Research Article - Der Pharma Chemica ( 2025) Volume 17, Issue 2
Synthesis, Spectral, DFT, Molecular Docking and Admet Properties of Dibenzyloxy Chalcones
S. Prabha*S. Prabha, Department of Chemistry, Govt. Arts College, Chidambaram, Tamilnadu, India, Email: drsprabhacdm@gmail.com
Received: 07-Jul-2023, Manuscript No. DPC-23-105176; Editor assigned: 10-Jul-2023, Pre QC No. DPC-23-105176 (PQ); Reviewed: 24-Jul-2023, QC No. DPC-23-105176; Revised: 14-Mar-2025, Manuscript No. DPC-23-105176 (R); Published: 21-Mar-2025
Abstract
Present article has included the preparation of unsubstitted, p-methoxy and p-nitro substituted dibenzxyloxy chalcones (1-3) with the help of ultrasonic energy. The formation of chalcones was confirmed by their spectral data. The DFT analyses of chalcones (1-3) were done and the calculated values are compared with literature values. Further, molecular docking with SARS-CoV-2 (pdb id : 6w63) for all chalcones (1-3) were performed and the results are compared. The pre-ADMET properties, drug-likeness and toxicity parameters are observed and discussed.
Keywords
Chalcones; UV, IR and NMR spectra; DFT analysis; Molecular docking; ADMET property
Introduction
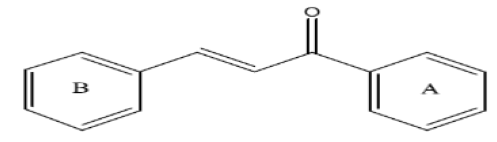
In 1899, the scientists namely Kostanecki and Tambor have given the compounds having α,β-unsaturated ketones as chalcones. Such compounds may be natural or synthetic which belong to the bicyclic flavonoid family [1]. They are both intermediates and end products in flavonide biosynthesis [2]. Chalcones has been targeted by several researchers in recent years due to their wide biological potential as well as their ability to serve as important intermediates for the synthesis of a large number of heterocyclic systems [3]. The structural skeleton of chalcones especially 1,3-diarylprop-2-en-1-ones are constituted by two aromatic rings linked through the open chain three-carbon unit α, β-unsaturated carbonyl system (Figure 1) [4].
The presence of chromophore (-CO-CH=CH-) and auxochromes give these compounds the colour, hence their ecological role in relation to plant colour. These bright yellow compounds are found in many plant organs [5,6]. Chalcones or 1,3-diarylpropenones are used as a starting material to synthesize many of the heterocyclic ring systems like pyrazolines, isoxazoles, pyrimidines and cyanopyridines. This compound forms a central core for a variety of important biological compounds which are used as an antioxidant, anticancer, antibacterial, antifungal, anti-inflammatory, and antiviral depending on the substitution made on them. The chemistry of chalcones has developed intensive scientific studies throughout the world. Chalcones exist as either E or Z isomers, the E-isomer being in most cases the thermodynamically most stable form and consequently, the majority of the chalcones is isolated as the E-isomer. The interesting electronic and nonlinear optical properties of chalcones derivatives act as a trigger towards exploring different and new applications. For assessing the spectral as well as structural properties of organic molecules, theoretical calculations like Density Functional Theory method (DFT) have developed as a powerful technique. Most of the DFT studies have been published describing a broad range of chalcones properties [15,16]. The characterization of synthesized novel chalcones by substitution groups in their derivative structures was also achieved using DFT [17,18]. Molecular docking is an effective strategy to gain insight into ligand-receptor interactions in the drug design industry. The vital role of molecular docking in the development of drug design is due to its ability to predict the best binding mode between drugs and the target protein. Molecular docking methods were used widely to analyze the biological and antibacterial activities of chalcones.
In pandemic period, SARS-CoV-2 is a virus from the Coronavirus group that spreads very quickly throughout the world and causes COVID-19 disease in millions of people worldwide. SARS-CoV-2 infects host cells by involving several proteins from the virus and proteins from the host. Main protease SARS-CoV-2 or Chymotrypsin-Like protease (3CLpro) is a protein that plays a role in the viral replication process and is very important for the life cycle of life SARS-CoV-2. Therefore, 3CLpro main protease is one of the potential targets in treating COVID-19. Until now, a drug has not been explicitly found used to treat COVID-19. Treatment therapy for COVID-19 patients is administering drugs that have been circulating. These drugs are antiviral drugs such as oseltamivir, ritonavir, lopinavir, and remdesivier. The other treatment is using antimalarial drugs such as chloroquine phosphate. As it is Covid time as well as a pandemic time, SARS-CoV-2 spike protease is chosen by several researchers in order to knowing their molecular docking study. Chalcone-derived compounds can be antivirals against the Coronavirus class through the primary protease inhibitory mechanism. Park et al., showed that chalcone-derived compounds isolated from the Angelica keiskei plant could inhibit the protease enzyme (3CLpro) SARS-CoV activity. Brousso chalcone A and B isolated from the roots of Broussonetia papyrifera were shown to inhibit 3CLpro SARS-CoV and 3CLpro MERS-CoV. Therefore, chalcone compounds and their derivatives may also have inhibitory activity against 3CLpro SARS-CoV-2. Several in silico studies through molecular docking of chalcone-derived compounds have been carried out, such as the indole-chalcone-derived compound that was docked against the main protease SARS-CoV-2 (GDP: 6YB7) and (E)-1-(2,4-dichlorophenyl)-3-[4-(morpholin-4-yl)phenyl]prop-2-en-1-one with main protease SARS-CoV-2 (GDP: 7BQY).
