Short Communication - Der Pharma Chemica ( 2018) Volume 10, Issue 7
Synthetic and Pharmacophoric Utility of Quinazoline Motif in Drug Design
Prakash Prajapat1*, Ganpat L Talesara2 and Shikha Agarwal2
1Department of Chemistry, Ganpat University, Mehsana-384012, Gujarat, India
2Department of Chemistry, MLS University, Udaipur-313001, Rajasthan, India
- *Corresponding Author:
- Prakash Prajapat
Department of Chemistry
Ganpat University
Mehsana-384012, Gujarat, India
Abstract
Heterocyclic motifs are pervasive in several areas of life sciences. These motifs are performing numerous important functions in drug, agriculture and technology. Nitrogen-containing heterocycle quinazoline motif is the subject of continuous interest in organic synthesis owing to the fact that they occur ubiquitously in biological active multipurpose oriented functional material as well as highly potent pharmaceuticals and agrochemicals. Quinazoline has been a popular heterocycle in pharmaceutical and medicinal chemistry allowing the construction of structurally different molecules with diverse pharmacophoric properties viz. antimicrobial, antimalarial, antiparasitic, anticonvulsant, anticancer, anti-inflammatory antiviral, analgesic, etc. This short communication is an effort to compile the literature of several synthetic approaches and medicinal properties of quinazoline analogues. This review helps in the further development of newer quinazolines for future drug discovery.
Keywords
Drug discovery, biophore, Quinazoline, Synthesis, Medicinal importance.
Introduction
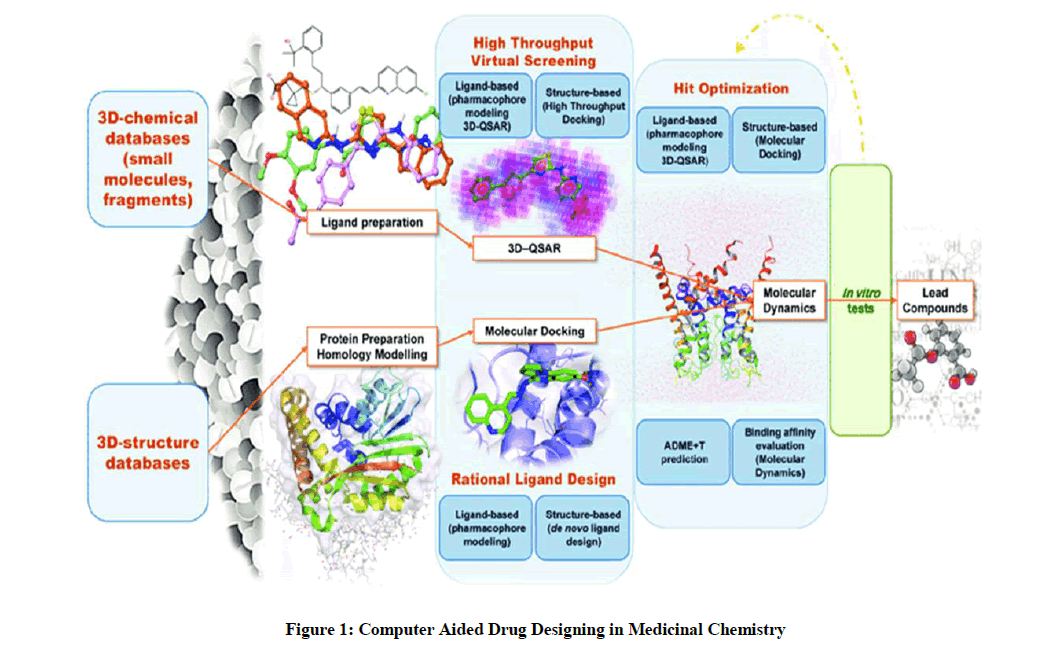
Medicinal chemistry has come of age in the last three decades. Before that, advances were often made as a result of trial and error, intuition or pure luck. Large number of analogs was synthesized based on the structure of a known active compound, defined as the Lead compound, but little was known about the detailed mechanism of drug action or the structure of targets with which they interacted. Advances in the biological sciences have how resulted in a much better understanding of drug targets and the mechanism of drug action. As a result, drug designing is as much target oriented as lead compound oriented. The medicinal chemistry covers three critical steps: A discovery step, consisting of (i) choice of therapeutic agents (ii) identification and production of new active substances interacting with selective targets. An optimization step which deals with improvement of lead structure on account of increasing potency, selectivity and decreasing toxicity. Its characteristics are the establishment and analysis of SAR, in an ideal context to enable the understanding of the molecular mode of action. A development step is the continuation of the improvement of pharmacokinetic properties and the fine tuning of chemical formulation of active substance is the main process of this step [1-7]. Similarly, computational techniques demonstrated to accelerate hit selection for a given drug target, and to significantly contribute to multiple stages of drug discovery (Figure 1). Computer Aided Drug Designing (CADD) techniques to assist organic and medicinal chemists in drug design, development, and hit-optimization steps during the drug discovery process.
