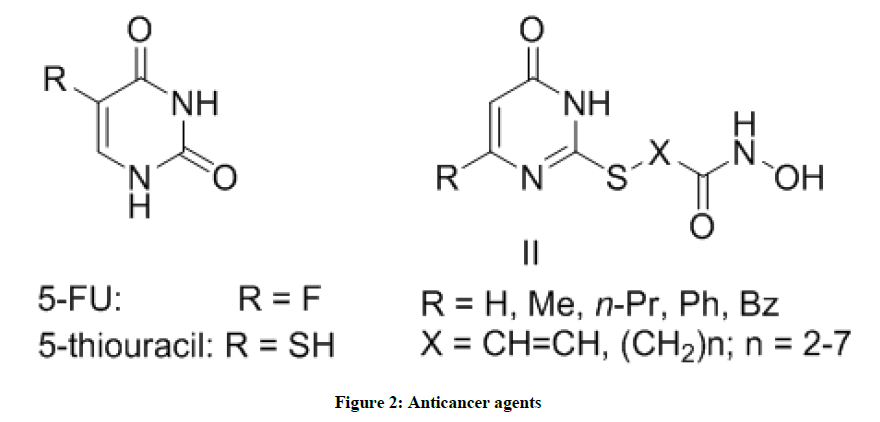
Moreover, pyrimidine derivatives display usefulness in the fields of chemotherapy. Pyrimidine-based antimetabolites structurally related to the endogenous substrates as 5-Fluorouracil (5-FU), 5-thiouracil and the uracil-based hydroxyamides were early recognized as effective therapies for cancer (Figure 2) [24-26]. It was reported that the chemotherapeutic efficacy of pyrimidine derivatives is related to their ability to inhibit vital enzymes responsible for DNA biosynthesis as dihydrofolate reductase (DHFR) [27], thymidylate synthetase (TSase), thymidine phosphorylase (TPase) and reverse transcriptase (RTase) [28]. Further, pyrimidine and fused pyrimidine derivatives show biologically important activities because of their structural resemblance to purine-pteridine systems [29]. Due to their severe toxicity and limited selectivity, the search for more potent and selective anticancer agents is highly interested.
Structural variations of the prepared compounds would be employed to trace possible changes in activity caused by changing the nature of substituents at C2 of the pyrimidine cornerstone. Substituents were chosen to cover diverse functionalities as hydrogen bond donor, acceptor, and rigidity. Our work was designed to encompass synthetic features to achieve the compounds of interest.
In this paper, we synthesized a new series of pyrimidines and fused pyrimidines derivatives via formation of fully substituted pyrimidine core structure through multi-component like reaction. Our objective was then directed to examine these compounds as antimicrobial and anticancer agents.
Materials and Methods
Experimental
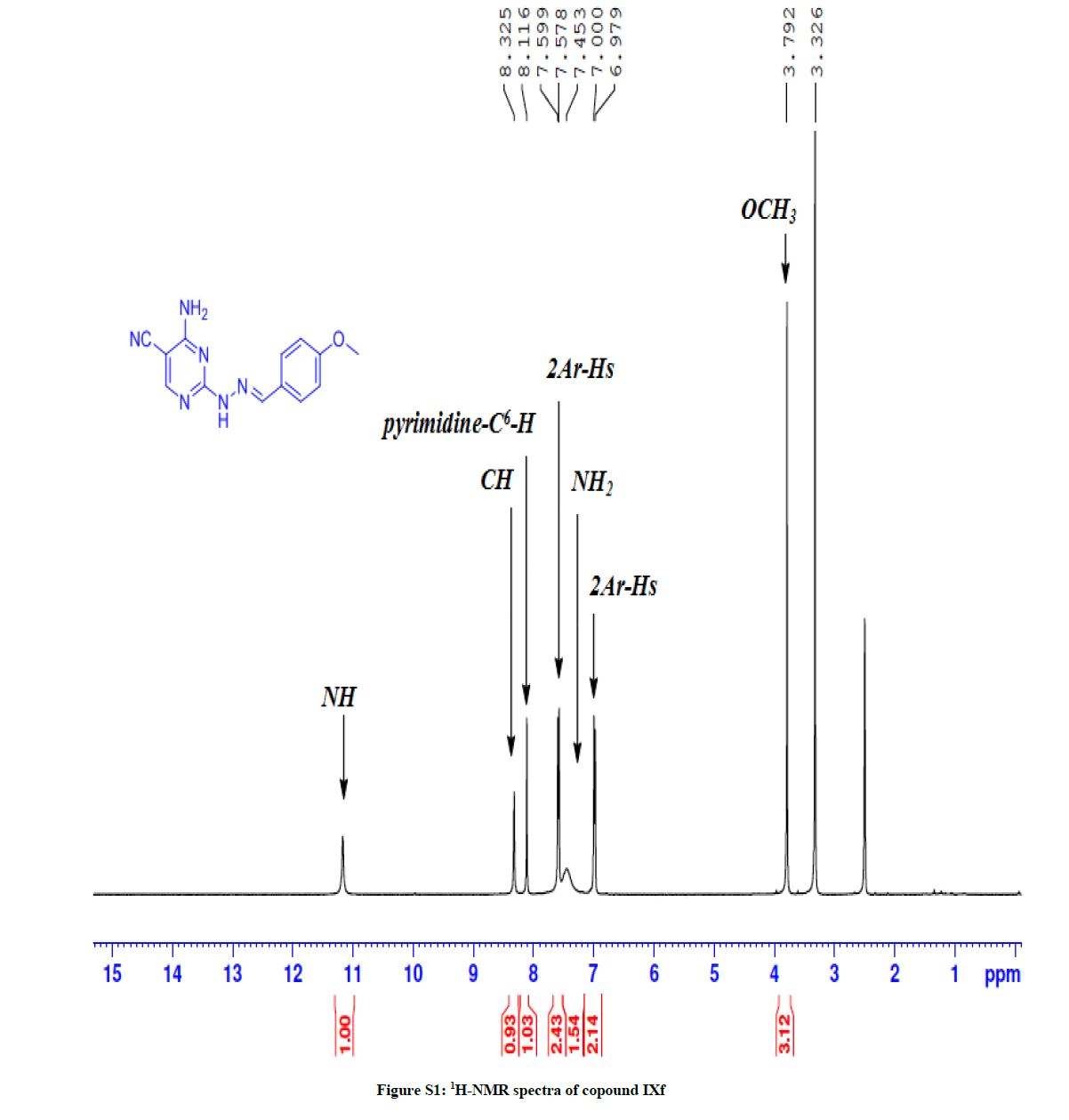
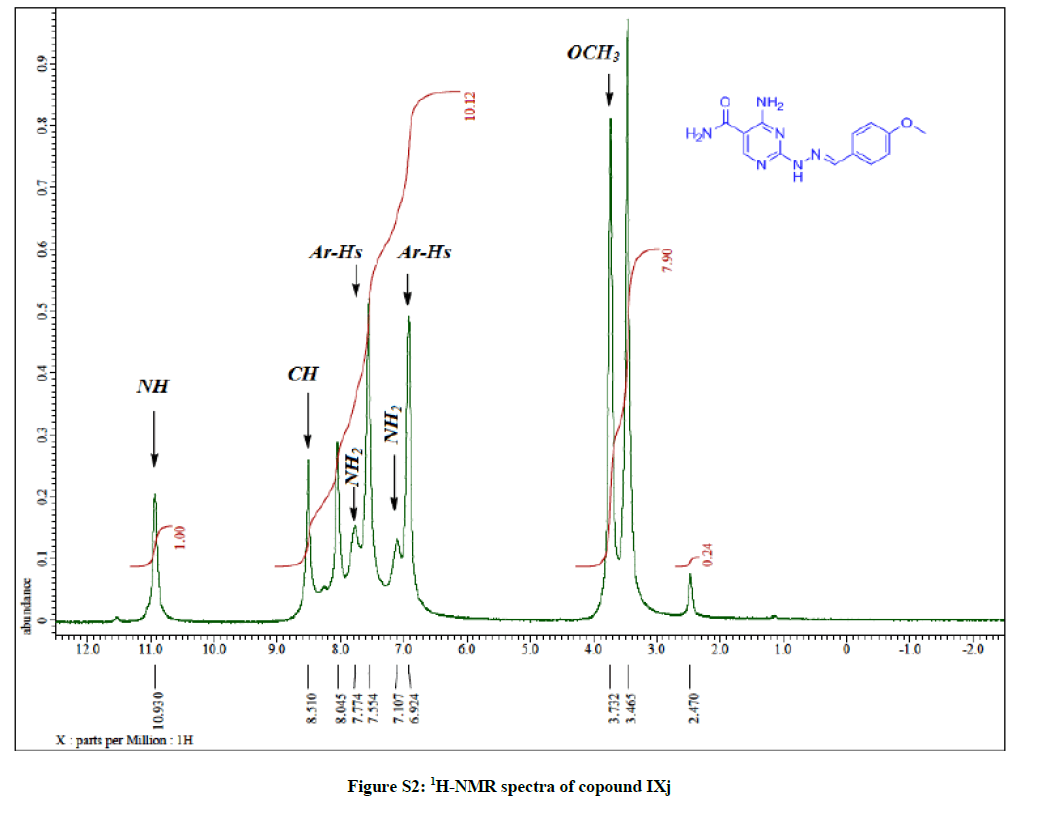
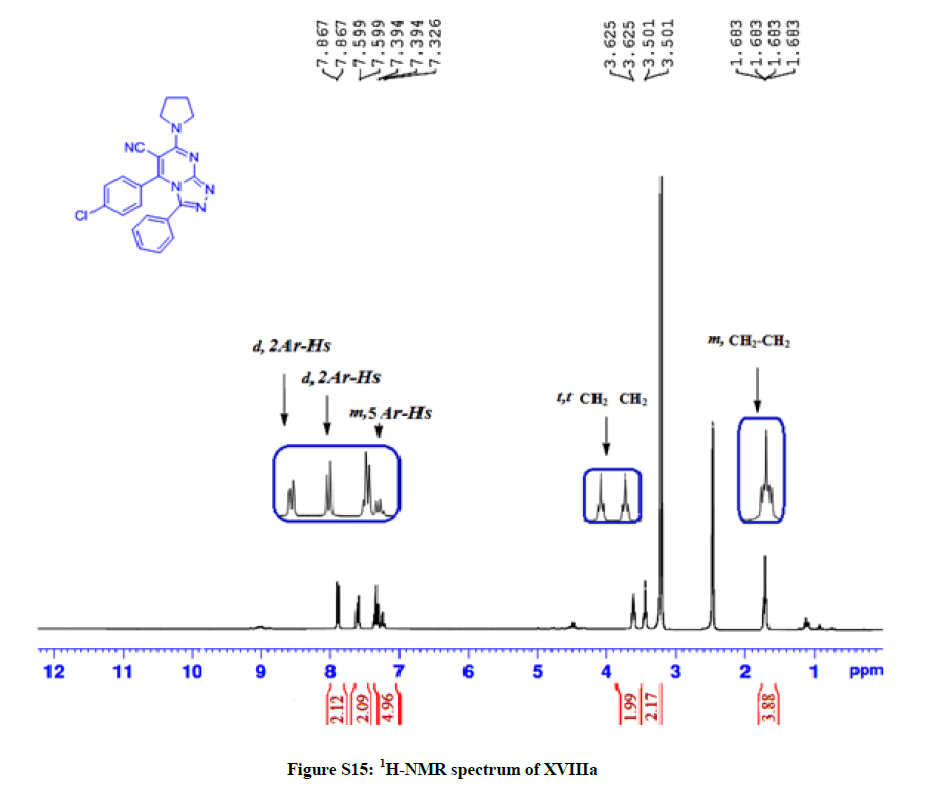
General: Melting points were determined in open-glass capillaries using an electro thermal melting point apparatus (Stuart Scientific, Model SMP1, UK) and were uncorrected. Infrared spectra (IR) were recorded, using KBr discs, υ (cm-1), on a Nicollet Infrared Spectrophotometer IR 470 at the Faculty of science, Assiut University. Nuclear magnetic resonance spectra, 1H-NMR wastaken using the following apparatus: Bruker Avance III apparatus 400 MHz; Central Laboratory Unit, Faculty of Pharmacy, Cairo and Bruker DRX 400 MHz, Central Laboratory Unit, Zagazig University, Egypt for 1H and 100 MHz for 13C-NMR. Chemical shifts are expressed as δ values (ppm) using tetramethylsilane (TMS) as internal reference. Signals are indicated by the following abbreviations: s=singlet, d=doublet, t=triplet, q=quartet, m=multiplet, and br=broad. Mass spectra (MS) were run on a gas chromatograph/mass spectrometer Shimadzu GCMS-Qp2010 plus, single quad (70 ev), Regional Centre for Mycology and Biotechnology, Al-Azhar University, Nasr City, Cairo. Elemental analyses were run on Vario EL III German CHN Elemental analyser model, Regional Centre for Mycology and Biotechnology, Al-Azhar University, Nasr City, Cairo (Figures S1-S16).
General procedure for the synthesis of compounds 7a-j and 8a-h: A solution of the 4-amino-2-hydrazinylpyrimidine-5-carbonitrile (2) (150 mg, 1 mmol) or 4-amino-2-hydrazinylpyrimidine-5-carboxamide (4) (168 mg, 1 mmol) in EG (2 ml) was treated with the equimolar amount of the appropriate benzaldehyde or acetophenone derivative in EG (2 ml) containing few drops gl. acetic acid. The reaction mixture was heated up at 120ºC for 30-60 min then left to cool to RT. The separated products were filtered, washed with petroleum ether, dried and crystallized from EtOH. Yields, melting points IR, 1H-NMR (DMSO-d6), 13C-NMR (DMSO-d6) and microanalysis of products 7a-j and 8a-h are depicted below:
4-Amino-2-(2-benzylidenehydrazinyl)pyrimidine-5-Carbonitrile (7a): Yield: (87%); M.p. 154-156ºC; IR (KBr): 3455-3343 (NH2, NH), 3127 (CH, Ar.), 2214 (C≡N), 1637 (C=N), 1654, 1595 (C=C) cm-1; 1H-NMR (400 MHz, DMSO-d6): 11.62 (s, 1H, NH, D2O exchanged), 8.58 (s, 1H, CH), 8.35 (s, pyrimidine-C6-H), 7.99 (NH2, D2O exchanged), 7.82-7.55 (m, 5Hs, Ar-Hs); 13C-NMR (DMSO-d6): 163.82, 162.83, 160.50, 144.36, 135.24, 129.91, 129.25, 127.15, 117.41, 80.92. Anal. calc. for C12H10N6 (238.25): C 60.50, H 4.23, N 35.27; found: C 60.40, H 4.22, N 35.27.
4-Amino-2-(2-(4-chlorobenzylidene)hydrazinyl) pyrimidine -5-carbonitrile (7b): Yield: (90%); M.p. 197-199°C; IR (KBr): 3516-3397 (NH2, NH), 2230 (C≡N), 1655 (C=N), 1597 (C=C) cm-1; 1H-NMR (DMSO-d6): 11.39 (s, IH,NH), 8.36 (s, pyrimidine-C6-H), 8.15 (s, 1H, N=CH), 7.67-7.65 (d, 2H, Ar-Hs, J=8 Hz), 7.51 (br, NH2, D2O exchanged), 7.47 (d, 2Hs, Ar-Hs, J=8 Hz); 13C-NMR (DMSO-d6): 163.30, 162.34, 159.94, 142.44, 133.68, 128.83, 128.20, 116.84, 80.92. Anal. calc. for C12H9ClN6 (272.69): C 52.85, H 3.33, N 30.82; found: C 52.85, H 3.31, N 30.70. MS: m/z (% relative abundance): m/z: 272.06 (33%), 274.05 (11%), 273.06 (6%), 43.01(100).