Hence, the continuation of synthesized chalcones (1-3), we have planned to study docking study using SARS-CoV-2 spike protease. This paper reports the synthesis, spectral characterization, DFT calculations of chalcones (1-3) and their molecular docking against spike protease.
Materials and Methods
All chemicals were purchased commercially and used without prior purification. Melting points were determined in open capillary tube and were uncorrected. Elemental analyzes were carried out on Variomicro V2.2.0 CHN analyzer. FT-IR spectrum of title compounds were recorded on a Shimadzu FTIR spectrophotometer in the range 400-4000 cm-1 using the KBr pellets. The proton and carbon NMR spectra were recorded on a BRUKER AVANCE III 500 MHz NMR spectrometer using CDC13 as solvent. Chemical shifts were reported in ppm. Tetramethylsilane (TMS) was used as internal reference for all NMR spectra, with chemical shifts reported in units (parts per million) relative to the standard. 1H NMR signal patterns are indicated as singlet (s), doublet (d), doublet of doublet (dd), triplet (t), quartet (q) and multiplet (m). The FT-IR are recorded at department of chemistry, Annamalai University and NMR spectra are recorded at SAIF, IIT, Chennai.
Synthesis of compounds
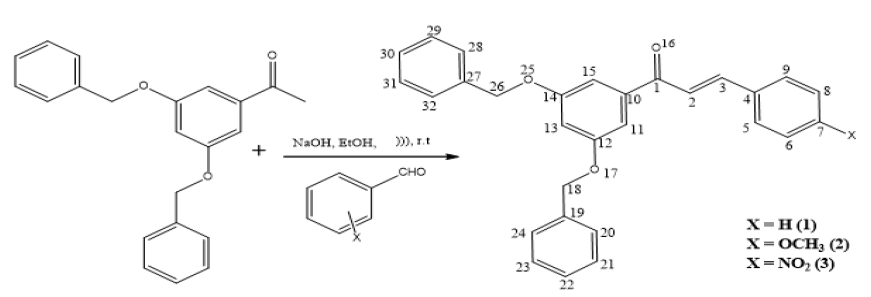
Synthesis of (E)-1-(3,5-bis(benzyloxy)phenyl)-3-phenylprop-2-en-1-one (1): In a 100 mL conical flask, equimolar quantity of 1-(3,5-bis(benzyloxy)phenyl)ethanone (2.5 mmol) and benzaldehyde (2.5 mmol) were taken with absolute ethanol (5 mL) and 40% sodium hydroxide (20 mmol diluted to 2 mL). The mixture was then immersed to ultrasonic bath at room temperature for 2 min. The completion of the reaction was monitored by thin-layer chromatography (eluent 20:80: ethyl acetate: hexane). After completion, the resulting mixture was acidified by using dilute HCl and the precipitate was filtered. The product formed was recrystallized from hot ethanol. The general scheme for preparation of compounds 1, 2 and 3 are given in Figure 2. The synthesized pure products were characterized by UV, FT-IR, 1HNMR, 13C NMR and Mass analytical methods.
Computational study: Geometry optimizations were carried out according to density functional theory using B3LYP/6-31 G(d,p) basis set in the Gaussian-09 package. The polarizabilities and hyperpolarizabilities have been derived from the DFT optimized structure by using finite discipline technique using the B3LYP/6-31 G basis set available in Gaussian-09.
Results and Discussion
Spectral analysis
The physical and spectral measurements of the synthesised compounds are given below.
Spectral analysis of (E)-1-(3,5-bis(benzyloxy)phenyl)-3-phenylprop-2-en-1-one (1): Yield 83%; mp 121-122°C; m/z 420; UV (CH3OH, λmax nm) 318.00, IR (KBr, νcm-1) 3061-2886 (Ar. CH str), 1660.71 (C=O), 1585.49 (Ar C=C str), 1215.15 (CHip), 918.12 (CHop), 1024.20 (Ali. CH=CHop), 565.14 (Ali. C=Cop). 1H NMR (500 MHz, CDCl3, δ/ppm) d 6.864(1H, d, J=15 Hz), 7.251 (1H, d, J=15 Hz), 5.139 (4H), 7.285-7.662 (16H, overlapping m, ArH); 13C-NMR (125 MHz, CDCl3, δ/ppm) 122.066 (Cα), 144.966 (Cβ), 189.989(CO), 29.713 (O-CH2-), 106.660-160.084 (Ar. Carbons).