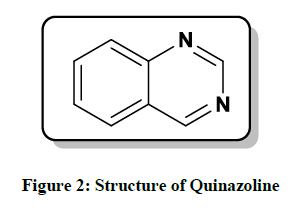
Quinazoline is an important pharmacophore in medicinal chemistry. It is formed by two fused six-member heterocyclic aromatic rings: benzene and pyrimidine (Figure 2). Quinazoline is a fused bicyclic medicinal active scaffold earlier known as benzo-1, 3-diazine. It was earliest prepared in 1903 by chemist Gabriel.
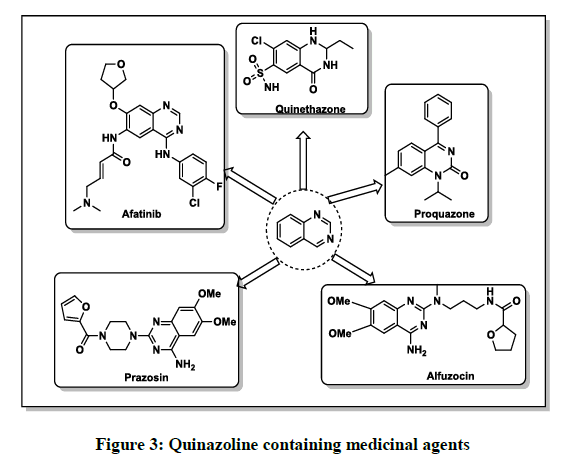
The beginning of the academic medicinal researches on the quinazoline moiety started with the discovery of febrifugine, a quinazolinone alkaloid, which has potent anti-malarial activity, from the Chinese plant aseru (Dichroa febrifuga Lour) [8]. Quinazoline derivatives have occupied a prominent place among various heterocyclic compounds by virtue of their diverse medicinal values. Its derivatives encompass a broad spectrum of pharmaceutical activity profile such as antitumor [9-13], anti-HIV [14-16], antimicrobial [17-19], anti-inflammatory [20-23], CNS activity [24-26], and cardiovascular activity [27-30]. Quinazoline containing drugs (Figure 3) like prazosin was an effective medicine as adrenergic blockers for the treatment of high blood pressure, panic disorder and anxiety, alfuzocin was used to treat solid tumor and other analogues of quinazoline have been used as active ingredients in drugs like afatinib as antifugal, quinethazone as anti-hypertensive and proquazone as non-steroidal anti-inflammatory agent.
Synthetic aspects
The synthetic chemistry of quinazoline motif was investigated by scientist Williamson in 1957 and then by Lindquist in 1959 and brought up to date by Armarego in 1963. Quinazolines can be obtained from a wide variety of starting materials and also largely distributed in natural sources [23]. For example by Cascade Imino-Diels-Alder reaction [31].
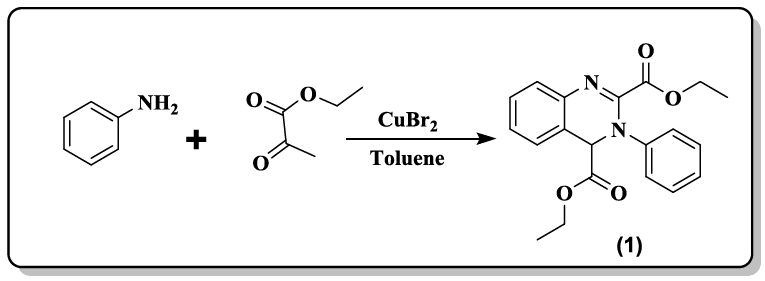
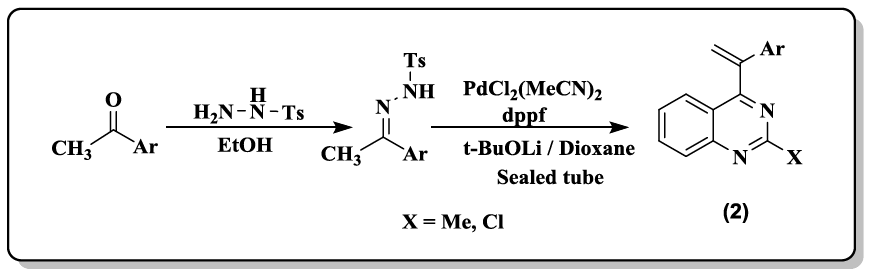
A series of isocombretaquinazolines (isoCoQ) (3.69) were prepared [32] by coupling N-toluenesulfonylhydrazones with 4-chloroquinazolines under palladium catalysis.