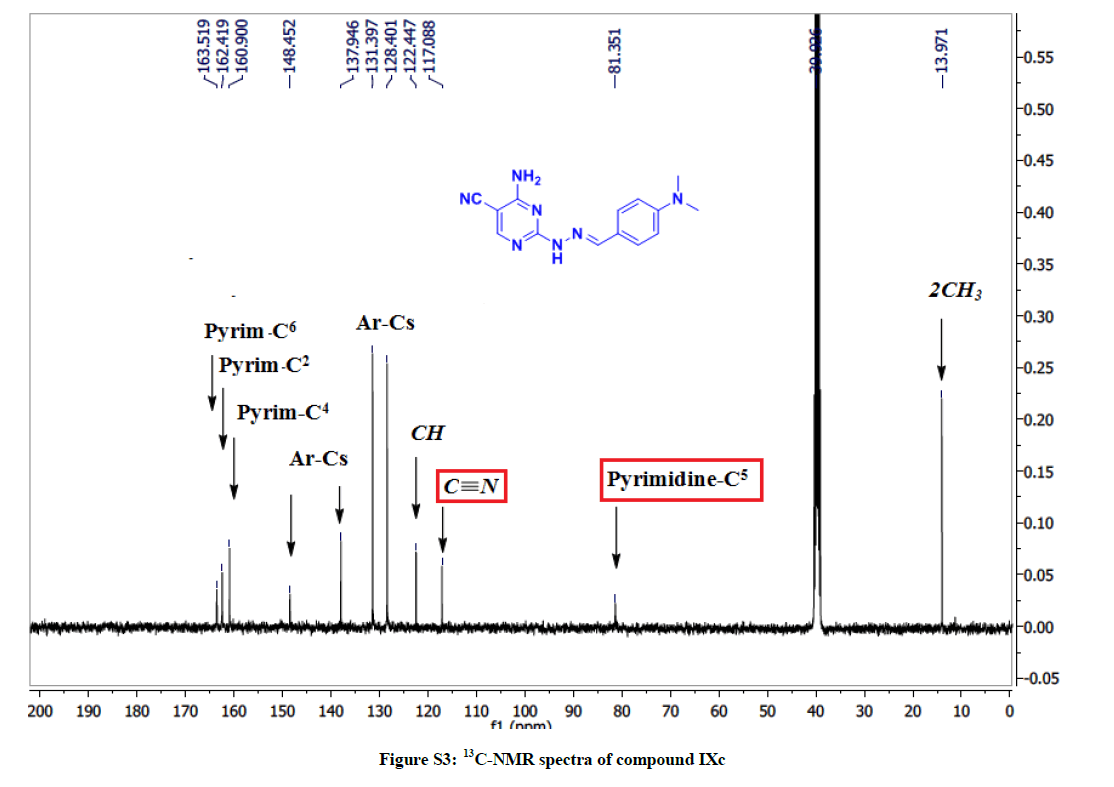
4-Amino-2-(2-(4-(dimethylamino) benzylidene) hydrazinyl) pyrimidine-5-carbonitrile (7c): Yield: (81%); M.p. 133-135ºC; IR (KBr): 3396- 3155 (NH2, NH), 2208 (C≡N), 1654 (C=N), 1585 (C=C) cm-1; 1H-NMR (DMSO-d6): 11.02 (s, 1H, NH), 8.26 (s, pyrimidine-C6-H), 8.00 (s, H, CH), 7.44 (d, 2Hs, Ar-Hs, J=8 Hz), 7.36 (brs, 2H, NH2, D2O exchanged), 6.69 (d, 4Hs, Ar-Hs, J=8 Hz), 2.85 (d, 6Hs, 2CH3); 13C-NMR (DMSOd6): 163.51, 162.41, 160.90, 148.45, 137.94, 131.39, 128.40, 122.44, 117.08, 81.35, 13.97. Anal. calc. for C14H15N7 (281.32): C 59.77, H 5.37, N 34.85; found: C 59.72, H 5.33, N 34.86.
4-Amino-2-(4-(4-nitrobenzylidene)hydrazinyl)pyrimidine-5-carbonitrile (7d): Yield: (92%); M.p. 167-169ºC; IR (KBr): 3469-3340 (NH2, NH), 2222 (C≡N), 1651 (C=N), 1601 (C=C) cm-1; 1H-NMR (DMSO-d6): 10.22 (s, IH, NH), 8.61 (s, 1H, N=CH), 8.35 (s, pyrimidine-C6-H), 7.70 (d, 2Hs, 2Hs, Ar-Hs, J=8 Hz), 7.55 (d, 2Hs, 2Hs, Ar-Hs, J=8 Hz), 7.45 (h, 2H, D2O exchanged NH2); 13C-NMR (DMSO-d6): 162.51, 160.30, 145.57, 128.51, 122.61, 117.68, 80.91. Anal. calc. for C12H9N7O2 (283.25): C 50.88, H 3.20, N 34.62; found: C 50.77, H 3.19, N 34.73.
4-Amino-2-(4-(2-nitrobenzylidene)hydrazinyl)pyrimidine-5-carbonitrile (7e):Yield: (92%); M.p. 167-169ºC; IR (KBr): 3469-3340 (NH2, NH), 2222 (C≡N), 1651 (C=N), 1601 (C=C) cm-1; 1H-NMR (DMSO-d6): 11.63 (s, 1H, NH), 8.55 (s, 1H, N=CH), 8.37 (s, pyrimidine-C6-H), 8.05 (d, 2Hs, 2Hs, Ar-Hs), 7.45 (s, 2H, D2O exchanged NH2), 7.58-7.56 (m, 3Hs, Ar-Hs); 13C-NMR (DMSO-d6): 163.83, 162.97, 160.47, 148.22, 139.23, 134.03, 130.44, 129.71, 128.21, 125.20, 80.91. Anal. calc. for C12H9N7O2 (283.25): C 50.88, H 3.20, N 34.62; found: C 50.77, H 3.19, N 34.73.
4-Amino-2-(2-(4-methoxybenzylidene) hydrazinyl) pyrimidine-5-carbonitrile (7f): Yield: (76%); M.p. 143-14ºC; IR (KBr): 3464-3297 (NH2, NH), 2210 (C≡N), 1639 (C=N), 1589 (C=C) cm-1. 1H-NMR (DMSO-d6): 11.34 (s, 1H, D2O exchanged, NH), 8.32 (s, 1H, N=CH), 811 (s, pyrimidine-C6-H), 7.59 (d, 2Ar-Hs, J= 8.4 Hz), 7.45 (s, 2Hs, D2O exchanged, NH2), 7.00 (d, 2Ar-Hs, J= 8.4 Hz), 3.79 (OCH3). 13C-NMR (DMSO-d6): 163.75, 162.73, 162.65, 160.85, 160.44, 144.42, 128.70, 127.82, 114.76, 80.93, 55.77. Anal. calc. for C13H12N6O (268.27): C 58.20, H 4.51, N 31.33; found: C 58.00, H 4.53, N 31.20.
4-Amino-2-(2-benzylidenehydrazinyl)pyrimidine-5-carboxamide (7g): Yield: 77%; M.p. 178-180ºC; IR (KBr): 3461, 3422 (2NH2), 3312 (NH), 1672 (C=O), 1643 (C=N), 1600 (C=C) cm-1. 1H-NMR (DMSO-d6): 11.28 (s, 1H, D2O exchanged, NH), 8.32 (s, 1H, N=CH), 8.14 (s, pyrimidine-C6 H), 7.67 (brs, D2O exchanged, NH2), 7.61 (d, 2Hs, Ar-Hs, J= 8.4 Hz), 7.55 (s, D2O exchanged, NH2), 7.32-7.31 (m, 3Hs, Ar-Hs); 13C-NMR (DMSO-d6): 169.34, 163.91, 160.23, 158.29, 141.51, 134.42, 133.99, 129.13, 128.48, 100.91. anal.calc. for C12H12N6O (256.26): C 56.24, H 4.72, N 32.79; found: C 56.26, H 4.75, N 32.80.
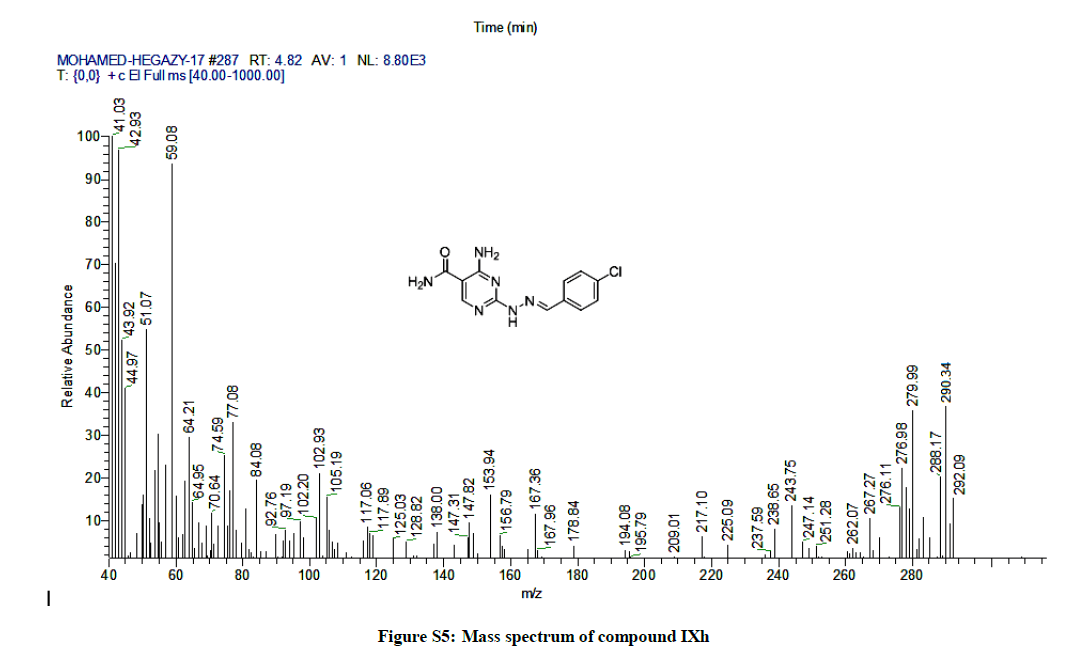
4-Amino-2-(2-(4-chlorobenzylidene) hydrazinyl) pyrimidine-5-carboxamide (7h): Yield: 83%; M.p. 213-215ºC; IR (KBr): 3488 (NH2), 3461 (NH2), 3427 (NH), 1669 (C=O), 1600 (C=N), 1605 (C=C) cm-1; 1H-NMR (DMSO-d6): 11.17 (s, 1H, D2O exchanged, NH), 8.29 (s, 1H, N=CH), 8.11 (s, pyrimidine-C6 H), 7.84 (d, 2Hs, 2Ar-Hs, J= 8 Hz), 7.75 (s, 4Hs, D2O exchanged, 2NH2), 6.95 (d, 2Hs, 2Ar-Hs, J= 8 Hz). Anal. calc. for C12H11ClN6O (290.71): C 49.58, H 3.81, N 28.91; found: C 49.60, H 3.82, N 28.93. MS: m/z (% relative abundance): m/z: 290.34 (36%), 292.09 (12%), 41.03(100).
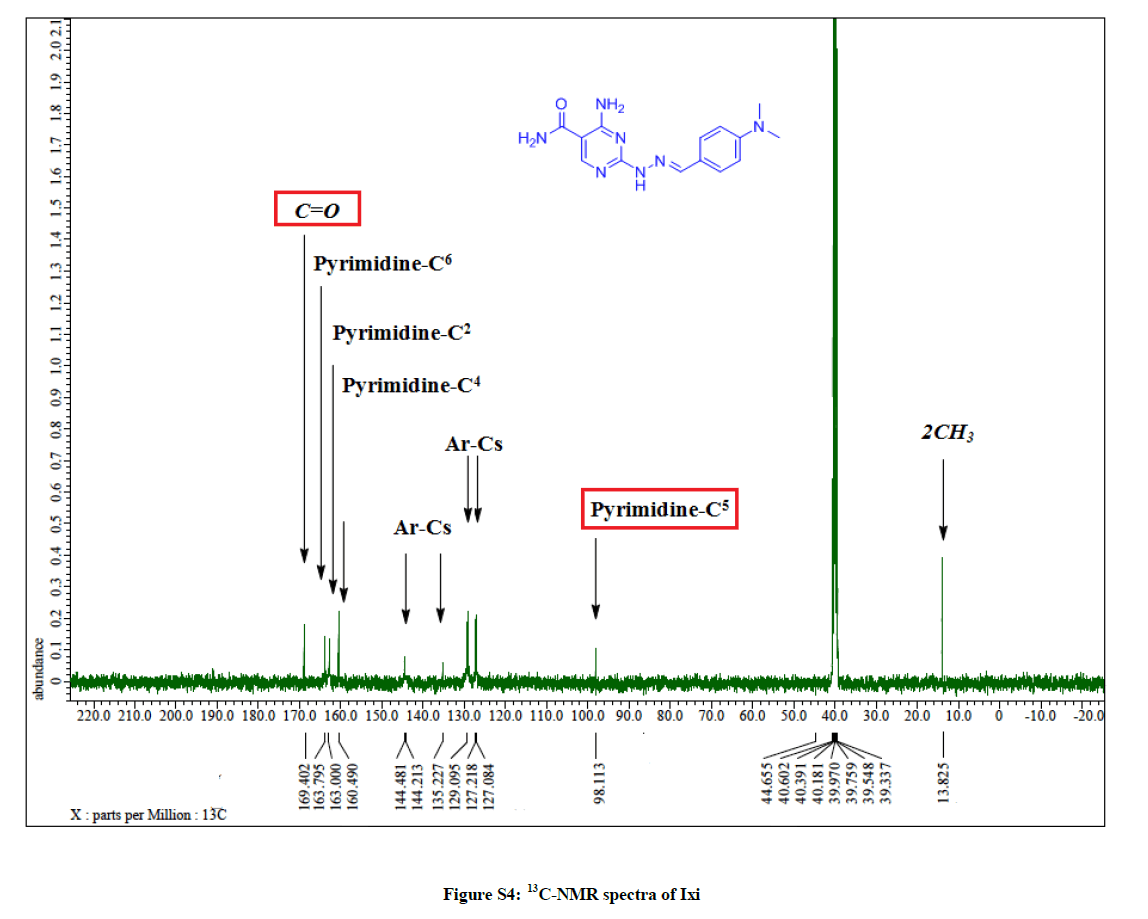
4-Amino-2-(2-(4-methoxybenzylidene) hydrazinyl) pyrimidine-5-carboxamide (7i): Yield: 74%; M.p. 156-158ºC; IR (KBr): 3522 (NH2), 3417 (NH2), 3331 (NH), 1644 (C=O), 1601 (C=C) cm-1; 1H-NMR (DMSO-d6): 10.93(s, 1H, D2O exchanged, NH), 8.51 (s, 1H, N=CH), 8.04 (s, pyrimidine-C6 H), 7.77 (s, 2Hs, D2O exchanged, NH2), 7.55 (d, 2Hs, Ar-Hs, J= 8.4 Hz), 7.10 (s, 2Hs, D2O exchanged, NH2), 6.92 (d, 2Hs, Ar- Hs, J= 8.4 Hz), 3.73 (s, 3Hs, OCH3); 13C-NMR (DMSO-d6): 169.48, 164.01, 160.58, 158.28, 143.00, 142.76, 128.61, 128.41, 128.12, 114.93, 114.45, 100.47, 55.85. Anal. calc. for C13H14N6O2 (286.26): C 54.54, H 4.93, N 29.35; found: C 54.59, H 4.98, N 29.42.