Spectral analysis of (E)-1-(3,5-bis(benzyloxy)phenyl)-3-(4-methoxyphenyl)prop-2-en-1-one (2): Yield 76%; mp 126-127°C; m/z 450; UV (CH3OH, λmax nm) 327.00, IR (KBr, νcm-1) 3072-2848 (Ar. CH str), 1664.57 (C=O), 1587.42 (Ar C=C str), 1253.73 (CHip), 939.33 (CHop), 1043.49 (Ali. CH=CHop), 526.57 (Ali. C=Cop). 1H NMR (500 MHz, CDCl3, δ/ppm) d 7.252(1H, d, J=15 Hz), 7.791, (1H, d, J=15 Hz), 5.114 (4H), 7.334-8.470 (15H, overlapping m, ArH); 13C-NMR (125 MHz, CDCl3, δ/ppm) 125.645 (Cα), 142.847 (Cβ), 189.187(CO), 30.927 (O-CH2-), 29.705 (O-CH3) 107.483-160.176 (Ar. Carbons).
Spectral analysis of (E)-1-(3,5-bis(benzyloxy)phenyl)-3-(4-nitrophenyl)prop-2-en-1-one (3): Yield 79%; mp 131-132°C; m/z 465; UV (CH3OH, λmax nm) 342.00, IR (KBr, νcm-1) 3082-2880 (Ar. CH str), 1664.57 (C=O), 1593.20 (Ar C=C str), 1168.86 (CHip), 979.84 (CHop), 1041.56 (Ali. CH=CHop), 518.85 (Ali. C=Cop). 1H NMR (500 MHz, CDCl3, δ/ppm) d 7.528(1H, d, J=15 Hz), 7.792 (1H, d, J=15 Hz), 5.118 (4H), 6.861-8.474 (15H,overlapping m, ArH); 13C-NMR (125 MHz, CDCl3, δ/ppm) 122.434 (Cα), 141.746 (Cβ), 189.181(CO), 29.698 (-O-CH2-), 107.017-160.181 (Ar. Carbons).
DFT analysis
In the present study, the author has synthesized three number of diaryl prop-2-en-1-ones and characterized them with the help of spectral techniques. The Density Functional Theory analysis of these compounds (1-3) were performed using the Guassian 09 programme with 6-31G(d,p) basis set and the compounds are visualized at Gaussview 5.0.8 software. The obtained results are discussed below.
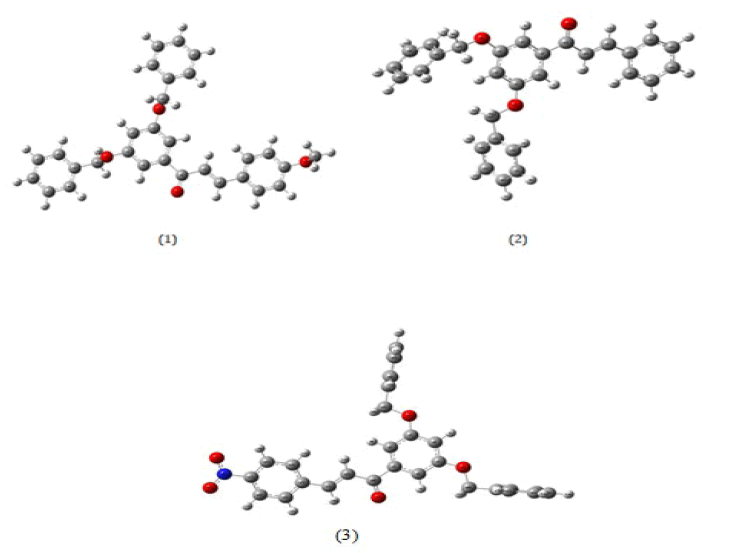
Geometry and its parameters: All the synthesized chalcones or diaryl prop-2-en-1-ones (1-3) are optimized and their optimized structures are given in Figure 3. Some of the selected bond lengths calculated for these systems (1-3) is displayed in Table 1 along with bond angles and dihedral angles.
| Bond length (A°) | 1 | 2 | 3 | Bond angle (°) | 1 | 2 | 3 |
|---|---|---|---|---|---|---|---|
| C4-C5 | 1.4 | 1.4 | 1.4 | C5-C6-C7 | 120 | 120 | 120 |
| C1-O16 | 1.26 | 1.26 | 1.26 | C10-C1-C2 | 119 | 119 | 120 |
| C1-C2 | 1.47 | 1.47 | 1.48 | O16-C1-C2 | 120 | 120 | 120 |
| C2-C3 | 1.35 | 1.35 | 1.35 | C1-C2-C3 | 120 | 120 | 120 |
| C2-H2 | 1.08 | 1.08 | 1.08 | C2-C3-C4 | 128 | 120 | 127 |
| C3-H3 | 1.08 | 1.08 | 1.08 | C12-O17-C18 | 119 | 119 | 119 |
| C10-C1 | 1.49 | 1.49 | 1.49 | O17-C18-C19 | 107 | 107 | 107 |
| C12-O17 | 1.39 | 1.39 | 1.38 | C11-C12-O17 | 120 | 120 | 120 |
| O17-C18 | 1.47 | 1.46 | 1.47 | C7-O33-C34 | 117 | ||
| C18-C19 | 1.5 | 1.5 | 1.5 | C7-N33-O34/35 | 118 | ||
| C18-H18 | 1.09 | 1.09 | 1.09 | ||||
| C7-O33/C7-N33 | 1.38 | 1.46 | |||||
| O33-C34/N33-34 | 1.45 | 1.26 | |||||
| Dihedral angle (°) | 1° | 2° | 3° | ||||
| C10-C1-C2-C3 | 128 | 129 | 176 | ||||
| C1-C2-C3-C4 | 179 | 179 | 179 | ||||
| O16-C2-C3-H3 | 177 | 180 | 174 | ||||
| H2-C2-C3-H3 | 180 | 180 | 179 | ||||
| C14-H25-C26-C27 | 180 | 178 | 179 | ||||
| C12-O17-C18-C19 | 180 | 180 | 179 | ||||
| O17-C18-C19-C20 | 96 | 93 | 96 |
Table 1: Selected geometrical Parameters of the compounds 1-3
The bond length between the carbonyl group C1-O2 is calculated as ∼1.26A° for all the t compounds (1-3). The vinyl bond length C2-C3 and C2/3-H2/3 in α and β positions are calculated as 1.35 and 1.08 respectively. These calculated lengths are agreed with literature values [29]. The bond angles between C10-C1-O16 are calculated as 119 by DFT method. The other calculated angles O16-C1-C2 and C1-C2-C3 ∼120 are agreed with literature values. The angle between C7-O33-C-34 is calculated nearly 117 for compound 2 and it is slightly distorted from the phenyl ring. The dihedral angles C1-C2-C3-C4 and C10-C1-C2-C3 of compounds 1-3 shows nearly 179 and it clearly indicates that all the synthesized compounds (1-3) have exhibited in Trans form and cal. values are agreed good with previous values.