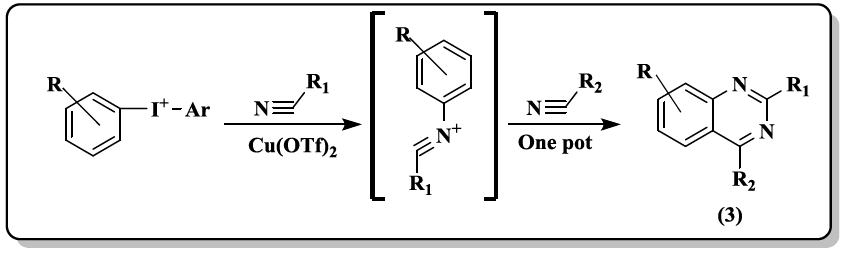
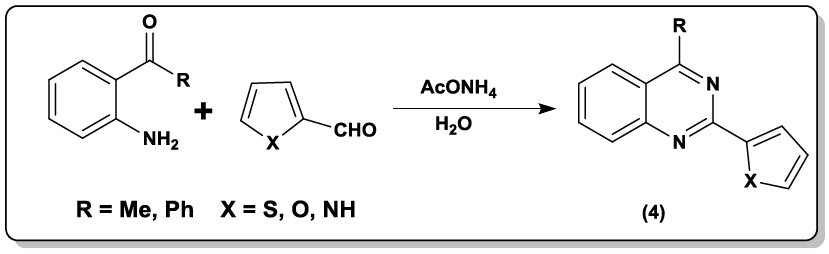
An efficient one-pot approach to multiple substituted quinazolines (3) with diaryliodoniumsalt, and two nitriles has been synthesized by Chen et al. [33]. Catalyst free reaction for quinazoline derivatives (4) was carried out [34] in aqueous medium between 2-aminoacetophenone, various aldehyde and AcONH4 as substrates.
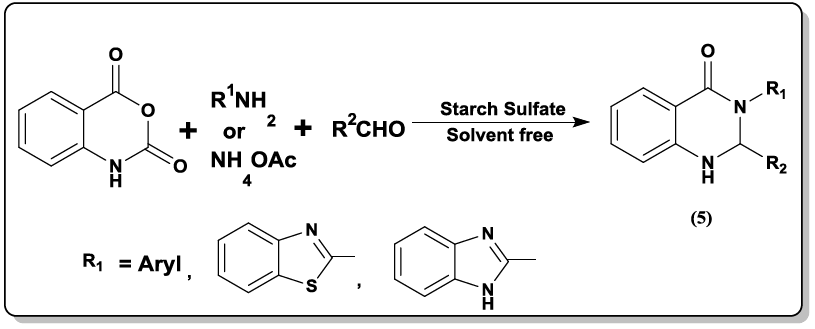
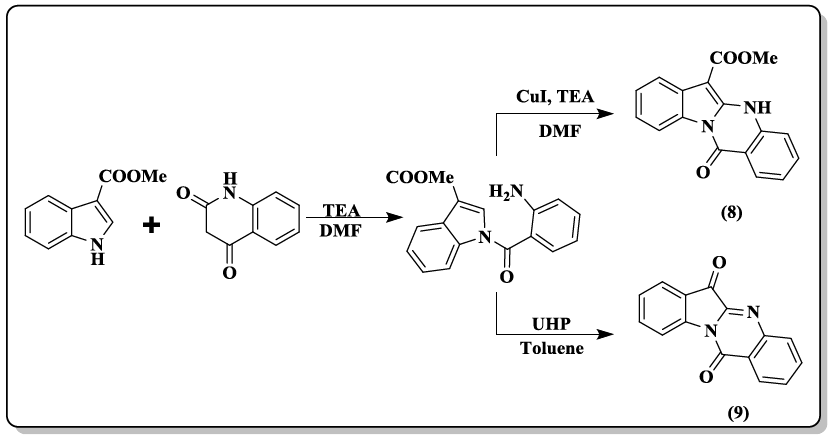
Synthesis of 2,3-dihydroquinazolin-4(1H)-one, (5) 2-substituted-1,2,3,4-tetrahydro-4-quinazolinones (6) using starch sulfate as a biodegradable solid acid catalyst was carried out [35] under thermal solvent-free conditions.
