Research Article - Der Pharma Chemica ( 2021) Volume 13, Issue 2
TBAI/CS2 Mediated Cleaner, Greener Synthesis and Anticancer Activity of 3-Substituted- (4-Oxo-2-Thioxo-Thiazolidin-5-Ylidene)-Methyl Ethanoates
Nitin Srivastava1, Ram Kishore2, Namrata Kalia2, Jatinder Singh2 and Devdutt Chaturvedi3*2Department of Molecular Biology & Biochemistry, Guru Nanak Dev University, Amritsar, India
3Department of Chemistry, School of Physical Sciences, Mahatma Gandhi Central University, Motihari (East Champaran), Bihar-845401, India
Devdutt Chaturvedi, Department of Chemistry, School of Physical Sciences, Mahatma Gandhi Central University, Motihari (East Champaran), Bihar-845401, India, Email: devduttchaturvedi@gmail.com
Received: 28-Sep-2020 Accepted Date: Feb 20, 2021 ; Published: 26-Feb-2021
Abstract
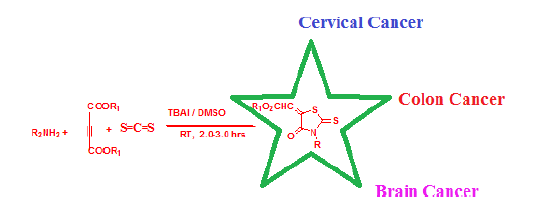
Owing to the key importance of 3- substituted- (4-oxo-2-thioxo-thiazolidin-5- ylidene)-methyl ethanoates in various biological activities, a new greener, cleaner and cheaper method is being put forward here employing primary amines, but-2-yne dioic acid dimethyl ester, CS2 and phase transfer catalyst tetrabutyl ammonium iodide (TBAI) in dimethyl sulphoxide. The synthesized compounds have been found to possess good in-vitro anti-cancer activities against cervical cancer cells, colon cancer cells and brain cancer cells.
Keywords
Carbon disulfide, Primary amines, Acetylenic ester, Tetrabutyl ammonium iodide (TBAI), Anti-cancer activities
Introduction
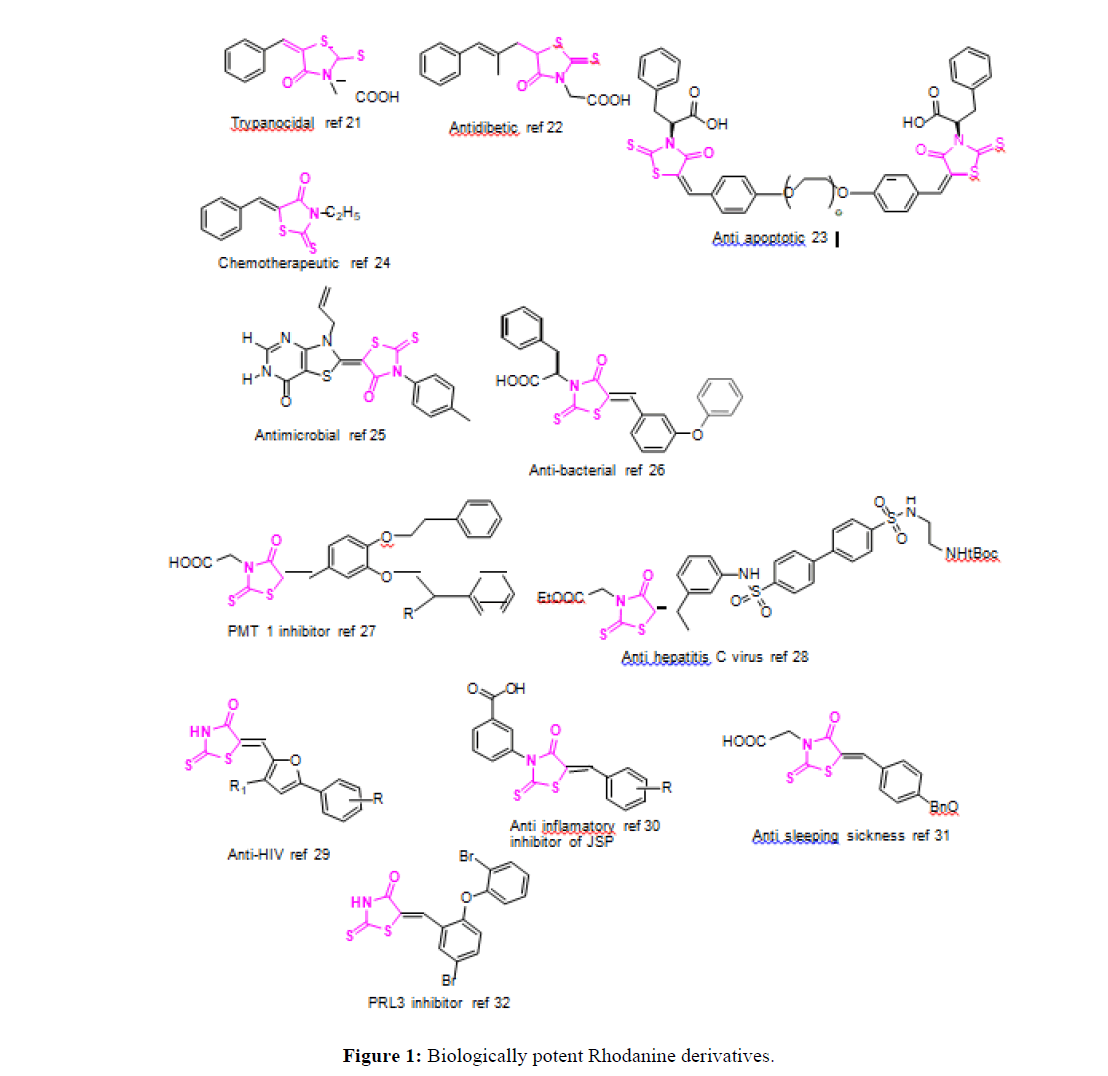
Biological activities of key importance are being shown by Rhodanine-based compounds. The rhodanine derivatives have been proved to possess fungicidal activities [1-5]. Such compounds have been found to bind with proteins which are set as target in many types of illness. Such compounds have positively inhibited proteins found in hepatitis C virus. These compounds have also deactivated viral non structural protein (p-70) [6], dolichyl-phosphate-mannose-protein mannosyltransferase [7], Phosphatase of regenerating liver, JSP-1 phosphatase [7,8] and beta lactamase [9].