Vibrational analysis
The experimental IR spectra of the compounds (1-3) were recorded and compared with the theoretical frequencies calculated by DFT method and the values are given in Table 2.
| DFT scaled (cm-1) | Exp. IR (cm-1) | DFT scaled (cm-1) | Exp. IR (cm-1) | DFT scaled (cm-1) | Exp. IR (cm-1) | Assignment |
|---|---|---|---|---|---|---|
| 3135 | 3134 | 3136 | sym str CH | |||
| 3131 | 3131 | 3131 | sym str CH | |||
| 3101 | 3099 | 3100 | sym str CH | |||
| 3099 | 3094 | 3097 | sym str CH | |||
| 3079 | 3061 | 3087 | 3072 | 3077 | 3082 | sym str CH |
| 3061 | 2924 | asy str CH3 | ||||
| 2989 | asy str CH3 | |||||
| 3029 | 3031 | 2965 | 2846 | 2972 | 2880 | asy str -CH2- |
| 2961 | 2924 | 2952 | 2959 | asy str -CH2- | ||
| 1646 | 1660 | 1631 | 1664 | 1639 | 1664 | sym str C=O |
| 1609 | 1585 | 1603 | 1587 | 1603 | 1593 | sym str C=C |
| 1606 | 1591 | 1592 | sym str C=C | |||
| 1602 | 1583 | 1590 | sym str C=C | |||
| 1580 | 1580 | 1575 | sym str C=C | |||
| 1574 | 1556 | 1573 | sym str C=C | |||
| 1550 | 1534 | 1542 | sym str C=O, C=C | |||
| 1500 | 1490 | 1507 | 1440 | 1507 | 1442 | CH2-def |
| 1496 | 1498 | 1499 | CH2-def | |||
| 1495 | 1495 | 1491 | ßCH (phenyl) | |||
| 1492 | 1488 | 1462 | ßCH (phenyl) | |||
| 1469 | 1473 | 1455 | ßCH (C=C) | |||
| 1238 | 1253 | sym str C-O (-C-OCH3) | ||||
| 1427 | 1359 | asy NO2 | ||||
| 1392 | asy NO2 | |||||
| 1235 | sym NO2 | |||||
| 1165 | 1165 | 1167 | 1165 | 1178 | 1168 | ?CH (phenyl) |
| 1131 | 1139 | 1125 | ?CH (phenyl) | |||
| 1345 | 1354 | 1345 | ?CH (phenyl) | |||
| 1339 | 1336 | 1335 | ?CH (phenyl) | |||
| 1021 | 1024 | 1024 | 1043 | 1082 | 1041 | ?CH (C=C) |
| 1007 | 1008 | 1073 | 912 | ?CH (C=C) | ||
| 1007 | 918 | 1014 | 939 | 1014 | ?CH op | |
| 574 | 565 | 574 | 526 | 576 | 518 | ?C=C op |
Table 2: IR frequencies of experimental and DFT (scaled) method of compounds 1-3.
The carbonyl group has characteristic band at 1660-1664 cm-1 for compounds 1-3 in experimental. The frequencies are calculated at 1631-1646 cm-1 by B3LYP method. The aromatic C-H stretching are observed nearly 3082-3062 cm-1 by experimentally and calculated in the region of 3150-3077 cm-1. The aromatic -C=C- stretching is observed at 1585, 1587, 1593 cm-1 and calculated at 1646, 1631 and 1639 respectively for compounds 1-3. The -CH in plane bending deformaions for phenyl ring are observed experimentally nearly 1215-1168 cm-1 and the values are calculated nearly 1213-1226 cm-1. For compound 2, the C-O-C stretching for methoxy group is observed at 1253 cm-1 and calculated at 1238 cm-1. The NO2 stretching for 3 is calculated 1285 and 1485 cm-1 and observed at 1359 cm-1.