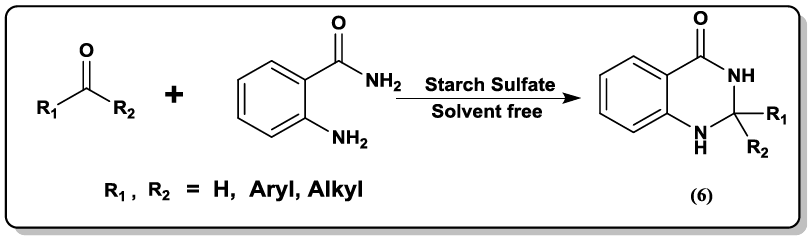
Synthesis of bis(quinazolinon-4(1H)-one) derivatives (7) by using one-pot pseudo five-component condensation of two molecules isatoic anhydrides, two molecules orthoesters, and diamines in high yields is described by Mohammadi et al. [36].
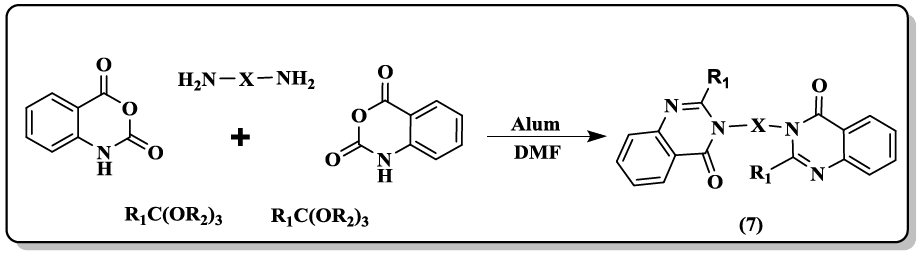
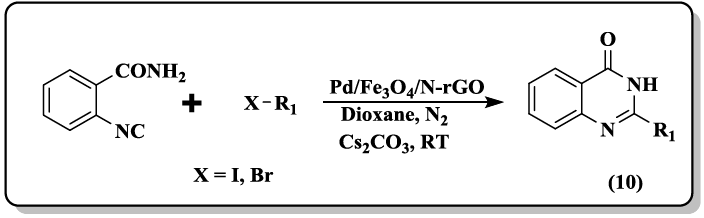
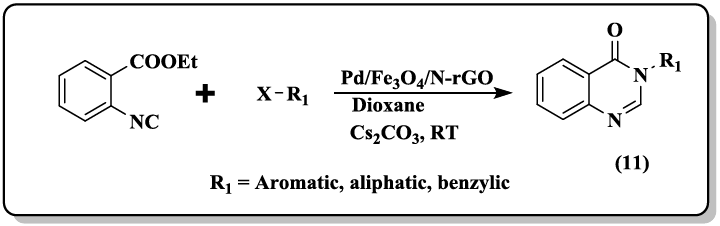
A one-pot protocol of phaitanthrin E (8, 9) starting from methyl indole-3-carboxylate and isatoic anhydride through intermolecular condensation/intramolecular aryl C-H amination cascade was developed [37]. Pd/Fe3O4 supported on N-doped reduced grapheme oxide (N-rGO) catalyst was reported [38] to carry out the synthesis of quinazolinones (10, 11) by isocyanide insertion reactions. The supported Pd/Fe3O4 nanoparticles easily recovered from the reaction mixture and reused several times without any loss in catalytic activity.
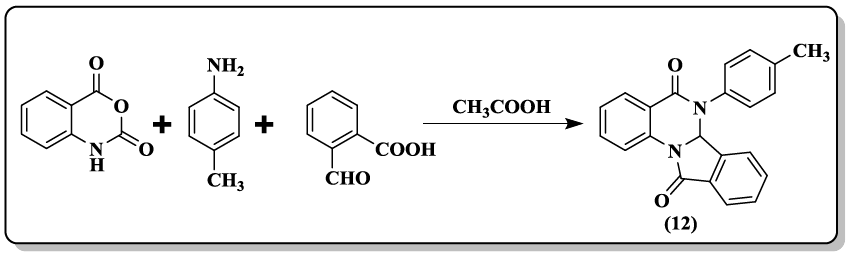
An efficient synthesis for the construction of 6, 6a-dihydroisoindolo[2,1-a]quinazoline 5,11-diones and 5-phenylisoindolo[2,1-a]quinazolin- 11(6aH)-one derivatives (12) in acetic acid under catalyst-free conditions have been performed by Sashidhara and co-workers [39].