Such compounds have also been found to be hypoglycemic [10], a cure for diabetes mellitus by decreasing the blood glucose concentration in the blood and found to possess antiviral [11] behavior. Rhodanine based compounds have been used for curing sleeping sickness [12], apoptosis [13,14]. They have also been found to possess antimicrobial activities [15,16] and have been proved to be anti HIV [17]. The earlier methods of the synthesis of such important class of compounds involved many steps [18] which used to make to synthesis process tedious (Figure 1).
Arylidene rhodanine were synthesized by Taran and coworker [19] by reaction of dithiocarbamates and arylpropiolates employing catalyst Bu3P in isopropyl alcohol. The derivatives of amine on reaction with dialkyl acetylendicarboxylate and isocyante gave maleimide derivatives as proposed by Abdolali Alizadeh and coworker [20,21]. Such important class of compounds has proved to be potential drugs for many illnesses, so many scientists have put forward their synthesis with different procedures [22-27]. These synthetic procedures are not free from the use of harsh chemicals, extreme conditions, tedious work-up and lesser yields. So there is a scope of more cleaner, greener, easier synthesis of such important class of compound. We have already reported the formation of these class of compound with the help of Triton-B [28]. We are working upon the improvement of the process in the synthesis of such important class of compounds and in this journey we found that TBAI is a little bit better in performance than Triton-B.
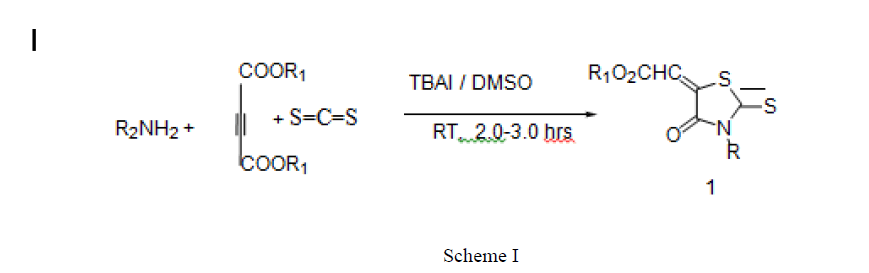
In this paper we are reporting the new, efficient, greener, cleaner and cheaper method for the synthesizing 3- substituted- (4-oxo-2-thioxo-thiazolidin-5-ylidene)-acetic acid methyl ester by single flask chemical interaction among but-2-ynedioic acid dimethyl ester, carbon disulphide and primary amines in DMSO and in presence catalyst Tetra butyl ammonium Iodide (TBAI). The reported process took place at room temperature and made the use of easily available cheaper chemical.