Non-linear optical property: In the field of telecommunications, optical interconnections and optical memory for emerging in the area of signal processing, the nonlinear optical organic materials has significant role. These organic materials exhibit high polarizability and delocalized π conjugated electronic structure. These structures enable passage of electron contributor and acceptor groups from single side of molecule to the other side and provide widespread range of usages in NLO, engineering and synthesizing the chemicals. Due to the charge distribution or transfer from one side to another side makes the molecule as polarized. In the present study, the calculated dipole moments (μ), polarizabilities (αtot) and first order hyperpolarizabilities (βtot) are given in Table 3.
| Parameters | 1 | 2 | 3 |
|---|---|---|---|
| Dipole moments (Dynes) | 4.495 | 2.616 | 4.304 |
| Polarizability | |||
| axx | -146.64 | -143.74 | -255 |
| axy | 8.32 | 14.89 | 12.21 |
| ayy | -190.42 | -176.93 | 186.01 |
| axz | 2.71 | -0.98 | 3.92 |
| ayz | -2.21 | -2.83 | 0.38 |
| azz | -187.9 | -196.14 | -193.17 |
| (a.u) | 174.99 | 172.27 | 87.39 |
| atot x 10-24(esu) | 25.93 | 25.53 | 12.95 |
| Hyperpolarizabilities | |||
| ßxxx | 148.08 | -408.67 | 945.13 |
| ßxxy | 37.38 | -254.7 | -51.01 |
| ßxyy | -18.1 | -40.56 | -15.98 |
| ßyyy | 115.28 | 93.71 | 146.66 |
| ßxxz | 47.71 | -18.1 | -32.61 |
| ßxyz | -8.28 | -20.48 | -8.82 |
| ßyyz | 19.04 | 21.82 | -0.73 |
| ßxzz | -54.4 | 8.64 | 17.75 |
| ßyzz | -19.36 | 30.19 | 13.33 |
| ßzzz | 0.3 | -40.56 | 3.31 |
| ßtot (a.u) | 167.26 | 89.63 | 953.63 |
| ßtot x 10-30(esu) | 1.445 | 3.983 | 8.238 |
Table 3: Dipole moments and polarizabilities of compounds 1-3.
The dipole moments clearly indicate that all the compounds (1-3) are mainly attributed to an overall imbalance in charge. All the compounds have significant NLO property when compared with standard urea and compound 3 is higher NLO activity than others. The observed βo values are several times greater than standard urea which is a typical NLO material.
Frontier molecular orbital energies
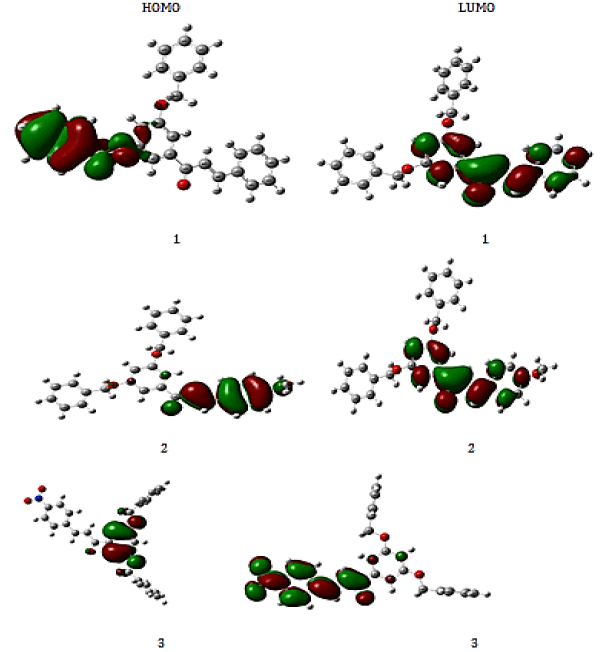
Molecular orbital energies are an effective tool that plays an important role in both electrical and optical properties of quantum chemistry. The two significant orbitals called HOMO and LUMO define the conjugated molecules. These are referred to as FMOs and are found at the outermost boundaries of electron molecules. The HOMO-LUMO energy was used to find out the energetic activity of the molecule using the B3LYP/6-31G (d,p) package. For compounds 1 and 2, the HOMO is located over phenyl ring of ketone moiety and it is widespread throughout the chalcone moiety for 2. LUMO is distributed over α,β-unsaturated ketone and phenyl ring of aldehyde taken. The diagram and energies of HOMO’s and LUMO’s are given in Figure 4 and Table 4 with the energy gap level.
| Parameters | Calculated values | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| EHomo(a u) | -6.436 | -6.435 | -6.234 |
| ELumo(a.u) | -2.534 | -2.272 | -3.306 |
| Energy gap(a.u) | 3.902 | 4.163 | 2.928 |
| Ionization energy(I) | 6.436 | 6.435 | 6.234 |
| Electron affinity(A) | 2.534 | 2.272 | 3.306 |
| Global hardness(η) | 1.951 | 2.081 | 1.464 |
| Chemical potential(µ) | -4.485 | -4.353 | -4.77 |
| Electrophilicity index(ω) | 5.154 | 4.552 | 7.772 |
| Chemical softness(s) | 0.513 | 0.4805 | 0.68315 |
Table 4: FMO energies of compounds 1-3.