Medicinal aspects
For the last few years, the quinazoline motif has drawn an immense attention owing to its diversified utility in the field of medicinal and pharmaceutical chemistry. Diverse structural modifications were carried out in quinazoline ring system. These structural diversities resulted in some fruitful biological activities of the molecules. Some of these important activities are mentioned here.
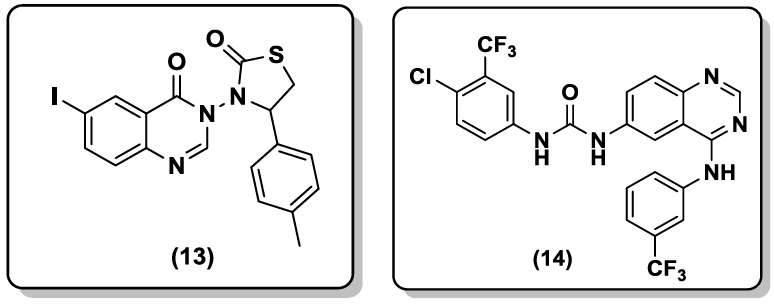
A series of 6-iodo-2-phenylquinazolin-4(3H)-one derivatives were synthesized by Adnan Kadi et al. [40]. The potential antibacterial effects of the prepared compounds were examined against Staphylococus aureus; Bacillus subtilis; Escherichia coli; Psudomonas eruginosa; Candida albicans strains. Compound (13) attained maximum activity within the series. Series 6-ureido-4-anilinoquinazolines derivatives were prepared by Madapa et al. [41]. These compounds were screened for their in vitro anti-malarial activity against chloroquine-sensitive P. falciparum. One of the compounds (14) had IC50 value of 2.27 mg/ml which was equipotent to the standard drug chloroquine used in the bioassay.
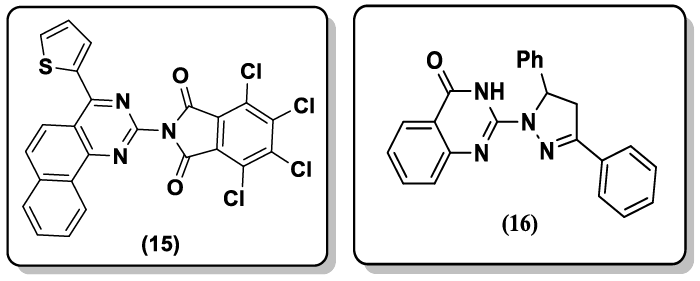
Some quinazoline and thioxopyrimidine derivatives (15) have been reported [42] by using 4-(thiophen-2-yl)-3, 4, 5, 6- tetrahydrobenzo[h]quinazoline-2(1H)-thione as synthon and evaluated their anti-HIV and cytotoxicity. Niraj and coworkers [43] have reported the synthesis of pyrazoline bearing 4(3H)-quinazolinone derivatives (16) and evaluated their analgesic and anti-inflammatory activities.
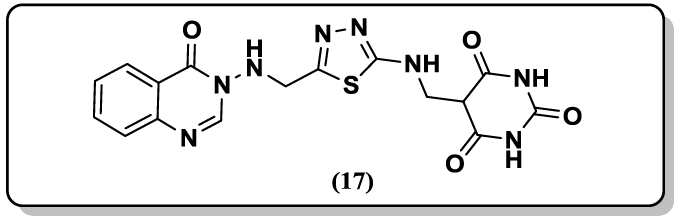
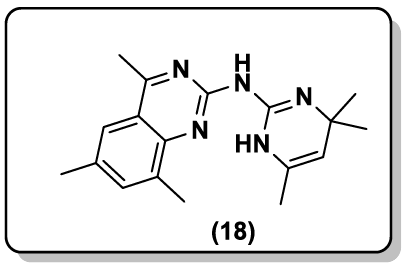
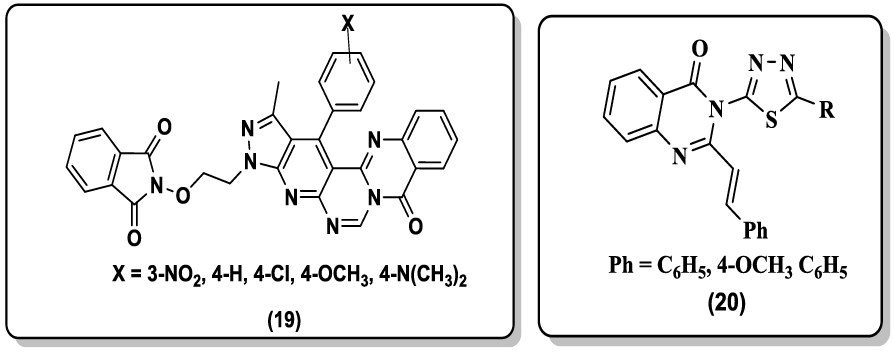
Archana et al. [44] synthesized some thiazolidinonyl quinazoline-4(3H)-ones derivative (17) and screened for their anticonvulsant activity against maximal electroshock (MES) induced convulsions in animal models. Matthew and coworkers [45] reported the synthesis of 2- guanidinoquinazolines and studied for their selective STAT3 pathway inhibitors, led to a more potent analog (18) that demonstrated improved anti-proliferative activity against a panel of HNSCC cell lines. A convenient synthesis of N-ethoxyphthalimido-3-methyl-4-substituted phenylpyrazolo[4',3':5,6] pyrido[2,3-d] pyrimido[6,1-b]quinazolin-10-one (19) via Niementowski reaction are reported by Hussain et al. [46].