4-Amino-2-(2-(1-phenylethylidene)hydrazinyl)pyrimidine-5-carbonitrile (8a): Yield: (78%); M.p. 187-189°C; IR (KBr): 3400-3278 (NH2, NH), 2218 (C≡N), 1647 (C=N), 1588 (C=C) cm-1; 1H-NMR (DMSO-d6): 10.20 (s, 1H D2O exchanged, NH), 8.35 (s, pyrimidine-C6-H), 7.76 (d, 2Hs, 2Ar-Hs, J= 8.1 Hz), 7.47 (D2O exchanged NH2), 7.35-7.34 (m, 3Hs, 3Ar-Hs), 2.28 (s, 1H, CH3); 13C-NMR (DMSO-d6): 163.62, 162.80, 161.21, 150.21, 139.78, 136.85, 129.56, 129.08, 126.70, 126.62, 117.51, 81.37, 14.31. Anal. calc. for C13H12N6 (252.27): C 61.89, H 4.79, N 33.31; found: C 61.89, H 4.75, N 33.28.
4-Amino-2-(2-(1-p-tolylethylidene)hydrazinyl)pyrimidine-5-carbonitrile (8b): Yield: 71%; M.p. 170-172ºC; IR (KBr): 3422-3281 (NH2, NH), 2213 (C≡N), 1639 (C=N), 1590 (C=C) cm-1; 1H-NMR (DMSO-d6): 10.13 (s, 1H, D2O exchanged, NH), 8.33 (s, pyrimidine-C6 H), 7.64 (d, 2Ar-Hs, J=8 Hz), 7.45 (br, 2Hs, D2Oe xchanged, NH2), 7.17 (d, 2Ar-Hs, J=8 Hz), 2.28 (s, 3Hs, CH3), 2.25 (s, 3Hs, CH3); 13C-NMR (DMSO-d6): 163.82, 162.44, 161.21, 150.21, 138.88, 136.25, 129.56, 129.08, 126.70, 126.62, 117.51, 80.97, 21.36, 14.42. Anal. calc. for C14H14N6 (266.30): C 63.14, H 5.30, N 31.56; found: C 63.12, H 5.28, N 31.50.
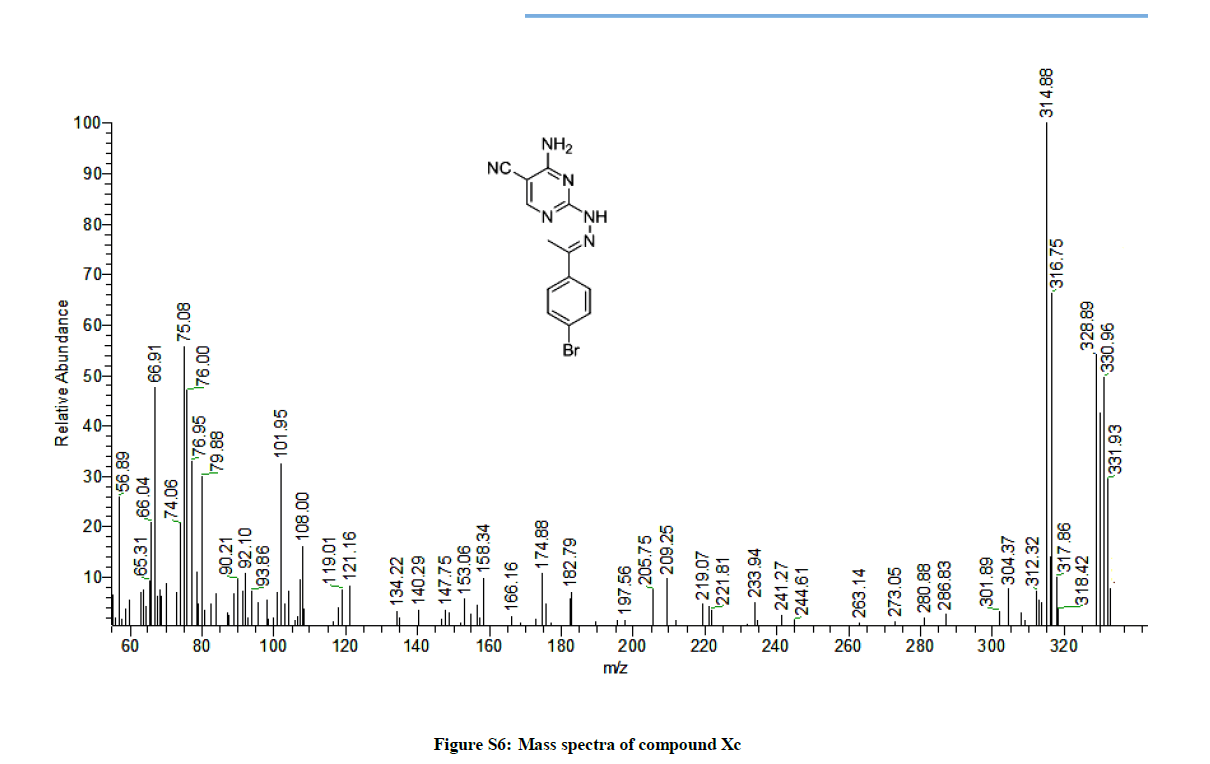
4-Amino-2-(2-(1-(4-bromophenyl)ethylidene)hydrazinyl)pyrimidine-5-carbonitrile (8c): Yield: 46%; M.p. 208-210ºC; IR (KBr): 3451-3397 (NH2, NH), 2211 (C≡N), 1636 (C=N), 1587 C=C) cm-1; 1H-NMR (DMSO-d6): 10.13 (s, 1H, D2O exchanged, NH), 8.33 (s, pyrimidine-C6 H), 7.65 (d, 2Hs, 2Ar-Hs, J= 8 Hz), 7.44 (s, 2Hs, D2O exchanged, NH2), 7.16 (d, 2Hs, 2Ar-Hs, J= 8 Hz), 2.24 (s, 3Hs, CH3); 13C-NMR (DMSOd6): 163.81, 162.79, 162.44, 161.20, 150.21, 138.88, 136.25, 129.56, 129.70, 126.70, 117.51, 81.25, 14.42. Anal. calc. for C13H11BrN6 (331.17): C 47.15, H 3.35, N 25.38; found: C 47.12, H 3.36, N 25.42; MS: m/z (% relative abundance): 329.95 (38%), 330.96 (42%), 331.93 (28%), 314.88(100).
4-Amino-2-(2-(1-(4-methoxyphenyl) ethylidene) hydrazinyl) pyrimidine-5-carbonitrile (8d): Yield: (79%); M.p. 159-161ºC; IR (KBr): 3421-3280 (NH2, NH), 2213 (C≡N), 1640 (C=N), 1590 (C=C) cm-1; 1H-NMR (DMSO-d6): 10.09 (s, 1H, D2O exchanged NH), 8.32 (s, pyrimidine-C6-H), 7.70 (d, 2H Ar-Hs, J= 8 Hz), 7.42 (brs, D2O exchanged, NH2), 6.91 (d, 2H, Ar-Hs, J= 8 Hz), 3.78 (s, 3Hs, OCH3), 2.24 (s, 3Hs, CH3); 13C-NMR (DMSO-d6): 163.84, 162.74, 161.18, 160.44, 150.29, 131.46, 128.53, 128.18, 127.84, 113.82, 81.07, 55.82, 14.40. Anal. calc. for C16H16N4O (280.32): C 68.55, H 5.75, N 19.99; found: C 59.50, H 4.98, N 29.75.
4-Amino-2-(2-(1-phenylethylidene)hydrazinyl)pyrimidine-5-carboxamide (8e): Yield: 67%. M.p 123-125ºC; IR (KBr): 3429 (NH2), 3381 (NH), 1673 (C=O), 1651 (C=C) cm-1; 1H-NMR (DMSO-d6): 9.87 (s, 1H, D2O exchanged, NH), 8.54 (s, pyrimidine-C6 H), 7.76 (d, 2Hs, 2Ar- Hs), 7.16-7.13 (m, 4Hs, D2O exchanged, 2NH2), 7.34-7.31(m, 3Hs, Ar-Hs), 2.26 (s, 3Hs, CH3); 13C-NMR (DMSO-d6): 169.46, 164.03, 161.16, 158.43, 158.12, 147.98, 139.28, 129.23, 128.89, 128.52, 126.55, 100.84, 14.17. Anal. calc. for C13H14N6O (270.29): C 57.77, H 5.22, N 3109; found: C 57.83, H 5.20, N 31.14.
4-Amino-2-(2-(1-p-tolylethylidene)hydrazinyl)pyrimidine-5-carboxamide (8f): Yield: (77%); M.p. 171-173ºC; IR (KBr): 3443 (NH2), 3324 (NH), 1654 (C=O), 1615 (C=C) cm-1; 1H-NMR (DMSO-d6): 9.81 (s, 1H D2O exchanged, NH), 8.59 (s, pyrimidine-C6-H), 7.77 (brs, 2Hs, D2O exchanged, NH2), 7.66 (d, 2Hs, 2Ar-Hs, J= 8 Hz), 7.39 (s, 2Hs, D2O exchanged, NH2), 7.16-7.14 (d, 2Hs, 2Ar-Hs, J= 8 Hz), 2.28 (s, 1H, CH3), 2.23 (s, 3Hs, CH3); 13C-NMR (DMSO-d6): 169.43, 164.00, 161.09, 158.29, 157.99, 148.10, 138.51, 136.49, 129.54, 126.45, 126.15, 100.72, 21.34, 14.10. Anal. calc. for C14H16N6O (284.32): C 59.14, H 5.67, N 29.56; found: C 59.20, H 5.70, N 29.60.
4-Amino-2-(2-(1-(4-bromophenyl)ethylidene)hydrazinyl) pyrimidine-5-carboxamide (8g): Yield: (63%); M.p. 201-203ºC. IR (KBr): 3487, 3476 (2NH2), 3336 (NH), 1668 (C=O), 1606 (C=C) cm-1; 1H-NMR (DMSO-d6): 9.90 (s, 1H, D2O exchanged, NH), 8.53 (s, pyrimidine-C6-H), 8.06 (s, 2Hs, D2O exchanged, NH2), 7.70 (s, 2Hs, 2Ar-Hs), 7.50 (s, 3Hs, 3Ar-Hs), 7.11 (s, 2Hs, D2O exchanged, NH2), 2.23 (s, 1H, CH3); 13C NMR (DMSO-d6): 169.41, 163.98, 161.05, 158.26, 146.69, 138.42, 131.61, 128.50, 122.36, 101.04, 13.90. Anal. calc. for C13H13BrN6O (349.19): C 44.72, H 3.75, N 24.07; found: C 44.75, H 3.80, N 24.20.
4-Amino-2-(2-(1-(4-methoxyphenyl)ethylidene)hydrazinyl)pyrimidine-5-carboxamide (8h): Yield: (59%); M.p. 194-196ºC; IR (KBr): 3457, 3398 (2NH2), 3376 (NH), 1682 (C=O), 1615 (C=C) cm-1; 1H-NMR (DMSO-d6): 9.74 (s, 1H, D2O exchanged, NH), 8.51 (s, pyrimidine-C6-H), 7.98 (s, 2Hs, D2O exchanged, NH2), 7.71 (d, 2Hs, 2Ar-Hs, J= 8 Hz), 7.08 (s, 2Hs, D2O exchanged, NH2), 7.91-7.89 (d, 2Hs, 2Ar-Hs, J= 8 Hz), 3.74 (OCH3), 2.22 (s, 1H, CH3); 13C-NMR (DMSO-d6): 169.47, 164.02, 161.18, 160.20, 158.40, 158.32, 158.09, 148.08, 131.78, 127.99, 120.97, 100.58, 55.82, 14.10. Anal. calc. for C14H16N6O (300.13): C 55.99, H 5.37, N 27.98; found: C 55.95, H 4.40, N 27.90.
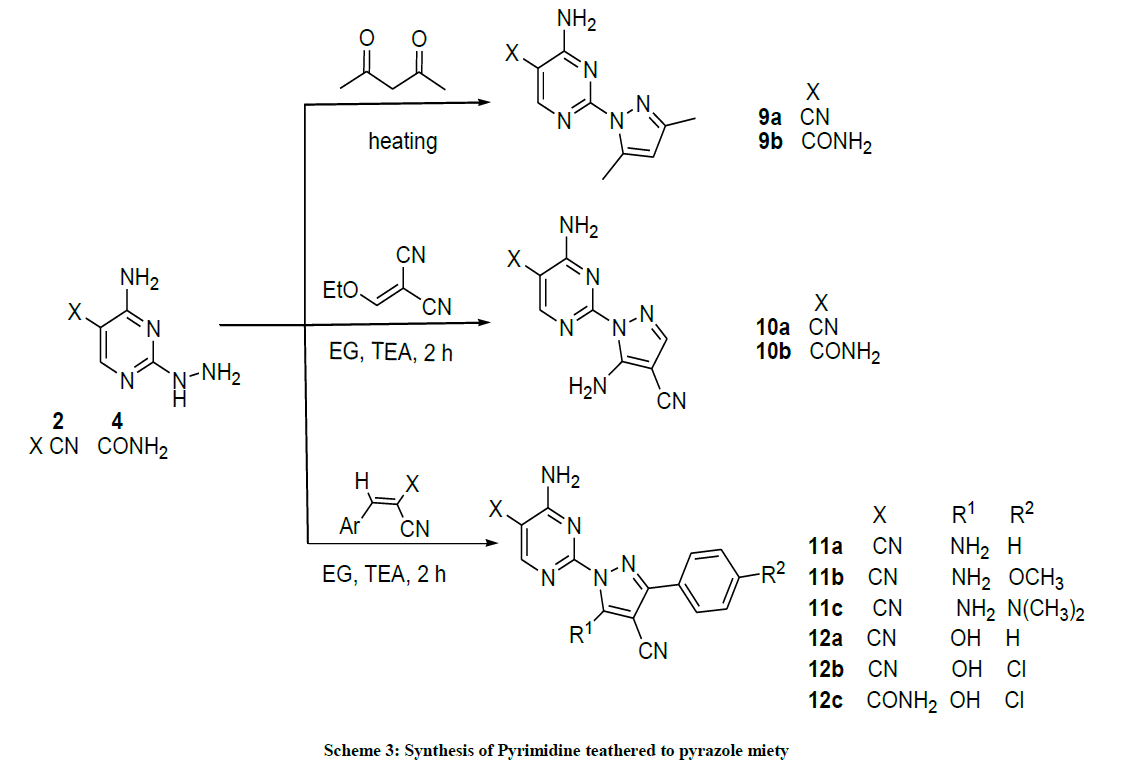
Synthesis of 4-amino-2-(3,5-dimethyl-1H-pyrazol-1-yl)pyrimidine-derivatives (9a,b): A mixture of 4-amino-2-hydrazinylpyrimidine-5- carbonitrile (2) (150 mg, 1 mmol) or 4-amino-2-ydrazinylpyrimidine-5-carboxamide (4) (168 mg, 1 mmol) and acetyl acetone (100 mg, 1 mmol) in EG (5 ml) was heated for 3 h at 120ºC. The solution was diluted with ice-cold H2O (5 ml) and refrigerated overnight. The obtained precipitate was filtered, dried and crystallized from EtOH.