The energy gap between HOMO and LUMO is an important factor for studying their rigidity and reactivity. The compound 3 has lowest energy gap and it is highly reactive than others. From the orbital energies, other parameters like ionisation energy, hardness, electrophilicity index, chemical potentials and softness are calculated and displayed in same Table 4. The hardness of the compound 2 is higher and is chemically less reactive than others. This is attributed to the presence of electron donating methoxy group in molecule 2. The electrophilicity index of molecule 2 is higher and is better bioactivity than others.
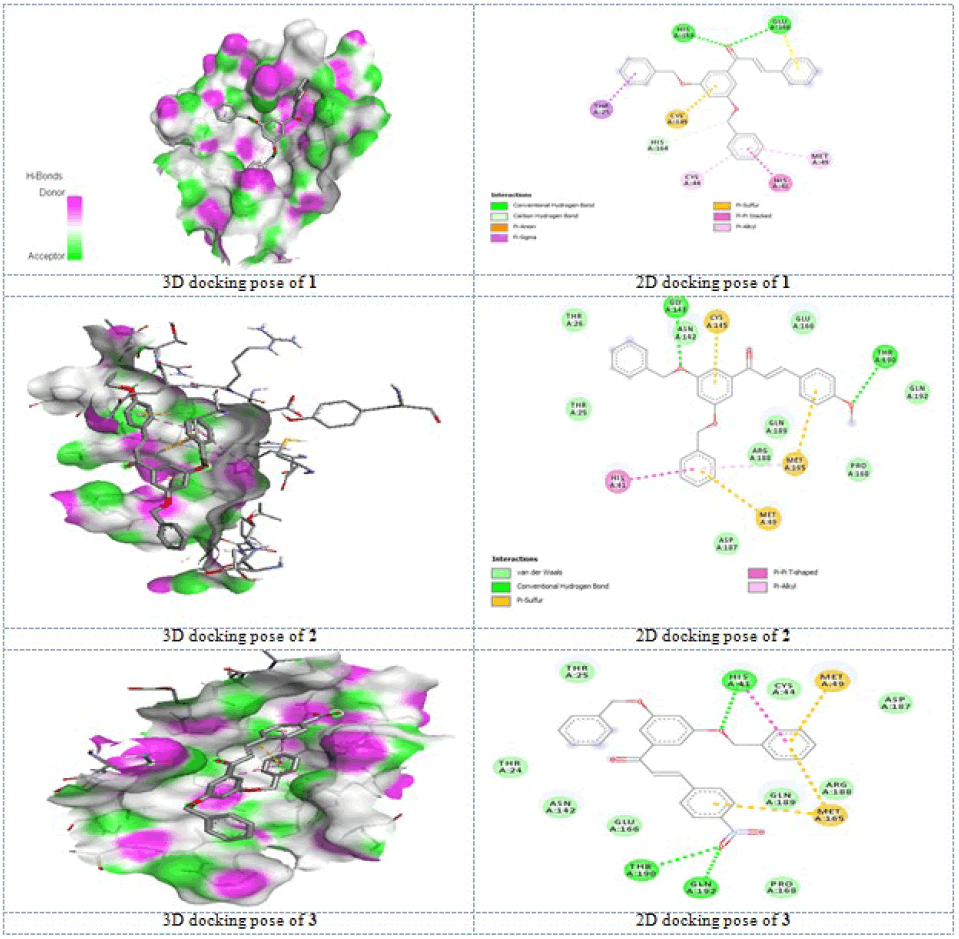
Molecular docking study: Now-a-days, the drug design for the molecular docking study plays a vital role. For the purpose of docking, many more number of docking programs is available. Generally, these programs examine the interactions and movements during the binding of ligand with protein. For the study of binding interactions, we need the three dimensional structure of ligands and proteins. In the present study, the selected protein has been downloaded as 3D structure from RCSB (Protein Data Bank) according to the determined activities and the ligands are prepared and converted to pdb file. AutoDock Vina program has been used for docking study of present investigation.
In the downloaded pdb structure of the protein, the water molecules and ligand are removed then polar hydrogen atoms were added. The optimized geometries of molecules (1-3) were used for PDB format as ligand. In this step, all preparations for both ligands and targets were made by with Discover Studio Visualizer 17.0 software.
The docking studies as described in literature, with the help of AutoDock Vina program, the molecular docking result outputs computed for 9 different conformational poses of the each ligand compounds (1-3) docked into the target receptor macromolecules (6w63) are listed in Table 5. For molecular docking analysis, the starting ground state molecular conformational forms of the ligand compounds (1-3) were obtained by using DFT-B3LYP/6-311++G(d,p) computational level of theoretical model. The comments on the interaction results were performed for the best conformational poses with the lowest interacting energies obtained of the ligand compounds docked into the target macromolecules 6w63. Additionally, in these tables inhibition constants and the number of hydrogen bonding were given in the bottom lines.