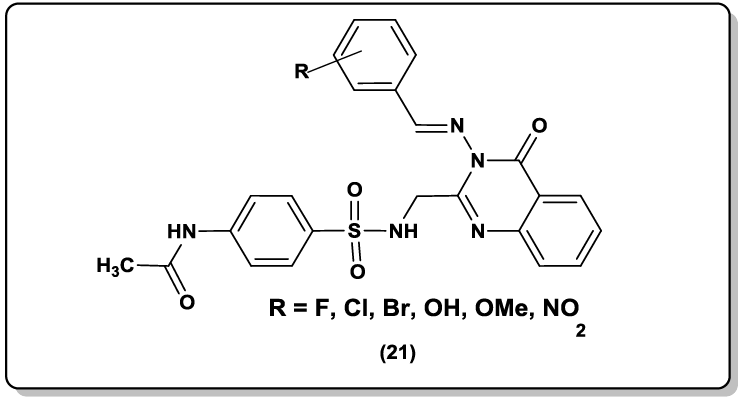
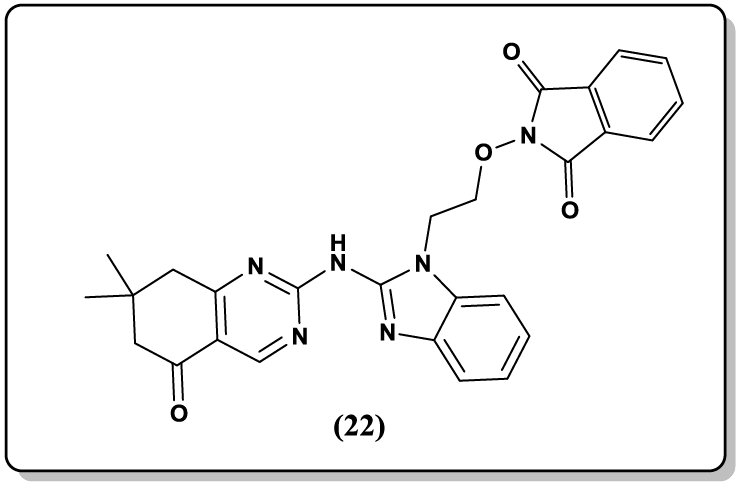
Similarly, Varsa Jatav et al. [47] synthesized 3- [5-substituted-1, 3, 4-thiadiazole-2-yl]-2- styryl quinazoline-4(3H)-ones (20) and their CNS depressant activity was screened with the help of forced swim pool method. Stereoselective 2,3-disubstituted quinazoline-4(3H)-one derivatives (21) derived from glycine were reported as a potent anti-malarial agents [48]. Prajapat et al. [49-51] reported the synthesis of ethoxyphthalimide linked benzimidazoloquinazolinone (22) and benzimidazolopyrimidopyrimidne derivatives as anti-inflammmatory agent and antimicrobial agents respectively.
Conclusion
The quinazoline ring is a significant pharmacophore in drug discovery. These derivatives are very useful for the development of drug leads and molecules of pharmaceutical and agrochemical interests. Owing to the great synthetic and medicinal importance of this heterocyclic core, synthesis of quinazolines has long been an area of intense growth, and still constitutes an active domain from academic and industrial points of view. The idea of this work is to collect the literature reported by researchers on quninazoline for its diverse pharmacophoric properties and also report present synthetic efforts made on this motif. Finally, the current review highlights the use of quinazoline motif as a template for of new development of synthetic methodology and newer therapeutic agents.