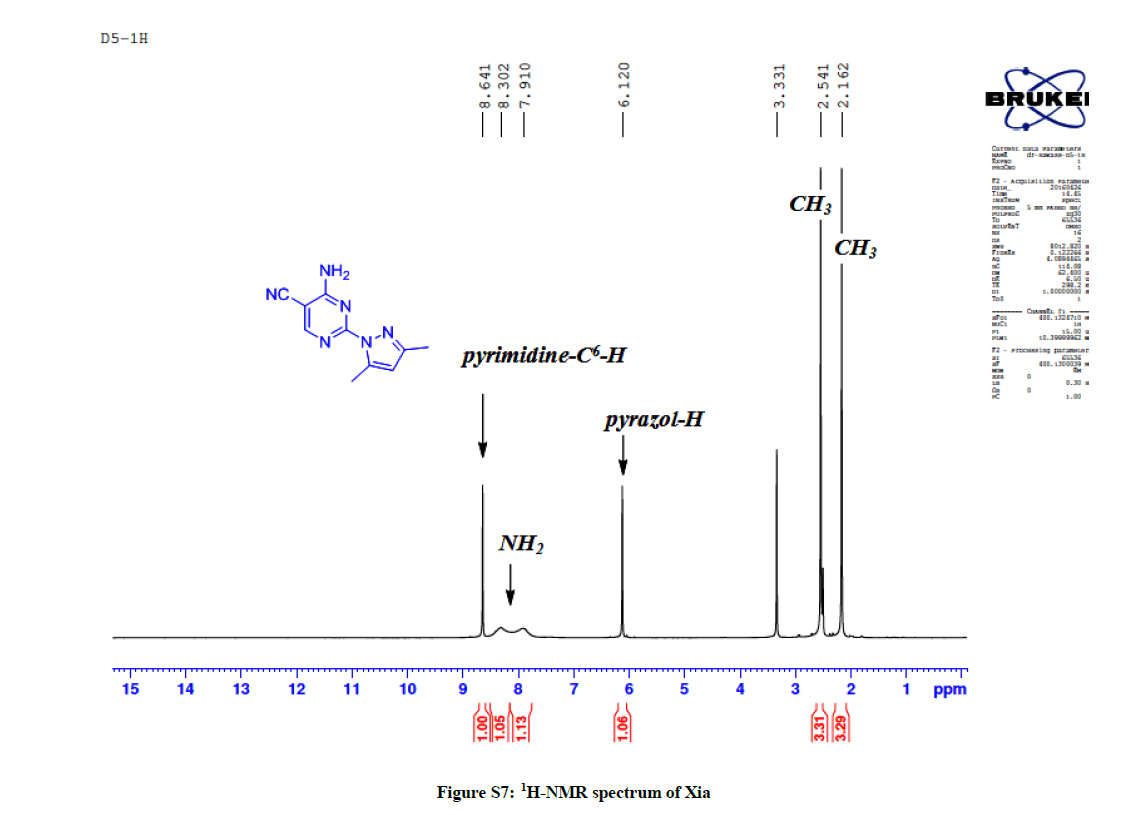
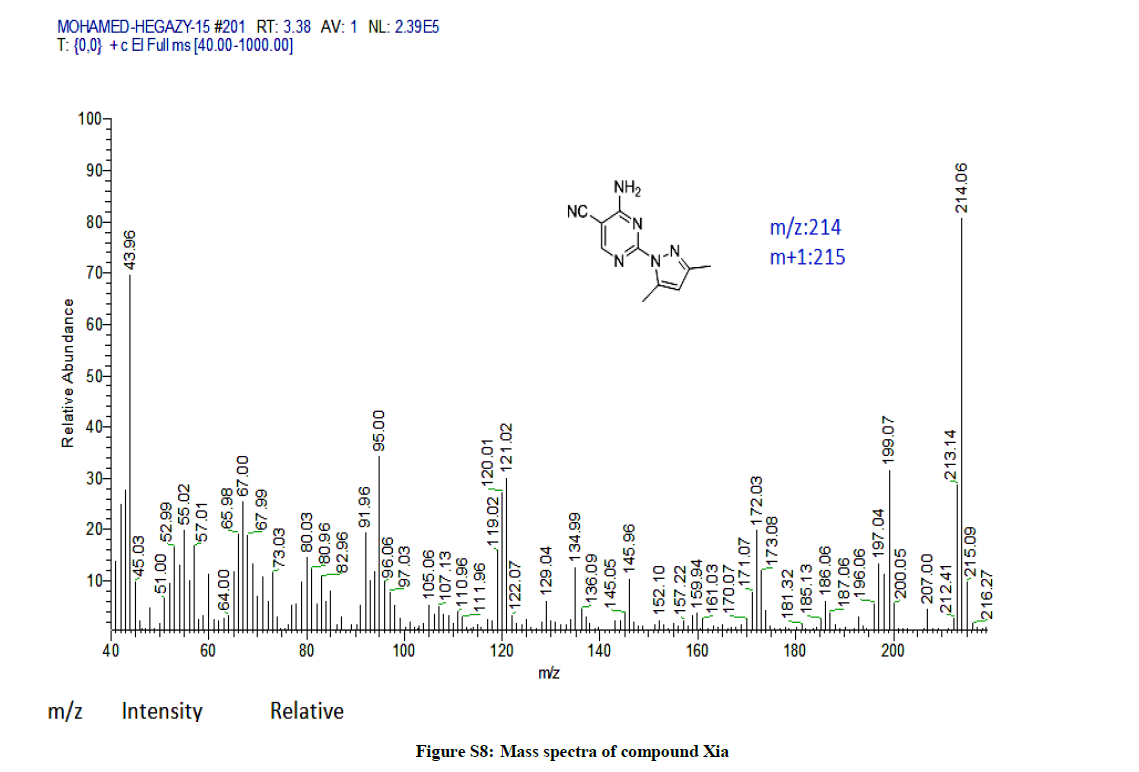
4-Amino-2-(3,5-dimethyl-1H-pyrazol-1-yl)pyrimidine-5-carbonitrile (9a): Yield: 63%; M.p. 112-114ºC; IR (KBr): 3477 (NH2), 2926 (aliph, C-H), 2220 (C≡N), 1632 (C=N), 1585 C=C) cm-1; 1H-NMR (DMSO-d6): 8.64 (s, pyrimidine-C6-H), 8.30-7.91 (s, 2H, D2O exchanged, NH2), 6.12 (s, 1H, pyrazol H), 2.54 (s, 3Hs, CH3), 2.16 (s, 3Hs, CH3); 13C-NMR (DMSO-d6): 163.88, 163.14, 158.01, 150.68, 143.36, 115.97, 110.86, 86.89, 15.68, 13.94. Anal. calc. for C10H10N6 (214.23): C 56.07, H 4.71, N 39.23; found: C 56.10, H 4.73, N 39.25.
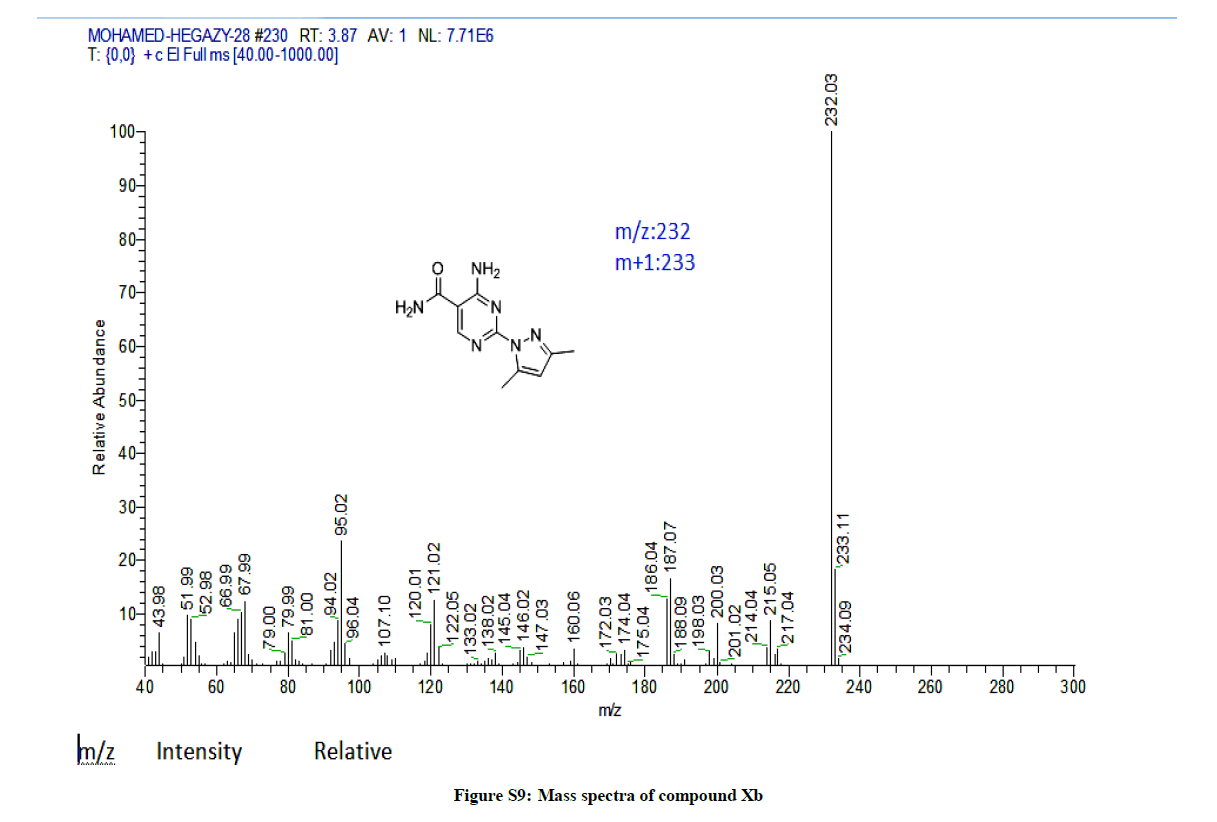
4-Amino-2-(3,5-dimethyl-1H-pyrazol-1-yl)pyrimidine-5-carboxamide (9b): Yield: (65%); M.p. 177-179ºC; IR (KBr): 3543-3417 (2NH2), 1674 (C=O), 1601 (C=C) cm-1; 1H-NMR (DMSO-d6): 8.61 (s, pyrimidine-C6-H), 8.32-8.87 (4Hs, D2O exchanged, 2NH2), 6.08 (s, 1H, pyrazol- H), 2.56 (s, 3Hs, CH3), 2.17 (s, 3Hs, CH3). Anal. calc. for C10H12N6O (332.24): C 51.72, H 5.21, N 36.19; found: C 51.76, H 5.28, N 36.30. MS: m/z (% relative abundance): m/z: 232.03 (100%), 233.11 (16%), 234.09 (2%).
General Procedure for the Synthesis of Compounds 10a,b, 11a-c, 12a-c: A mixture of 4-amino-2-hydrazinylpyrimidine-5-carbonitrile 2 (150 mg, 1 mmol) with equimolar amount of 2-(ethoxymethylene)malono-nitrile, 2-(benzylidene)malono-nitrile, 2-(4-ethoxy-benzylidene) malononitrile, 2-(4-(dimethyl-amino) benzylidene) malono-nitrile, Ethyl (2-cyano)-3-phenyl-acrylate or Ethyl 3-(4-chlorophenyl)-2-cyano acrylate in EG (3 ml) containing 3 drops of TEA, heated at 120ºC for 2 h. the reaction monitored by TLC till completed. The solution was diluted with icecold H2O (5 ml) and refrigerated overnight. The obtained precipitate was filtered, dried and crystallized from EtOH afforded 10a,b, 11a-c and 12a-b respectively. Following the same procedure; a mixture of 4-amino-2-hydrazinylpyrimidine-5-carboxamide (168 mg, 1 mmol) with Ethyl 3-(4-chlorophenyl)-2-cyano acrylate (337 mg, 1 mmol) afforded 12c. Yields, melting points and microanalysis of products 10a,b, 11a-c and 12a-c are listed.