According to the affinity binding energies, the best bindings were determined for compound 1 and compound 3 with same -8.7 kcal/mol energy, and two hydrogen bonding for compound 1 and three hydrogen bonding for 3 as shown in Table 5.
| Compounds | 1 | 2 | 3 |
|---|---|---|---|
| Binding energy | -8.7 | -8.6 | -8.7 |
| Hydrogen bond | 2 | 2 | 3 |
| Electrostatic interaction | 1 | - | - |
| Other interactions (Hydrophobic/pi-alkyl/pi-anion/pi-cation) | 5 | 6 | 5 |
| Amino acid residue involved interaction | HIS163, GLU166, HIS164, THR25, CYS145, HIS41, CYS44 and MET49 | GLY143, THR190, MET49, CYC145, MET165, HIS41 | HIS41, THR190, GLN192, MET49 and MET165 |
| Hydrogen Bond Distance (A°) | 2.48, 2.98, 3.57 | 2.743. 2.744 | 2.74, 2.58, 2.34 |
Table 5: Docking parameters of compounds 1-3 with Sars-Cov-19 protease.
The results implied that the carbonyl group oxygen strongly binded with HIS 163 and GLU 166 residues for compound 1. The compound 2 has binding energy of -8.6 kcal/mol with protein residue including two hydrogen bonding interactions. The other interactions like pi-alkyl, pi-sulphur, pi-anion, pi-cation, etc., interactions are seen in all compounds (1-3). The compound 3 has significantly strong affinity than 1 with the low hydrogen bonding distance. All the active groups are strongly involved in hydrogen bonding interactions with protease chosen. The pi-electrons of the phenyl groups, nitro group, oxygen atom of carbonyl group, methylene oxygen atoms and methoxy oxygen atom are involved in interactions with protease. On comparing these three chacolnes (1-3), the compound 3 has potentially active than other two chalcones (1-2). The interactions obtained from molecular docking results as 3D (a) and 2D (b) forms are shown in Figure 5.
Although we cannot say the statement precisely because we do not have experimental data, these results concluded that the compounds are potent inhibitor?
ADMET evaluation: In this section, the pharmacokinetic parameters of compounds (1-3) were obtained and tabulated as in Table 6 in accordance with the criteria defined above. These parameters were obtained with the help of Pre-ADME web page (http://www.swissadme.ch/) and molinspiron tool. According to the obtained results, the synthesized chalcone compounds (1-3) meets these criteria in other words as can be seen the theoretical ADME results of the synthesized are in agreement with the five rule of Lipinski.
All the molecules studied showed a number of hydrogen bond acceptors and hydrogen bond donors less than ten and five, respectively. Also, the number of rotatable bonds in each molecule was less than or equal to 10. Topological Polar Surface Area (TPSA) is a very useful descriptor for prediction of transport of drug molecule across the biological barriers and was observed less than 130 Å2 in the range of 80-96Å2 (% ABS). The partition coefficient between n-octanol and water (LogPo/w) is the classical descriptor for hydrophobicity. All the compounds (1-3) studied were found to have Log P values nearly 5.08 to 5.81 indicating moderate permeability across the cell membrane. These parameters exhibited that the studied compounds obeyed Lipinski's rule of five with one violation and thus could be good orally active agents (Table 6).
| Pre-ADMET | Compounds | Drug likeness | Compounds | ||||
|---|---|---|---|---|---|---|---|
| Property | 1 | 2 | 3 | Property | 1 | 2 | 3 |
| BBB Cbrain/Cblood | 0.67 | 0.35 | 0.41 | CLogP | 5.76 | 5.81 | 5.08 |
| In vitro Caco-2 (nm/sec) | 26.22 | 55.66 | 27.74 | M.Wt | 420.51 | 450.53 | 465.5 |
| CYP2C19 inhibitor | Yes | Yes | Yes | HBA | 3 | 4 | 5 |
| CYP2D6 inhibitor | No | No | No | HBD | 0 | 0 | 0 |
| Volume | 396.24 | 421.79 | 419.58 | MDDR like Rule | Drug-like | Drug-like | Drug-like |
| TPSA | 35.54 | 44.77 | 81.36 | Lipinski violations | 1 | 1 | 0 |
| %ABS | 96.74 | 93.55 | 80.93 | Synthetic Accessibility | 3.51 | 3.66 | 3.59 |
| HIA (%) | 99.03 | 98.14 | 97.65 | WDI like Rule | 90% | 90% | 90% |
| MDCK (nm/sec) | 9.75 | 0.07 | 0.06 | Toxicity | Compounds | ||
| iPPB | 100 | 100 | 100 | Property | 1 | 2 | 3 |
| C LogP | 5.76 | 5.81 | 5.08 | hERG inhibition | Medium risk | Low risk | Low risk |
| Log S | -6.58 | -6.65 | -6.64 | Toxic Carcino Rat | Negative | Negative | Negative |
| PAINS/alerts | 0 | 0 | 0 | ||||
| Note: HBA hydrogen bond acceptor, ≤ 10; HBD hydrogen bond donor, ≤ 5; n-rotb no. of rotatable bonds, ≤ 10; TPSA topological polar surface area, ≤ 130 Å2; Log Po/w octanol/water partition coefficient, -0.7 to +5.0; Log S, aqueous solubility scale: Insoluble <−10 |
|||||||
Table 6: ADMET and Drug likeness properties of compounds 1-3
The solubility of a molecule is a significant property influencing absorption. Also, a water-soluble compound facilitates the ease of formulation and oral administration to deliver a sufficient quantity of active ingredient. The computational data revealed that none of the compounds crosses the blood-brain barrier and hence less possibility to produce neurotoxicity. CYP2D6 belongs to the superfamily of cytochrome P450 (CYP450) which is the most crucial isoenzyme that is responsible for metabolizing most of the drugs in the liver. Inhibition of these enzymes causes drug-drug interaction leading to toxic effects due to accumulation and reduced elimination of drugs or its metabolites. The results revealed that none of the compounds is inhibiting this essential enzyme.