Acknowledgement
I thankful to chemistry staff members of the Mehesana Urban Institute of Sciences, Ganpat University, Gujarat for their kind support.
References
- P. Prajapat, H. Vaghani, S. Agarwal, G.L. Talesara, Ann. Med. Chem. Res., 2017,3(1), 1021.
- P. Prajapat, S. Agarwal, G.L. Talesara, J. Med. Org. Chem., 2017, 1(1), 1.
- P. Prajapat, Mod. Chem. Appl., 2017, 5, 123
- P. Prajapat, Mod. Appro. Drug. Des., 2018, 1(4), MADD.000517.
- P. Prajapat, Mod. Chem. Appl., 2018, 6, 124.
- P. Prajapat, Arc. Org. Inorg. Chem. Sci., 2018, 1(1).
- P. Prajapat, Curr. Trends. Med. Chem., 2018, 1(1), 1001.
- M. Fishman, P.A. Cruckshank, J. Med. Chem., 1970, 13, 155-156.
- A.M. Al-Obaid, S.G. Abdel-Hamide, H.A. El-kashef, A.A. Abdel-Aziz, A.S. El-Azab, H.A. Al-khamees, H.I. El-Subbagh, Eur. J. Med. Chem., 2009, 44, 2379-2391.
- F.A. Al-Omary, L.A. Abou-Zeid, M.N. Nagi, S. Habib, A.A. Abdel-Aziz, A.S. El-Azab, S.G. Abdel-Hamide, M.A. Al-Omar, A.M. Al-Obaid, H.I. El-Subbag, Bioorg. Med. Chem., 2010, 18, 2849-2863.
- A.S. El-Azab, M.A. Al-Omar, A.A. Abdel-Aziz, N.I. Abdel-Aziz, M.A. El-Sayed, A.M. Aleisa, A.M.M. Sayed, S.G. Abdel-Hamide, Eur. J. Med. Chem., 2010, 45, 4188-4198.
- G.G. Berest, O.Y. Voskoboynik, S.I. Kovalenko, O.M. Antypenko, I.S. Nosulenko, A.M. Katsev, O.S. Shan-drovskaya, Eur. J. Med. Chem.,2011, 46, 6066-6074.
- M.R. Yadav, F. Grande, B.S. Chouhan, P.P. Naik, R. Giridhar, A. Garofalo, N. Neamati, Eur. J. Med. Chem., 2012, 24, 231-243.
- N. Mulakayala, B. Kandagatle, R.K. Ismail, P. Rao, C. Mulakayala, C.S. Kumar, J. Igbal, S. Oruganti, Bioorg. Med. Chem., 2012, 22, 5063-5066.
- Z. Zeng, Q. He, Y. Liang, X. Feng, F. Chen, E.D. Clercq, J. Balzarini, C.P. Couque, Bioorg. Med. Chem., 2010, 18:5039-5047.
- Y.A. Mohamed, A. Amr, S.F. Mohamed, M.M. Abdalla, M.A. Al-Omar, S.H. Shafik, J. Chem. Sci., 2012, 124, 693-702.
- K. Vijayakumar, A.G. Ahamed, G. Thiruneelakandan, J. Applied. Chem., 2013, 5, 187-191.
- P. Sharma, A. Kumar, P. Kumar, J. Singh, M.P. Kaushik, Med. Chem. Res., 2011, 21, 1136-1148.
- R.V. Anand, B. Narasimhan, R.V.P. Chandran, K.N. Jayaveera, Med. Chem. Res., 2011, 21, 2831-2836.
- Ryu C, Kim YH, Im AA, Kim JY, Yoon JH, Kim A. Bioorg Med Chem Lett 22:500-503, 2012.
- M.R. Yadav, S.T. Shirude, A. Parmar, R. Balaraman, R. Giridhar, Chem. Heterocyclic Compds., 2006, 42, 1038-1045.
- P.M. Chandria, T. Yakaiah, A.R.R. Rao, B. Narsaiah, N.C. Reddy, V. Sridhar, J.V. Rao, Eur. J. Med. Chem., 2008, 43, 846-852.
- A.M. Alafeefy, A.A. Kadi, A.S. El-Azab, S.G. Abdel-Hamide, M.H. Daba, Archiv der Pharmazie., 2008, 341, 377-385.
- E. Manivannan, S.C. Chaturvedi, Bioorg. Med. Chem., 2011, 19, 4520-4588.