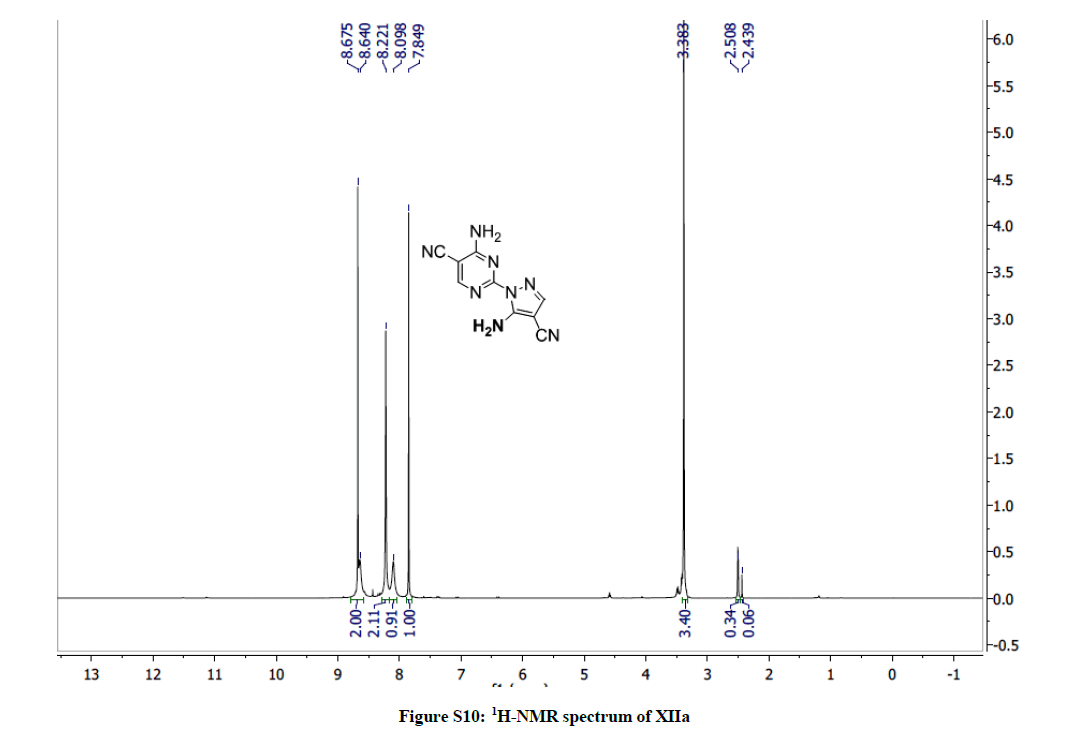
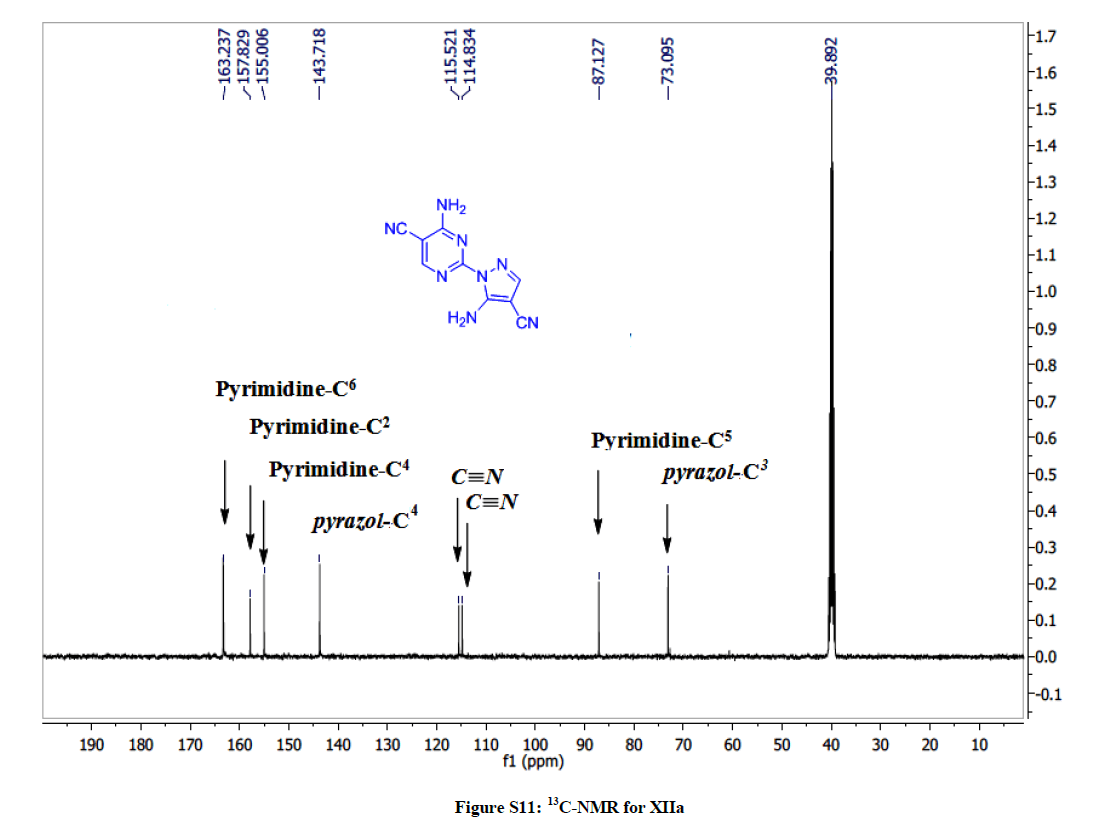
4-Amino-2-(5-amino-4-cyano-1H-pyrazol-1-yl)pyrimidine-5-carbonitrile (10a): Yield: (49%); M.p. 212-214ºC; IR (KBr): 3417 (NH2), 2221 (C≡N), 2216 (C≡N), 1614 (C=C), 1601 (C=C) cm-1; 1H-NMR (DMSO-d6): 8.67 (s, 2Hs, D2O exchanged, NH2), 8.22 (s, 2Hs, D2O exchanged, NH2), 8.09 (s, pyrimidine-C6-H), 7.84 (s, 1H, pyrazole H); 13C-NMR (DMSO-d6): 163.23, 157.82, 155.00, 143.71, 115.52, 114.83, 87.12, 73.09. Anal. calc. for C9H6N8 (226.20): C 47.79, H 2.67, N 49.54; found: C 47.73, H 2.71, N 49.62.
4-Amino-2-(5-amino-4-cyano-1H-pyrazol-1-yl) pyrimidine-5-carboxamide (10b): Yield: 43%; M.p. 143-145ºC; IR (KBr): 3417-3310 (3NH2), 2213 (C≡N), 1644 (C=O), 1601 (C=C) cm-1; 1H-NMR (DMSO-d6): 8.38 (s, pyrimidine-C6 H), 8.33-8.18 (m, 4Hs, D2O exchanged, 2NH2), 7.78 (s, 1H, pyrazole-H), 7.49 (s, 2Hs, D2O exchanged, NH2). Anal. Calc. for C9H7N9O (257.21): C 42.03, H 2.74, N 49.01; found: C 42.10, H 2.76, N 49.15.
4-Amino-2-(5-amino-4-cyano-3-phenyl-1H-pyrazol-1-yl)pyrimidine-5-carbonitrile (11a): Yield: (67%); M.p. 185-187ºC; IR (KBr): 3562 (NH2), 3407 (NH2), 2234 (C≡N), 2212 (C≡N), 1643 (C=N), 1518 C=C) cm-1; 1H-NMR (DMSO-d6): 8.33 (s, pyrimidine-C6-H), 7.96 (s, 2Hs, D2O exchanged, NH2), 7.82-7.36 (m, 7Hs, 2D2O exchanged NH2 + Ar-Hs); 13C-NMR (DMSO-d6): 163.85, 162.82, 162.47, 161.23, 150.10, 139.07, 129.53, 129.13, 128.90, 128.54, 126.72, 117.27, 114.50, 81.38, 73.67. Anal. calc. for C15H10N8 (302.29): C 59.60, H 3.33, N 37.07.
4-Amino-2-(5-amino-4-cyano-3-(4-methoxyphenyl)-1H-pyrazol-1-yl)pyrimidine-5-carbonitrile (11b): Yield: 64%; M.p. 234-236ºC; IR (KBr): 3454 (NH2), 3293 (NH2), 2213 (C≡N), 2212 (C≡N), 1638 (C=N), 1596 C=C) cm-1; 1H-NMR (DMSO-d6): 8.08 (s, pyrimidine-C6- H), 7.88 (s, 2Hs, D2O exchanged, NH2), 7.56, 7.54 (d, 2Ar-Hs, J= 8 Hz), 7.41 (h, 2Hs, D2O exchanged, NH2), 6.81, 6.79 (d, 2Ar-Hs, J= 8 Hz), 3.91 (s, 3Hs, OCH3); 13C-NMR (DMSO-d6): 162.45, 160.62, 160.20, 144.22, 128.47, 127.56, 117.27, 114.50, 80.92, 72.08, 55.52. Anal. Calc. for C16H12N8O (332.32): C 57.83, H 3.64, N 33.72; found: C 57.80, H 3.62, N 33.75.
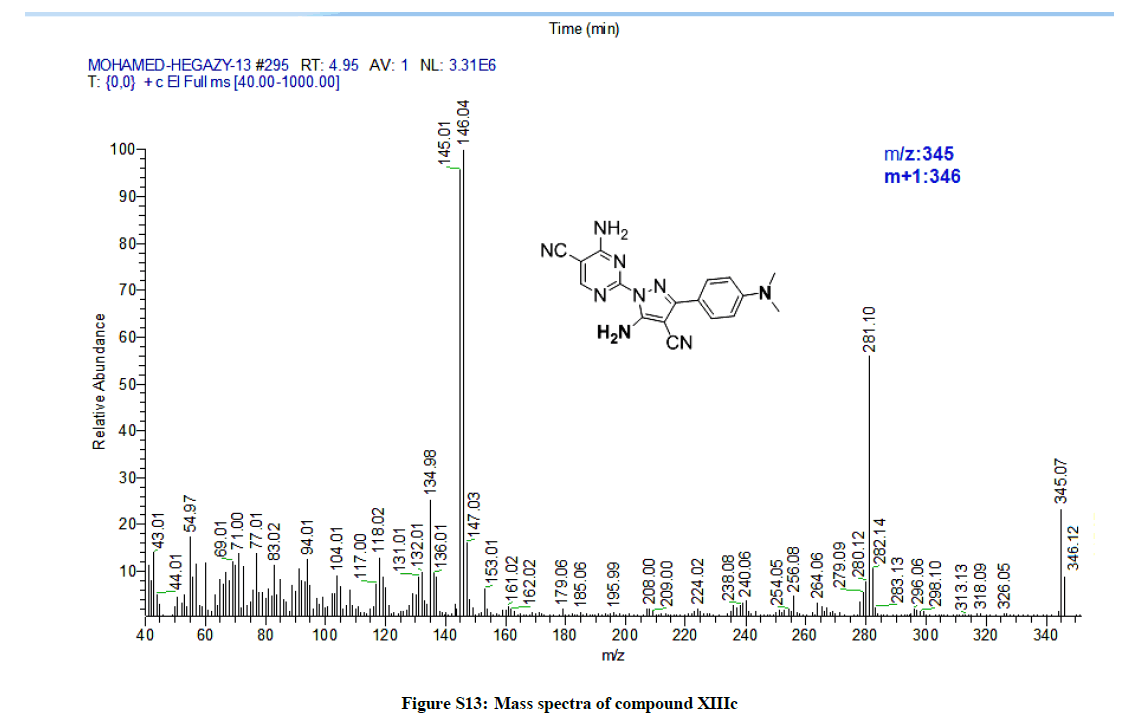
4-Amino-2-(5-amino-4-cyano-3-(4-(dimethylamino)phenyl)-1H-pyrazol-1-yl)pyrimidine-5-carbonitrile (11c): Yield: 47%. M.p. 267- 269ºC; IR (KBr): 3566 (NH2), 3338 (NH2), 2217 (C≡N), 2215 (C≡N), 1644 (C=N), 1596 C=C) cm-1; 1H-NMR (DMSO-d6): 8.14 (s, pyrimidine-C6-H), 7.83 (s, 2Hs, D2O exchanged, NH2), 7.61 (d, 2Hs, 2Ar-Hs, J= 8 Hz), 7.48 (s, 2Hs, D2O exchanged, NH2), 7.38-7.31(m, 3Hs, 3Ar-Hs), 2.28 (s, 3Hs, CH3). 2.22 (s, 3Hs, CH3); 13C-NMR (DMSO-d6): 163.85, 162.99, 162.54, 160.43, 143.09, 134.26, 134.17, 129.18, 128.99, 128.76, 117.25, 113.82, 80.92, 73.36, 2 1.11, 21.08. Anal. calc. for C17H15N9 (345.36): C 59.12, H 4.38, N 36.50; found: C 59.13, H 4.40, N 36.48. MS: m/z (% relative abundance): m/z: 345.07 (21%), 346.12 (7%), 146.04 (100).
4-Amino-2-(4-cyano-5-hydroxy-3-phenyl-1H-pyrazol-1-yl)pyrimidine-5-carbonitrile (12a): Yield: 55%; M.p. 188-190ºC; IR (KBr): 3454 (OH), 3295 (NH2), 2216, 2213 (C≡N, C≡N), 1639 (C=N), 1596 C=C) cm-1; 1H-NMR (DMSO-d6): 11.36 (s, 1H, D2O exchanged OH), 8.32 (s, pyrimidine-C6 H), 8.11-7.41 (m, 7Hs, D2O exchanged, NH2 and Ar-Hs). Anal. calc. for C15H9N7O (303.28): C 59.40, 2.99, 32.33; found: C 59.44, H 2.98, N 32.30.
4-Amino-2-(3-(4-chlorophenyl)-4-cyano-5-hydroxy-1H-pyrazol-1-yl)pyrimidine-5-carbonitrile (12b): Yield: 62%; M.p. 289-291ºC; IR (KBr): 3516 (OH), 3343 (NH2), 2230, 2225 (C≡N), (C≡N), 1654 (C=N), 1598(C=C) cm-1; 1H-NMR (DMSO-d6): 11.15 (s, 1H, D2O exchanged OH), 8.08 (s, pyrimidine-C6 H), 7.56, 7.54 (d, 2Ar-Hs, J= 8 Hz), 6.41 (s, 2Hs, 2D2O exchanged, NH2), 6.93 (d, 2Ar-Hs, J= 8 Hz); 13C-NMR (DMSO-6): 163.83, 162.82, 160.44, 142.99, 134.27, 134.16, 129.31, 128.71, 117.34, 112.33, 80.97, 72.10. Anal. calc. for C15H8ClN7O (337.72): C 53.35, H 2.39, N 29.03 found; C 53.33 H 2.41, N 29.12.
4-Amino-2-(3-(4-chlorophenyl)-4-cyano-5-hydroxy-1H-pyrazol-1-yl)pyrimidine-5-carboxamide (12c): Yield: (85%); M.p.193-195ºC; IR (KBr): IR 3516 (OH), 3417 (NH2), 1614 (C=O), 1601 (C=C) cm-1; 1H-NMR (DMSO-d6): 11.30 (s, 1H, D2O exchanged, OH), 8.14 (s, pyrimidine-C6-H), 7.79 (s, 2Hs, D2O exchanged, NH2), 7.60 (d, 2Ar-Hs, J= 8 Hz), 7.40 (s, 2Hs, D2O exchanged, NH2), 7.38-7.31 (m, 3Hs, 3Ar- Hs); 13C-NMR (DMSO-d6): 169.34, 163.83, 162.82, 160.44, 142.99, 134.27, 134.16, 129.31, 128.71, 117.34, 100.91, 80.96, 73.16. Anal. calc. for C15H10ClN7O2 (355.74): C 50.64, H 2.83, N 27.56; found: C 50.70, H 2.82, N 27.49.
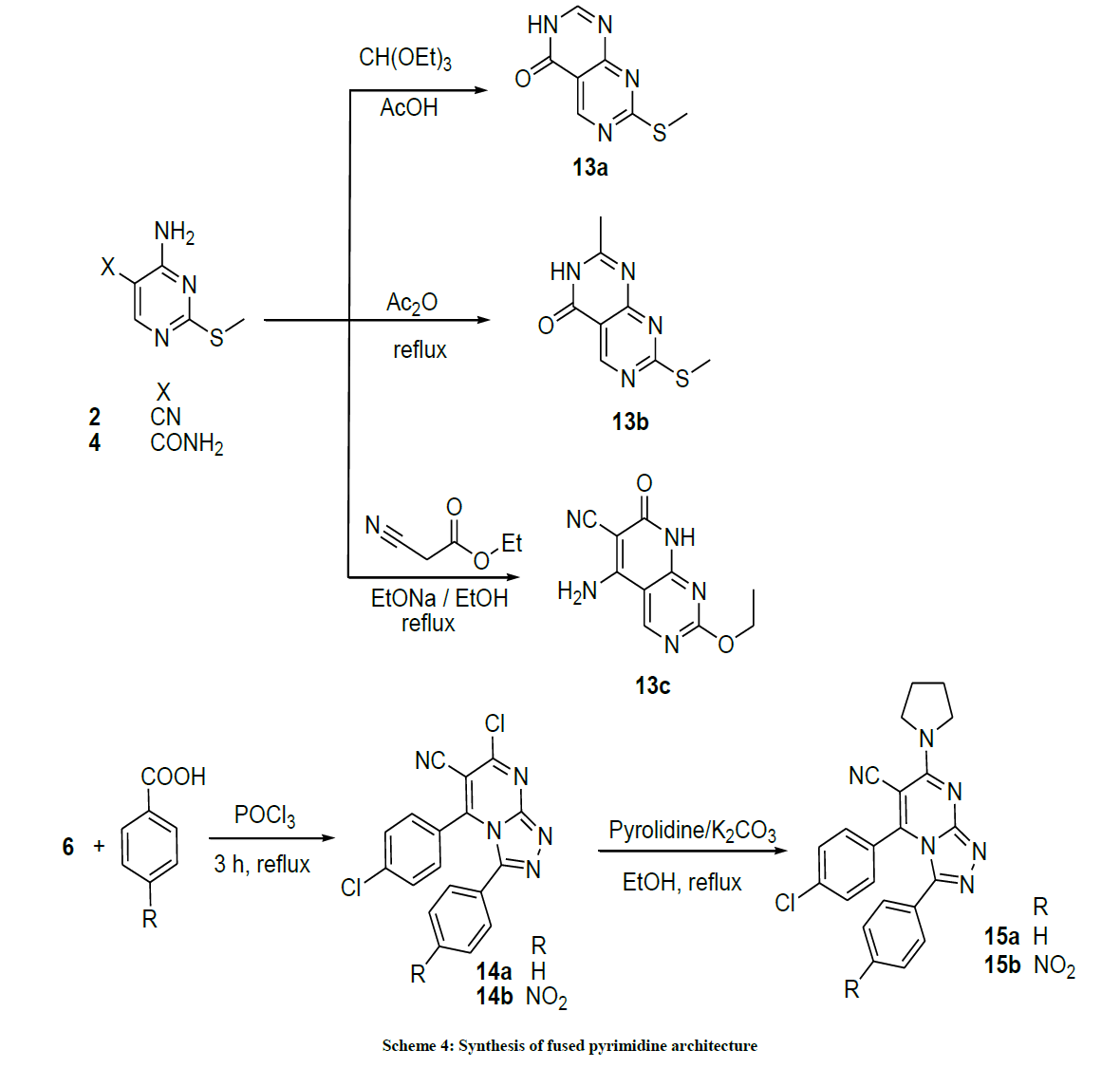
Synthesis of 7-(Methylthio)pyrimido[4,5-d]pyrimidin-4(3H)-one (13a): A mixture of the hydrazine 4 (1 mmol, 184 mg) and triethyl orthoformate (1 mmol, 138 mg) was heated under reflux in gl. AcOH (2 ml). The reaction was monitored by TLC (CHCl3/MeOH 10: 1). After the reaction was complete (ca. 4 h), the mixture was cooled and poured into ice-cold H2O. The resulting precipitate was filtered off, dried, and recrystallized from dioxane.