Diabetic patients are at higher risk of developing cardiovascular disease as it is a common diabetes associated complication. The inhibition of human Ether-a-go-go-Related Gene (hERG) causes prolongation of QT interval leading to fatal ventricular tachyarrhythmia. Compounds 2 and 3 were found to be at low-risk of hERG inhibition whereas 1 at moderate risk. Carcinogenicity is a toxicity that causes cancer in the body. The results specify that all the compounds (1-3) were non-carcinogenic thus stating the safety of compounds in the thriving drug development. Human Intestinal Absorption (HIA) data are the sum of bioavailability and absorption estimated from the ratio of excretion or cumulative excretion in urine, bile, and feces. The compounds are considered as well absorbed if the absorption is in the range of 70-100%. All the compounds evaluated were found to exhibit High Intestinal Absorption (HIA) in the range of 97.65 to 99.03 and excellent Plasma Protein Binding (PPB) nature (100%). Then the MDR like rule of these compounds reveals that all has drug-like property (Supplementary figures).
Conclusion
In the present research, three substituted chalcones 1-3 have been synthesized by ultrasonic energy utilization method results better yields with low time. Componds 1-3 are characterized by UV, IR and NMR spectra. From these spectra, the position and nature functionalities, different carbons and protons are identified and confirmed their formation. The coupling constant values indicated the Trans isomer of chalcones is formed. The DFT calculation of dihedral angles also supported these isomer conformations. DFT analyses of 1-3 are carried out and the bond parameters are calculated and agreed with literature. The compound 2 has shown good NLO property than others two compounds. The FMOs are calculated with their energies and showed that all the compounds possessed the charge transfer tendency. Further the molecular docking is performed and resulted that all the compounds have shown better binding with protease selected. The ADMET properties are noted and discussed. The compound 3 has obeyed the entire Lipinkis rule and all are non-toxic. They showed drug like property.
Acknowledgment
The author thank to the Department of Chemistry, Govt. Arts College, Chidambaram for providing research facilities for doing this work.
References
- Sharma B. Orient J Chem. 2008; 24(1): p. 289.
- Michelini LJ, Castro MR, Custodio JM, et al. J Mol Structure. 2018; 1168: p. 309-315.
- Mandge S, Singh HP, Gupta SD, et al. Trends Appl Sci Res. 2007; 2(1): p. 52-56.
- Bonakdar AP, Vafaei F, Farokhpour M, et al. Iran J Pharm Res. 2017; 16(2): p. 565
[Google Scholar] [PubMed]
- Osorio TM, Delle Monache F, Chiaradia LD, et al. Bioorganic Med Chem Let. 2012; 22(1):p. 225-30.
[Crossref] [Google Scholar] [PubMed]
- Kuete V, Sandjo LP. Chin J Integr Med. 2012; 18(7): p. 543-547.
[Crossref] [Google Scholar] [PubMed]
- Gomez-Rivera A, Aguilar-Mariscal H, Romero-Ceronio N, et al. Bioorganic Med Chem Lett. 2013; 23(20): p. 5519-5522.
[Crossref] [Google Scholar] [PubMed]
- Wang YJ, Zhou DG, He FC, et al. Chinese Chem Lett. 2018; 29(1): p. 127-130.
- Chavan BB, Gadekar AS, Mehta PP, et al. Asian J Biomed Pharm Sci. 2016; 6(56): p. 01.
- Larsen M, Kromann H, Kharazmi A, et al. Med Chem Lett. 2005; 15(21): p. 4858-4861.
[Crossref] [Google Scholar] [PubMed]
- Kumar A, Kumar R, Gupta A, et al. J Mol Struct. 2017; 1150: p. 166-178.
- Zaini MF, Arshad S, Thanigaimani K, et al. J Mol Struct. 2019; 1195: p. 606-619.
- Kumar CC, Govindarasu K, Fun HK, et al. J Mol Struct. 2015; 1085: p.63-77.
- Yalcin G, Burmaoglu S, Yildiz I, et al. J Mol Struct. 2018; 1164: p. 50-56.
- Hussein RK, Elkhair HM. J Mol Struct. 2021; 1231: p. 129979.
[Crossref] [Google Scholar] [PubMed]
- Nayak PS, Narayana B, Sarojini BK, et al. Med Chem Res. 2015; 24: p. 4191-206.
- Khan SA, Zia K, Ashraf S, et al. J Biomol Struct Dyn. 2021; 39(7): p. 2607-2616.
[Crossref] [Google Scholar] [PubMed]
- Park JY, Ko JA, Kim DW, et al. J Enz Inhib Med Chem. 2016; 31(1): p. 23-30.