- J.J. Letourneau, C.M. Riviello, H. Li, A.G. Cole, K. Ho, H.A. Zonetakos, H. Desai, J. Zhao, D.S. Auld, S.E. Napier, F.J. Thomson, K.A. Goan, J.R. Morphy, M.H.J. Ohlmeyer, M.L. Webb, Bioorg. Med. Chem. Lett., 2010, 20, 5394-5397.
- A. Gupta, S.K. Kashaw, N. Jain, H. Jajak, A. Soni, J.P. Stable, Med. Chem. Res., 2011, 20, 1638-1942.
- S. Kumar, H. Kaur, A. Kumar, Arab J. Chem.,2012,5, 475-484.
- S.S. Laddha, S.G. Wadodkav, S.K. Meghal, Med. Chem. Res., 2009, 18, 268-276.
- S. Abou, K. Abouzid, E. Abou, Eur. J. Med. Chem., 2011, 46, 647-658.
- I. Khan, A. Ibrar, W. Ahmed, A. Saeed, Eur. J. Med. Chem., 2015, 90, 124-169.
- X. Chen, H. Wei, L. Yin, X. Li, Chin. Chem. Lett., 2010, 21, 782-786.
- M.A. Soussi, O. Provot, G. Bernadat, J. Bignon, D. Desravines, J. Dubois, J.D. Brion, S. Messaoudi, M. Alami, Chem Med. Chem., 2015, 10,31392-1402.
- X. Su, C. Chen, Y. Wang, J. Chen, Z. Lou, M. Li, Chem. Commun., 2013, 49, 6752-6754.
- M. Bandaru, N.M. Sabbavarapu, A.K. Akula, N.Y.V. Durga, Eur. J. Chem., 2012, 3, 252-257.
- H.R. Shaterian, F. Rigi, Res. Chem. Intermed., 2015, 41(2), 721-738.
- A.A. Mohammadi, S. Taheri, S. Askari, J. Heterocyclic Chem., 2017, 54(1), 484-488.
- T. Itoh, T. Abe, T. Choshi, T. Nishiyama, M. Ishikura, Heterocycles., 2016, 92, 132-1136.
- K. Singh, A.K. Singh, D. Singh, R. Singh, S. Sharma, Catal. Sci. Technol.,2016, 6, 3723-3726.
- K.V. Sashidhara, G.R. Palnati, R.P. Dodda, S.R. Avula, P. Swami, Synlett., 2013, 24, 105-113.
- A.K. Adnan, J. Saudi Chem. Soc., 2010, 15, 95-100.
- S. Madapa, Z. Tusi, A. Mishra, K. Srivastava, S.K. Pandey, R. Tripathi, S.K. Puri, S. Batra, Bioorg. Med. Chem., 2009, 17, 222-234.
- F.Y. Abdel, A.M. Amr, S.F. Mohamed, M.M. Abdalla, M.A. Al-Omar, S.H. Shfik, J. Chem. Sci., 2012, 124(3), 693-702.
- N.K. Sinha, A. Asnani, B. Dravyakar, Asian J. Pharm. Clin. Res., 2013, 6(3), 200-204.
- R. Chioua, F. Benabdelouahab, M. Chioua, A. Martinez, F. Herrera, Molecules., 2002,7, 507-510.
- G.L. Matthew, P.L. Dimas, Z. Feng, S. Malabika, R.G. Jennifer, C. Daniel, H. Yun, A.J. Paul, S.L. John, O.R. Lynn, W. Peter, M.H. Donna, Bioorg. Med. Chemi. Lett., 2014,24, 5081-5085.
- N. Hussain, R.R. Dangi, K.D. Sai, G.L. Talesara, Iran. J. Org. Chem., 2010, 2(1), 328-337.
- J. Varsa, M. Pradeep, K. Sushil, Stables. Eur. J. Med. Chem.,2008, 43, 135-141.
- T.S. Patel, S.F. Vanparia, S.A. Gandhi, U.H. Patel, R.B. Dixit, C.J. Chudasama, B.C. Dixit, New J. Chem., 2015, 39, 8638-8649.
- P. Prajapat, G.L. Talesara, J. Heterocyclic Chem., 2016, 53, 1603-1610.
- P. Prajapat, M. Kumawat, B. Kherodiya, G.L. Talesara, Chem. Inform., 2016, 47(52), 343.
- P. Prajapat, M. Kumawat, B. Kherodiya, G.L. Talesara, J. Indian Chem. Soc., 2016, 93, 539-544.