7-(Methylthio)pyrimido[4,5-d]pyrimidin-4(3H)-one (13a): C7H6N4OS (M. w. 194.03) Yield: (87%); M.p. 204-206ºC; IR (KBr): 3374 (NH), 3153 (CH, Ar-Hs), 1664 (C=O) cm-1; 1H-NMR (400 MHz, DMSO-d6): 11.72 (s, 1H, NH, D2O exchanged), 8.65 (s, 1H, CH), 8.84 (s, pyrimidine-C6-H), 2.63 (s, 3Hs, CH3); 13C-NMR (DMSO-d6): 177.68, 169.56, 162.78, 157.70, 114.04, 96.45, 24.01.
Synthesis of 2-methyl-7-(methylthio)pyrimido[4,5-d]pyrimidin-4(3H)-one (13b): 4-Amino-2-(methylthio)pyrimidine-5-carbonitrile (1) (1 mmol, 166 mg) was refluxed in Ac2O (2 ml) for 4 h. The reaction was monitored by TLC (CHCl3/MeOH 10: 1). After the reaction was complete, the mixture was cooled and poured into ice-cold H2O. The resulting precipitate was filtered off, dried, and recrystallized from EtOH.
2-Methyl-7-(methylthio)pyrimido[4,5-d]pyrimidin-4(3H)-one (13b): C8H8N4OS (M. w 208.04) Yield: (95%); M.p. 284-286ºC; IR(KBr): 3374 (NH), 3153 (CH, Ar-Hs), 1663 (C=O) cm-1; 1H-NMR (400 MHz, DMSO-d6): 11.21 (s, IH, NH, D2O exchanged), 8.93 (s, pyrimidine-C6- H), 2.56 (s, 3Hs, S-CH3), 2.18 (s, 3Hs, CH3); 13C-NMR (DMSO-d6): 176.72, 169.19, 162.17, 157.06, 112.01, 95.81, 23.35, 14.22.
5-Amino-2-ethoxy-7-oxo-7,8-dihydropyrido[2,3-d]pyrimidine-6-carbonitrile (13c): In dry flask, sodium metal (25 mg, 1.1 mmol) was dissolved in 5 ml absolute EtOH, (113 mg, 1 mmol) of ethyl cyanoacetate and (1 mmol, 166 mg) of 4-amino-2-(methylthio) pyrimidine-5- carbonitrile (1) was refluxed. The reaction was monitored by TLC (Hex/EA 3: 1). After the reaction was completed (ca. 5 h), the mixture was cooled, neutralized with AcOH and poured into ice-cold H2O. The resulting precipitate was filtered off, dried, and recrystallized from EtOH.
5-Amino-2-ethoxy-7-oxo-7,8-dihydropyrido[2,3-d]pyrimidine-6-carbonitrile (13c): Yield: (45%); M.p 231-233ºC; IR (KBr): 3374 (NH), 3213 (NH2), 3153 (CH, Ar-Hs), 2219 (C≡N), 1676 (C=O) cm-1; 1H-NMR (400 MHz, DMSO-d6): 8.96 (s, pyrimidine-C6-H), 8.57 (s, 1H, NH, D2O exchanged), 7.75 (s, 2H, NH2, D2O exchanged), 4.21 (q, 2Hs, CH2), 1.27 (t, 3Hs, J= 8 Hz, CH3).
Synthesis of 7-chloro-5-(4-chlorophenyl)-3-aryl-[1,2,4]triazolo[4,3-]pyrimidine-6-carbonitrile (14a,b): A mixture of 4-(4-chlorophenyl)-2- hydrazinyl-6-oxo-1,6-dihydropyrimidine-5-carbonitrile (6) (389 mg, 1.49 mmol), POCl3 (0.4 ml, 4 mmol), N,N-dimethylaniline (0.2 ml, 1.58 mmol) and equimolar amount of benzoic acid derivatives was heated at 60°C for 2 h. After completion of the reaction as indicated by TLC, the mixture was left to cool to RT, poured onto ice-cold H2O and the formed precipitate was filtered, dried and crystallized from EtOH. Yields, melting points and microanalysis of products are listed.
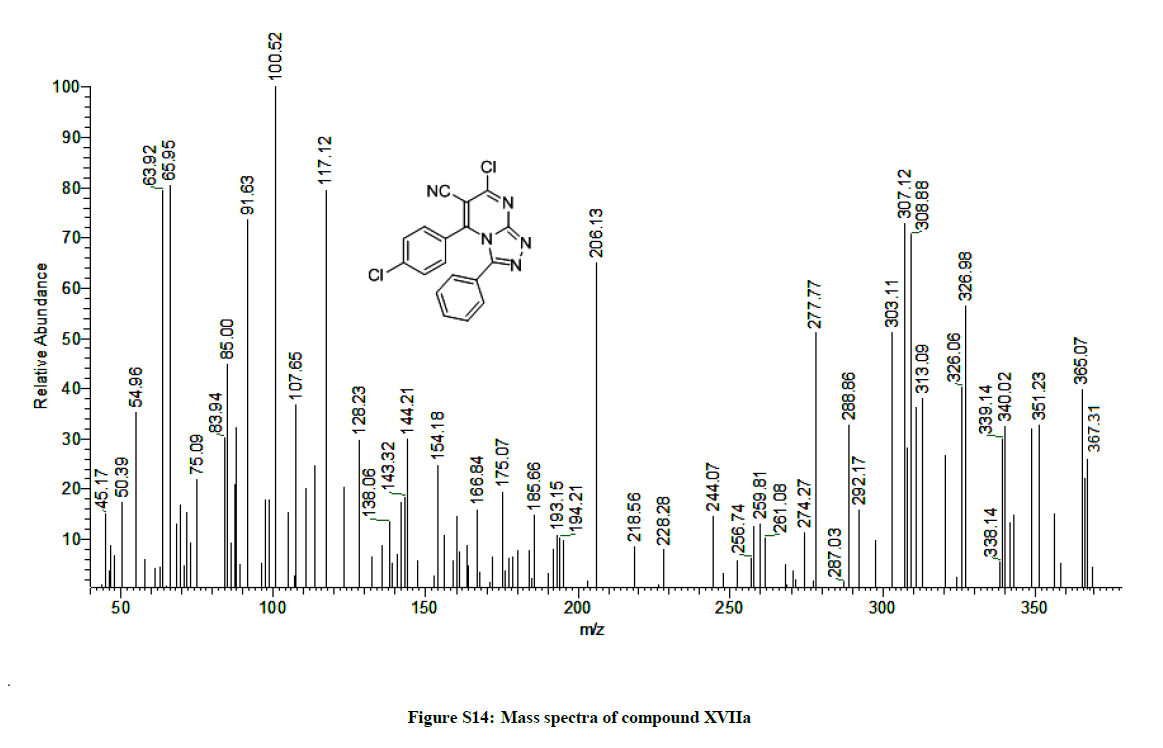
7-Chloro-5-(4-chlorophenyl)-3-phenyl-[1,2,4]triazolo[4,3-a]pyrimidine-6-carbonitrile (14a): Yield: 1.98 g (95%); M.p. 284-286ºC; IR (KBr): 2076 (CH, Ar), 2223 (C≡N), 1649 (C=N), 1605 (C=C) cm-1; 1H-NMR (DMSO-d6): 7.58-7.33 (m, 9Hs, Ar. Hs). Anal. calc. for C18H9Cl2N5 (366.20): C 59.04, H 2.48, N 19.12; found: C 59.10, H 2.54, N 19.18. MS: m/z (% relative abundance): m/z: 366.12 (23%), 367.31 (27%), 368.34 (6%), 100.52(100).
7-Chloro-5-(4-chlorophenyl)-3-(4-nitrophenyl)-[1,2,4] triazolo[4,3-a] pyrimidine-6-carbonitrile (14b): Yield: (46%). M.p. 236-238ºC; IR (KBr): 2106 (CH, Ar), 2225 (C≡N), 1649 (C=N), 1604 (C=C) cm-1; 1H-NMR (DMSO-d6): 7.98-7.06 (8Hs, Ar. Hs). Anal. calc. for C18H8Cl2N6O2 (411.20): C 52.58, H 1.96, N 20.44; found: C 52.60, H 1.94, N 20.48.
Synthesis of 5-(Aryl)-3-phenyl-7-(pyrrolidin-1-yl)-[1,2,4]triazolo[4,3-a]pyrimidine-6-carbonitrile derivatives (15a,b): A mixture of 7- chloro-5-(4-chlorophenyl)-3-aryl-[1,2,4] triazolo [4,3-a] pyrimidine-6-carbonitrile (2.86 mmol), pyrolidine (2.88 mmol) and anhydrous K2CO3 (0.4 g, 5.76 mmol) in EtOH (15 ml) was heated under reflux for 5 h. It was then cooled and filtered and the filtrate was concentrated to half its volume. The obtained precipitate was filtered and crystallized from EtOH-H2O (3: 1 v/v).
5-(4-Chlorophenyl)-3-phenyl-7-(pyrrolidin-1-yl)-[1,2,4]triazolo[4,3-a]pyrimidine-6-carbonitrile (15a): Yield: (87%); M.p. 211-213ºC; IR (KBr): 3106 (CH, Ar), 2966 (CH, aliphatic), 2225 (C≡N), 1649 (C=N), 1604 (C=C) cm-1; 1H-NMR (DMSO-d6): 7.86-7.32 (m, 9Hs, 9Ar-Hs), 3.62-3.50 (m, 4Hs, 2CH2 pyrolidine), 1.68-1.66 (m, 4Hs, pyrolidine Hs). Anal. calc. for C22H17ClN6 (400.86): C 65.92, H 4.27, N 20.96; found: 65.98, H 4.34, N 20.99.
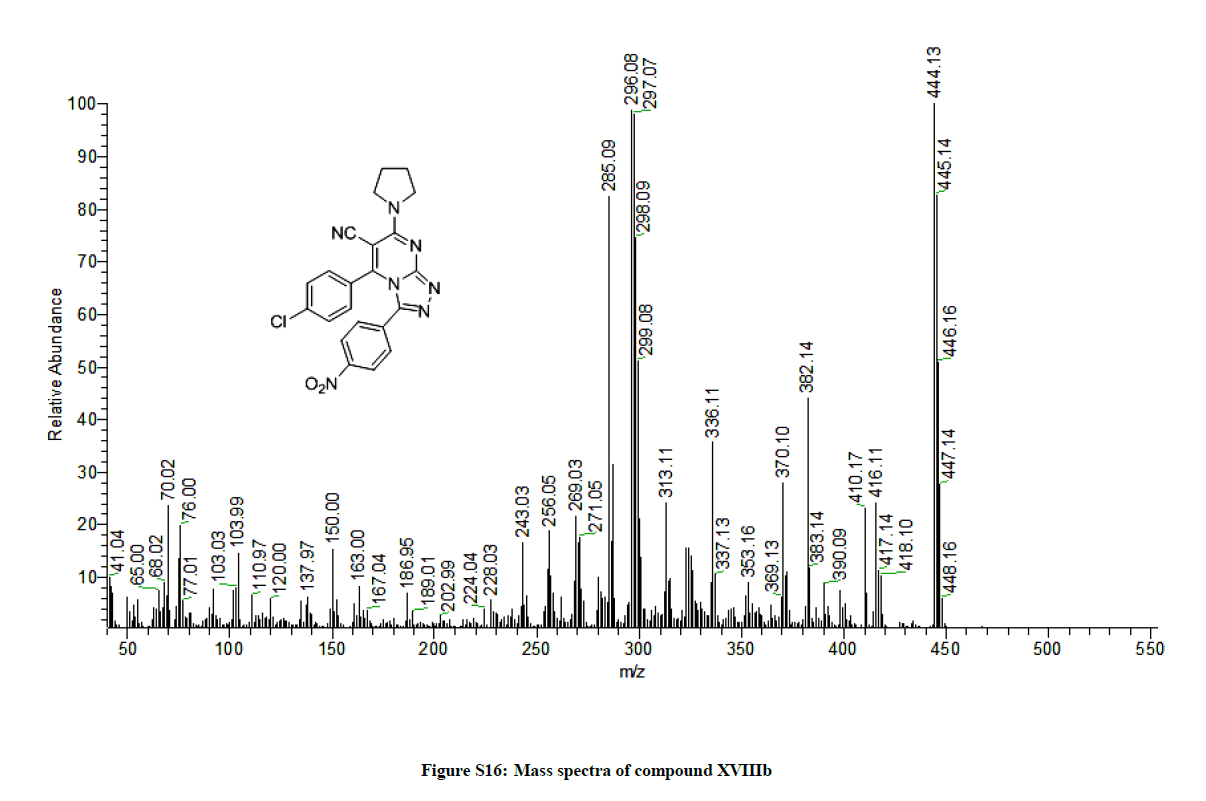
5-(4-Chlorophenyl)-3-(4-nitrophenyl)-7-(pyrrolidin-1-yl)-[1,2,4]triazolo[4,3-a]pyrimidine-6-carbonitrile (15b): Yield: (75%); M.p. 266- 268ºC; IR (KBr): 3106 (CH, Ar), 2966 (CH, aliphatic), 2225 (C≡N), 1649 (C=N), 1604 (C=C) cm-1; 1H-NMR (DMSO-d6): 7.54-7.40 (m, 8Hs, Ar-Hs), 3.65-3.52 (m, 4Hs, pyrolidine), 1.68-1.64 (m, 4Hs, pyrolidine Hs). Anal. calc. for C22H16ClN7O2 (445.86): C 59.26, H 3.62, N 21.99; found: C 59.36, H 3.72, N 21.91; MS: m/z (% relative abundance): 445.14 (83%), 446.16 (49%), 447.14 (28%), 444.13 (100).
Anticancer activity
Thirteen synthesized compounds were chosen by National Cancer Institute (NCI) to investigate the ant proliferative activity according to the protocol of the drug assessment branch. The prepared compounds were added at single concentration of 10-5 M and the culture was incubated for 48 h. End point determinations were made with a protein binding dye, sulforhodamine B (SRB). The human tumor cell lines were derived from nine different cancer types: leukemia, melanoma, lung, colon, CNS, ovarian, renal, prostate and breast cancers.
Results for each compound were reported as the growth percentage of the treated cells which are evaluated spectrophotometrically and compared to that of the untreated control cells.
Antimicrobial activity
Using the diffusion agar technique (at regional center of mycology and biotechnology, Alazhar University, Cairo, Egypt) the synthesized compounds were screened for their antifungal activity against Aspergillus flavus (RCMB0020002) and Candida albicans (RCMB005003 (1) ATTC10231). The antibacterial activity investigated against Gram positive bacteria; Staphylococcus aureus (RCMB010010) and Bacillus subtilis (RCMB015(1) NRRLB-543) and Gram negative bacteria; Salmonella typhimurium (RCMB006 (1)ATCC14028) and Escherichia coli (RCMB010052)ATCC25955. The test done on nutrient agar medium (bacteria) [30] and molt extract (fungi). The sterile medium (15 ml) in each petri-plate was uniformly smeared with cultures of Gram positive, Gram negative bacteria or Fungi. Sterile discs of 6 mm diameter were placed in the petriplates, to which (10 mg/ml, 100 μl/disc, DMSO as a solvent) of different synthesized compounds were added.
Ketoconazol [31] and gentamicin [32] were selected as antifungal antibacterial positive control respectively for comparison. For each treatment; three replicates were maintained. The plates were incubated at 37ºC ± 2ºC for 24 h and the zone of inhibition was determined. MIC investigated by the same previous technique used in the screening on the same organisms compared to the same controls.
Results and Discussion
Chemistry
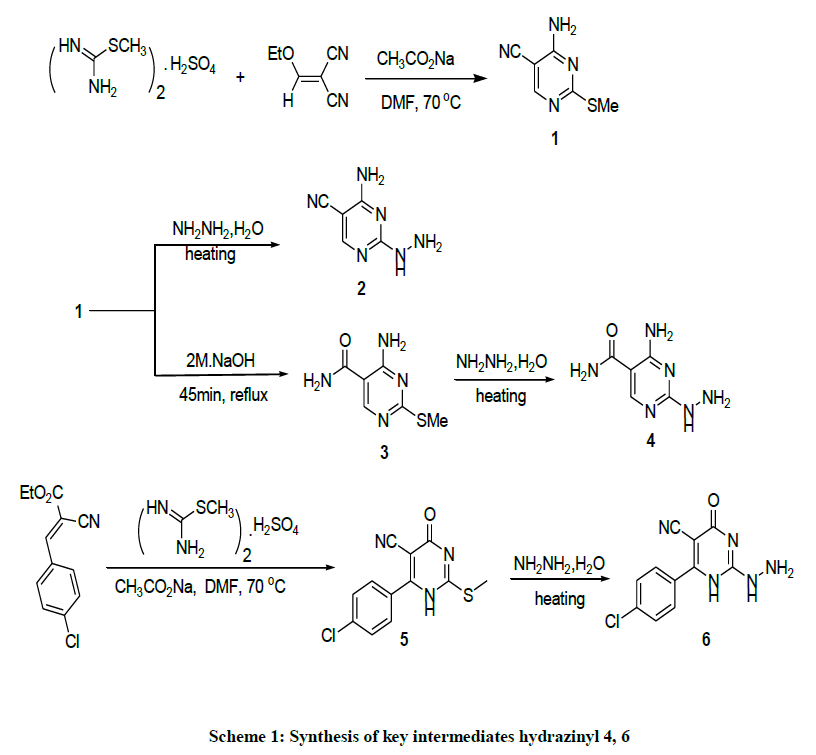
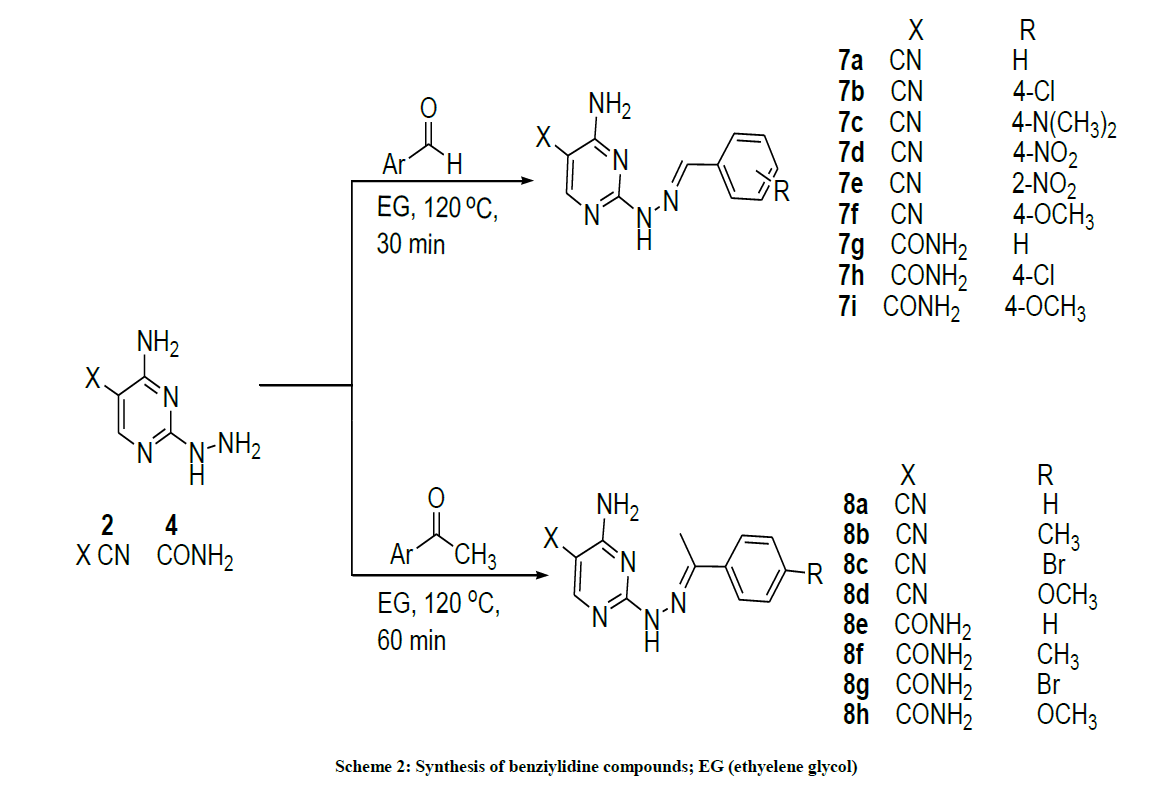
The requisite intermediates 2, 4, 6 were prepared according to procedures depicted in Scheme 1. Heating of S-methylisothiourea sulfate with 2- (ethoxymethylene) malononitrile [33] in the presence of anhydrous sodium acetate in DMF as a solvent to afford 4-amino-2- (methylthio)pyrimidine-5-carbonitrile 1 [34]. Basic hydrolysis of compound 1 generated the corresponding carboxamide derivative 3 [35]. Compounds 1 and 3 were treated with hydrazine hydrate to furnish the corresponding pyrimidyl hydrazine analogs 2 and 4 respectively [36]. In similar way, reaction of ethyl 3-(4-chlorophenyl)-2-cyano acrylate [37] with S-methylisothiourea sulfate in presence of anhydrous sodium acetate in hot DMF afforded 4-(4-chlorophenyl)-2-(methylthio)-6-oxo-1,6-dihydropyrimidine-5-carbonitrile 5 [38]. Displacing of the methyl thio group with hydrazine hydrate afforded 4-(4-chlorophenyl)-2-hydrazinyl-6-oxo-1,6-dihydropyrimidine-5-carbonitrile 6 (Scheme 1) [39]. Compounds 2, 4, and 6 are the key intermediates and building blocks for the newly synthesized compounds in this study. Having in hand the pyrimidpyl hydrazine as a key intermediate, our attention was directed to install substituent at C2. Condensation of compound 2 with aromatic aldehydes including benzaldehyde, 4-chlorobenzaldehyde, 4-nitrobenzaldehyde, 2-nitrobenzaldehyde and 4-methoxybenzaldehyde in ethylene glycol (EG) in presence of few drops of glacial acetic acid at 120°C afforded the corresponding hydrazones 7a-g (Scheme 2).
Similarly, compounds 7g-i were obtained from its precursor 4, (Scheme 2). Reaction of acetophenone, 4-methyacetophenone, 4- bromoacetophenone and 4-methoxyacetophenone with pyrimidyl hydrazine 2 or 4 led to the formation of compounds 8a-d and 8e-h respectively (Scheme 2). Cyclo condensation of hydrazine derivative with 1,3-dielectrophiles has been extensively used for the preparation of pyrazole heterocycles [40]. In this regard, heating the hydrazine derivatives 2 and 4 with 1,3-dielectrophile such as acetyl acetone in EG containing a few drops of glacial acetic acid afforded 2-pyrazolyl pyrimidine derivatives 9a,b. On the other hand, reaction of the hydrazine derivatives 2 and 4 with 2-(ethoxymethylene) malononitrile in EG containing a few drops of triethylamine (TEA) afforded the 2-pyrazolyl pyrimidine derivatives 10a,b. Additionally, 2-pyrazolyl pyrimidine bearing phenyl moiety 11a-c and 12a-c was generated (Scheme 3). In order to extend our strategy, different fused pyrimidine derivatives were explored (Scheme 4). The pyrimido-pyrimidinone 13a was prepared by the reaction of compound 3 with triethyl orthoformate in boiling AcOH [41].
Boiling compound 2 in acetic anhydride for 3 h generated also pyrimido-pyrimidinone, compound 13b (Scheme 4). The previous reaction can be rationalized by a Dimroth rearrangement which is an isomerization involving an O/N-translocation through a ring-opening ring-closure sequence [42]. Moreover, treatment of compound 1 with ethyl cyano acetate in boiling EtOH in presence of EtONa furnished pyrido-pyrimidinone compound 13c. Then, we thought to examine the pyrimidine fusion system in different place. The hydrazine derivative 6 was treated with POCl3 N,N-dimethylaniline and equimolar amount of benzoic acid or 4-nitrobenzoic acid to afford chlorinated triazolopyrimidine 14a,b. Compounds 14a, b were subjected to nucleophilic substitution reaction at the halogen of imine functionality with pyrolidine moiety in refluxing EtOH in the presence of K2CO3 to producetriazolo [4,3-a] pyrimidine-6-carbonitrile derivatives 15a,b.
Biology
Anticancer activity: Thirteen synthesized compounds were chosen by National Cancer Institute (NCI) to investigate the anti-proliferative activity according to the protocol of the drug assessment branch, Bethesda, USA, (http://www.dtp.nci.nih.gov).
Among the selected compounds, compound 7i exhibited best activity against leukemia, renal cancer and non-small lung cancer (Table 1). Compound 7h represent anticancer activity against ovarian cancer, breast cancer and melanoma. Compound 11c showed the best activity against colon cancer and CNS cancer. These active compounds belong to non-fused pyrimidine architecture with tethered to pyrazole moiety or aryl hydrazonyl moiety.
| Subpanel cancer cell Lines | %Growth Inhibition (GI%)a |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7g | 7h | 7a | 7i | 8b | 8c | 8e | 9a | 10b | 11b | 11c | 12a | 12c | |
| Leukemia | |||||||||||||
| CCRF-CEM | - | - | - | 15.18 | - | - | - | - | - | - | - | - | - |
| K-562 | - | - | 16.48 | 33.6 | 17.31 | 19.52 | - | - | - | 12.15 | 11.56 | - | 11.5 |
| MOLT-4 | 11.76 | 19.83 | 18.13 | - | - | - | - | - | - | 13.35 | - | - | 27.38 |
| RPMI-8226 | - | - | - | 23.16 | 23.17 | - | 16.13 | - | - | 14.21 | 11.39 | - | 29.5 |
| SR | - | 23.24 | - | - | 26.34 | 12.89 | - | - | - | 18.62 | 18.07 | - | 27.2 |
| Non-small cell lung cancer | |||||||||||||
| A549/ATCC | 17.46 | 29.21 | 12.78 | - | - | - | - | - | - | - | - | - | - |
| EKVX | - | - | - | - | - | - | - | - | - | - | - | - | - |
| HOP-62 | - | - | - | 12.57 | - | 11.77 | - | - | - | - | - | - | - |
| HOP-92 | 10.08 | - | - | 32.52 | - | - | - | - | - | - | - | - | - |
| NCI-H226 | - | - | - | - | - | - | - | - | - | - | - | - | 14.24 |
| NCI-H23 | 11.79 | 19.89 | - | - | 14.5 | - | - | - | - | - | 16.9 | - | 12.74 |
| NCI-H322M | 11.25 | 31.17 | 20.53 | 21.81 | - | - | 25.02 | - | - | - | - | - | 18.11 |
| NCI-H460 | - | 14.81 | - | 15.13 | - | - | - | - | - | - | - | - | - |
| NCI-H522 | - | 25.12 | 17.23 | 16.42 | - | - | 17.13 | 15.94 | 18.18 | - | - | - | - |
| Colon cancer | |||||||||||||
| COLO 205 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| HCC-2998 | - | - | - | - | - | - | - | - | - | - | - | 14.37 | - |
| HCT-116 | - | 21.98 | 11.61 | - | - | - | - | - | - | - | - | - | - |
| HCT-15 | 13.5 | 17.25 | - | - | - | - | - | - | - | - | - | - | - |
| HT29 | - | 24.37 | - | - | - | - | 14.11 | - | - | - | - | - | - |
| KM12 | - | - | - | - | 11.1 | - | - | - | - | 21.26 | 24.7 | - | 24.82 |
| SW-620 | - | - | - | - | - | - | - | - | - | - | 27.08 | - | - |
| CNS cancer | |||||||||||||
| SF-268 | 21.44 | 29 | 15.13 | - | 13.71 | - | 11.95 | - | - | - | 35.16 | - | 25.18 |
| SF-295 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| SF-539 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| SNB-19 | - | 18.54 | 12.92 | - | - | - | - | - | - | - | - | - | - |
| SNB-75 | 14.5 | 10.91 | 16.44 | - | - | - | - | - | - | 13.38 | 13.22 | - | 18.13 |
| U251 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Melanoma | |||||||||||||
| LOX IMVI | - | 12.62 | - | - | - | - | - | - | - | - | - | - | - |
| MALME-3M | - | - | - | - | 16.47 | - | - | - | - | - | - | - | - |
| M14 | - | 34.76 | 21.39 | 13.91 | - | - | - | - | - | - | 22.2 | - | - |
| MDA-MB-435 | - | 13.57 | - | - | - | - | - | - | - | - | - | - | - |
| SK-MEL-2 | - | 30.04 | 23.84 | 28.18 | - | - | - | - | - | 15.41 | - | - | - |
| SK-MEL-28 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| SK-MEL-5 | 14.06 | - | - | - | - | - | - | - | - | - | - | - | - |
| UACC-257 | 10.84 | 34.4 | - | - | - | - | - | 15.33 | - | - | - | - | - |
| UACC-62 | - | 32.86 | - | - | - | - | - | - | - | - | - | - | - |
| Ovarian cancer | |||||||||||||
| IGROV1 | - | 31.13 | 19.88 | 21.03 | 14.48 | - | - | - | - | - | 15.06 | - | 23.55 |
| OVCAR-3 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| OVCAR-4 | 11.02 | 21.45 | 12.71 | - | - | - | - | - | - | - | 12.11 | - | - |
| OVCAR-5 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| OVCAR-8 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| NCI/ADR-RES | - | - | - | - | - | - | - | - | - | - | - | - | - |
| SK-OV-3 | - | 23.53 | 14.06 | 20.37 | - | - | - | - | - | - | - | - | - |
| Renal cancer | |||||||||||||
| 786-0 | 16.19 | - | - | - | - | - | - | - | - | - | - | - | |
| A498 | 22.38 | 31.21 | 15.23 | 50.36 | 14.62 | - | 11.72 | - | 19.14 | 15.77 | 13.77 | - | 15.46 |
| ACHN | - | 15.05 | 10.13 | - | - | - | - | - | - | 21.21 | - | - | |
| XF 393 | - | 14.74 | 16.74 | 17.31 | - | - | - | - | - | - | - | - | - |
| SN12C | - | - | - | - | - | - | - | - | - | - | - | - | - |
| TK-10 | 11.92 | 37.93 | 26.57 | 27.9 | - | - | 17.28 | - | - | - | - | - | - |
| UO-31 | 15.93 | 36.11 | 26.68 | 29.71 | 12.71 | - | 17.61 | - | 11.97 | 13.63 | 28.61 | - | 18.48 |
| Prostate cancer | |||||||||||||
| PC-3 | - | 23.54 | - | 24.62 | - | - | - | - | - | - | - | - | - |
| DU-145 | - | 24.89 | - | - | - | - | - | - | - | - | - | - | 14.28 |
| Breast cancer | |||||||||||||
| MCF7 | 17.75 | 24.84 | 13.27 | - | 13.26 | - | 13.71 | - | - | 15.47 | - | - | 12.97 |
| MDA-MB-231/ATCC | - | - | - | - | - | - | - | - | - | - | - | - | - |
| HS 578T | - | - | - | - | 18.02 | - | - | - | - | - | - | - | - |
| BT-549 | - | - | - | - | - | - | - | - | - | 23.98 | - | - | |
| T-47D | 14.47 | - | 13.47 | 16.6 | 12.81 | - | - | - | - | - | - | - | - |
| MDA-MB-468 | 12.39 | - | - | - | - | - | - | - | - | - | - | - | 11.75 |
Table 1: Summarize the received data: Percentage growth inhibition (GI %) of in vitro subpanel tumor cell lines at 10 μM concentration of the selected compounds a (-=GI<10%) (nt: not tested)
Antimicrobial activity: Using the diffusion agar technique [38] the following compounds were tested as antimicrobial agents against the illustrated organisms Table 2.
| Fungi | Gram (+) bacteria | Gram (-) bacteria | ||||
|---|---|---|---|---|---|---|
| Asperigillus Flavus | Candida albicans | Staphylococcus aureus | Bacillus subtilis | Salmonella typhi | Escherichia coli | |
| 7f | - | - | 17 | 16 | 18 | - |
| 7h | - | - | - | - | - | - |
| 8a | - | - | 25 | 21 | 24 | 25 |
| 8b | - | - | 33 | 25 | 26 | 23 |
| 8c | - | - | 31 | 28 | 23 | 26 |
| 8d | - | - | 22 | 10 | 12 | 13 |
| 8e | - | - | - | - | - | - |
| 8f | - | - | 30 | 26 | 24 | 23 |
| 8g | - | - | - | - | - | - |
| 8h | - | - | - | - | - | - |
| 9a | - | - | 13 | 15 | 17 | - |
| 9b | - | - | 28 | 27 | 25 | 22 |
| 10a | - | - | - | - | - | - |
| 10b | - | - | 27 | 26 | 25 | 24 |
| 11a | - | - | - | - | - | - |
| 11b | - | - | 29 | 33 | 30 | 24 |
| 11c | - | - | 8 | - | 10 | - |
| 12a | - | - | 30 | 24 | 23 | 27 |
| 12b | - | - | 11 | - | 8 | - |
| 12c | 9 | - | - | 12 | 10 | 17 |
| 14a | 15 | 16 | - | 10 | 8 | 21 |
| 14b | 15 | 16 | 14 | - | - | - |
| 15a | - | - | 19 | 19 | - | - |
| 15b | - | - | - | - | - | - |
| CONTROL | 16 | 20 | 24 | 26 | 27 | 30 |
| Ketoconazol | Gentamicin | Gentamicin | ||||
Table 2: Antimicrobial activity of the synthesized compounds expressed as size of the inhibition zone (mm/mg sample)
Compounds 14a, 14b 8b, 8c, and 11c exhibited the best antifungal activity and antibacterial compounds among the synthesized compounds. So, these compounds were further screened to determine their MIC (minimum inhibitory concentration μg/ml).
From the data represented in Table 3, compound 14b was the most active compounds against C. albicans with MIC=625 μg/ml in comparison to ketoconazole (MIC=20 μg/ml). Compound 8c was the most active compound against S. aureus and E. coli with MIC=19.53 μg/ml in comparison of gentamicin with MIC=24 μg/ml and 30 μg/ml respectively. Compounds 11c was the most active compound against B. subtilis with MIC=19.53 μg/ml in comparison of gentamicin with MIC=26 μg/ml.
| species | MIC tested cpds | control | |||
|---|---|---|---|---|---|
| 12c | 14a | 14b | ketoconazol | ||
| Fungi | A. flavus | - | - | 1250 | 16 |
| C. albicans | 2500 | 1250 | 625 | 20 | |
| 8b | 8c | 11c | gentamicin | ||
| Grame (+) bacteria | S. aureus | 78.13 | 19.53 | 39.06 | 24 |
| B. subtilis | 19.53 | 39.06 | 19.53 | 26 | |
| Grame (-) bacteria | S. typhimurium | 78.13 | 78.13 | 156.25 | 27 |
| E. coli | 39.06 | 19.53 | 39.06 | 30 | |
Table 3: MIC of the highest active antifungal and antibacterial compounds
Conclusion
A new series of pyrimidine and fused pyrimidine derivatives was synthesized. These pyrimidyl derivatives were constructed from the key intermediates pyrimidyl hydrazine (2,4,6). The varieties of target compounds include differently substituted benzylidene functionalities, or planar aromatic heterocycles such as pyrazole ring. Fused ring systems such as triazolo [4,3-a] pyrimidine, pyrido [2,3-d] pyrimidine and pyrimido [4,5-d] pyrimidin systems. The synthetic courses were adopted to prepare pyrimidines and fused pyrimidines derivatives via formation of substituted pyrimidine core structure in very efficient and simple reactions. The structures of the synthesized compounds were assigned based on different spectroscopic techniques. The new compounds were evaluated for their antibacterial and antifungal activity compared to gentamicin and ketoconazol as standard drugs. Compound 14b was the most active compounds against C. albicans with MIC=625 μg/ml in comparison to ketoconazole (MIC=20 μg/ml). Compound 8c was the most active compound against S. aureus and E. coli with MIC=19.53 μg/ml in comparison of gentamicin with MIC=24 μg/ml and 30 μg/ml respectively. Compounds 11c was the most active compound against B. subtilis with MIC=19.53 μg/ml in comparison of gentamicin with MIC=26 μg/ml. Moreover, some compounds were selected and screened for their anticancer activity by National Cancer Institute (NCI), and exhibited moderate anticancer activity. From the obtained results, Compounds 7h, 7i and 12d revealed a significant anticancer activity and they are the most active compounds.
References
- H. Cho, M. Ueda, K. Shima, A. Mizuno, M. Hayashimatsu, Y. Ohnaka, Y. Takeuchi, M. Hamaguchi, K. Aisaka. J. Med. Chem., 1989, 32, 2399.
- K.S. Atwal, G.C. Rovnyak, S.D.Kimball, D.M. Floyd, S. Moreland, B.N. Swanson, J.Z. Gougoutas, J. Schwartz, K.M. Smillie, M.F. Malley, J. Med. Chem., 1990, 33, 2629.
- R. Kaur, B. Kaur, J. Applic. Chem., 2013, 2, 1102.
- J.S. Sandhu, ARKIVOC: Online J. Org. Chem., 2012, 66, 133.
- G.C. Rovnyak, K.S. Atwal, A. Hedberg, S.D. Kimball, S. Moreland, J.Z. Gougoutas, B.C. O'Reilly, J. Schwartz, M.F. Malle, J. Med. Chem.,1992, 35, 3254.
- J.G. Gary, S. Dzwonczyk, D.M. McMullen, Normandin, Diane E.; Parham, Charles S.; Sleph, Paul G.; Moreland, Suzanne. J Cardiovasc Pharmacol, 1995, 26, 289.
- E. Pery, A. Sheehy, N.M. Nebane, V. Misra, M.K. Mankowski, L. Rasmussen, E.L. White, R.G. Ptak, D. Gabuzd, Virology., 2015, 484, 276.
- patent: R.D. Appari, X. Chen, R. Chilukuri, A.P. Crew Amino, US 8399433 B2, 2013.
- C. Cherian, L. Wang, A. Wallace, S. Orr, Z. Hou, A. Gangjee, L.H. Matherly, cancer res., 2014, 74, 19.
- K.M. Abu-Zied, T.K. Mohamed, O.K. Al-Duiaj, M.E.A. Zaki, Heterocyclic Commun., 2014, 20, 93.
- J. Zhang, J.F. Peng, Y.B. Bai, P. Wang, T. Wang, J.M. Gao, Z.T. Zhang, Molec. Divers., 2016, 20, 887.
- D.I. Park, C. Dournes, I. Sillaber, M. Uhr, J.M. Asara, N.C. Gassen, T. Rein, M. Ising, C. Webhofer, M.D. Filiou, M.B. Müller, C.W. Turck, Sci. Rep., 2016, 6, 35317.
- S. Jain, K. Arya, N.N. Inamdar, N.B. Auti, P.A. Unawane, S.H. Puranik, H.S. Sanap, M.D. Inamke, A.J. Mahale, V.S. Prajapati, C. Curr. Top. Med. Chem., 2016, 16, 3133.
- M.A. Becker, Nucleosides, Nucleotides and Nucleic Acids.,2016, 35, 502.
- N.M. Khalifa, M.A. Al-Omar, A.El-G.E. Amr, A.R. Baiuomy, R.F. Abdel-Rahman, Russ. J. Bioorg. Chem., 2015, 41, 192.
- H.M. Ashoura, O.G. Shaabana, O.H. RizkaIbrahim, M. El Ashmawy, Eur. J. Med. Chem., 2013, 62, 341.
- I. Kostova, P.Y. Atanasov, Curr. Org. Chem., 2017, 21, 2096.
- A.D. Lopez, C.D. Mathers, M. Ezzati, D.T. Jamison, C.J. Murray, Lancet., 2006, 367, 1690-1747.
- R. Laxminarayan, A. Duse, C. Wattal, A.K. Mzaidi, H.F.L. wertheim, N. Sumpradit, E. Vlieghe, G.L. Hara, I.M. Gould, H. Goossens, C. Greko, D. Anthony, M. Bigdeli, G. Tomson, W.W. Housen, E. Ombaka, A.Q. Peralta, F.N. Qamar, O.Cars, Lancet Infect. Dis., 2013, 13, 1057.
- A.P. Johnson, N. Woodford, J. Med. Microbiol., 2013, 62, 499.
- B. Roth, E. Aig, B.S. Rauckman, J.Z. Strelitz, A.P. Phillips, R. Ferone, S.R.M. Bushby, C.W. Sigel, J. Med. Chem., 1981, 24, 933.
- O.N. Al Safarjalani, X.J. Zhou, R.H. Rais, J. Shi, R.F. Schinazi, F.N.M. Naguib, M.H. el Koun, Cancer Chemother. Pharm., 2005, 55, 541.
- L. Spáčilová, P. Džubák, M. Hajdúch, S. Křupková, P. Hradil, J. Hlaváč, Bioorg. Med. Chem. Lett. 2007, 17, 6647.
- K.S. Jain, T.S. Chitre, P.B. Miniyar, M.K. Kathiravan, V.S. Bendre, V.S. Veer, S.R. Shahane, C.J. Shishoo, Curr. Sci., 2006, 90, 793.
- M. Boisdron-Celle, G. Remaud, S. Traore, A.L. Poirier, L. Gamelin, A. Morel, E. Gamelin, Cancer lett., 2007, 249, 271.
- A. Mai, S. Massa, R. Pezzi, P. Botton, R. Scatena, J. Meraner, G. Brosch, Bioorganic. Med. Chem. Lett., 2005, 15, 4656.
- M. Wang, J. Yang, M. Yuan, L. Xue, H. Li, C. Tian, X. Wang, J. Liu, Z. Zhang, Eur. J. Med. Chem. 2017, 128, 88.
- K. Pomeisl, I. Votruba, R. Pohl, Collect. Czech. Chem. Commun., 2017, 7, 291.
- T.L.V. Ulbricht, In: Robert, R. (Edi.), Purines, pyrimidines and nucleotides and the chemistry of nucleic acids : Elsevier, Pergamon, 1964, 8854.
- S. Fustero, María Sánchez-Roselló, Pablo Barrio, and Antonio Simón-Fuentes. Chem. Rev., 2011, 111, 6984.
- M. Mohadeszadeh, M. Rahimizadeh, H. Eshghi, A. Shiri, M. Gholizadeh, A. Shams, Helv. Chim. Acta., 2015, 98, 474.
- B. Bonev, J. Hooper, J. Parisot, J. Antimicrob. Chemother.,2008, 61, 1295.
- E.C. Taylor, R.J. Knopf, R.F. Meyer, A. Holmes, M.L. Hoefle, J. Am. Chem. Soc., 1960, 82, 5711.
- W.L. Drew, A.L. Barry, R. O' Toole, J.C. Sherris, J. Appl. Microbiol., 1972, 24, 240.
- J. Heeres, L.J.J. Backx, J.H. Mostmans, J. Van Cutsem, J. Med. Chem., 1979, 22, 1003.
- C. Regamey, R.C. Gordon, W.M. Kirby, Clin. Pharmacol. Ther., 1973,14, 396.
- U. Nagahara, H. Kawano, S. Sasaoka, C. Ukawa, T. Hirama, A. Takada, H.B. Cottam, R.K. Robins, J. Heterocyclic Chem., 1994, 31, 239.
- T. Masquelin, D. Sprenger, R. Baer, F. Gerber, Y. Mercadal, Helv. Chim. Acta., 1998, 81, 646.
- Chemical book, 4-amino-2-(methylthio)pyrimidine -5-carboxamide https://www.chemicalbook.com accessed at December, 2017.
- M.K.H. Muhlstadt, G. Fischer, J. practical chem.--practical applic. appl. chem., 1970, 312, 254.
- C. Yue, A. Mao, Y. Wei, M. Lü, Catalysis Communications., 2008, 9, 1571.
- M.R. Ali, G. Verma, J. Taibah University Med. Sci., 2015, 10, 437.