Experimental
General Procedure
100 mL round bottom flask was charged with Amine (1mmol), 2.0 mL Tetrabutyl ammonium iodide (TBAI) and stirred. This mixture was slowly added with 2 mmols of CS2. Then but-2-ynedioic acid dimethyl ester (1mmol) was added slowly and mixed thoroughly for 2.5 hours. The reaction proceeding was observed on thin layer chromatography (TLC) and new compound formation and end of the reaction was verified. When the reaction got over it was added with distilled water and organic compound was extracted with ethyl acetate. The compounds obtained were assigned structures on the basis of elemental analysis, IR, NMR and HRMS and were collated with the compounds available in literature [28].
Data of synthesized compounds
[4-Oxo-2-thioxo-3-(3-trifluoromethyl-benzyl)-thiazolidin-5-ylidene]-acetic acid methyl ester (1a)
Yield (0.27g, 80%), yellow powder, melts at 72-74 0C; IR(cm-1): 1726 (O=C), 1689 (C=C) and 1194, (S=C) ; 1H NMR δ= 7.4-7.7 (m, aromatic 4H), δ= 6.6-6.9 (s, vinylic H),δ=5.3(s,2H,N-CH2),3.8(s,3HofCH3O);13CNMR:δ=198,168,142.3,136,130,128,123,119.1132,50.5; % element analysis of C14H10NO3S2F3, Calculated: C, 46.53%;H,2.79%;N,3.88;O,13.28%,S,17.75%,F,15.77%;observed:C,46.48;H,2.74;N,3.82;O,13.14; S, 17.62; F, 15.69; m/e = 361.00 (found), 360.98 (calc.).
2-[4-oxo-2-thioxo-3-(p-trifluoro methyl benzyl)-thiazolidine-5-yliden]-acetic acid methyl ester (1b)
Yield (0.27 g, 79%), yellow powder melts at 78-80 0C;IR (cm-1) 1702(O=C),1618 (C=C),1323and1212(S=C);1HNMR:δ=7.3-7.7(aromatic4H),6.80(s,vinylicH),5.30(s,2HN-CH2),3.87(s,3H,CH3O);13CNMR:δ=198,168,142.3,136,130,128,123,119.1, 132,50.5;%elemental analysis for C14H10NO3S2F3,Calculated:C,46.53;H,2.79;N,3.88;O, 13.28, S, 17.75, F, 15.77; Observed: C, 46.38; H, 2.71; N, 3.84; O, 13.17; S, 17.66;F,15.65; m/e = 360.79 (found), 360.98 (calc.).
2-[4-oxo-2-thioxo-3-(propyl phenyl methyl)- thiazolidine-5-yliden]-acetic acid methyl ester (1c)
Yield (0.268 g, 82%), yellow powder melts at 88-90 0C; IR (cm-1) 1702(O=C), 1618 (C=C), 1212, 1323 (S=C); 1H NMR: δ= 7.8 (m, aromatic H), 7.1 (s, vinylic proton), 4.6 (m, 2HN-CH2),4.30(s,OCH3), (t,2HCH2-Ph),2.2(m,4H),1.9(s,1H);13CNMR:δ=198,168, 142.3, 136, 130, 128.3, 123, 119.1 132, 50.5, 45.8, 35.5, 27.9, 29.9, ; % element analysis for C13H17NO3S2,Calculated:C,57.29;H,5.11;N,4.80;O,14.31,S,19.12;observed:C,56.98; H, 5.02; N, 4.82; O, 14.13; S, 18.99; m/e = 335.06 (found), 335.44 (calc.).
2-[4-oxo-2-thioxo-3-(cyclopentyl)-thiazolidine-5-yliden]-acetic acid methyl ester (1d)
Yield(0.24g 78%), thick oil, IR in cm-1, 1702(C=O), 1618 (C=C), 1212 and 1323 (S=C); 1H NMR: δ= 1.62-2.12 (5H cyclopentyl, multiplet), δ=3.76 (Singlet OCH3), δ= 3.86 (singlet NH) δ= 7.3 (vinylic H, singlet); 13C NMR: δ= 198, 165, 136, 50.5, 28.3, 20.5; elemental analysis for C11H13NO3S2, Calculated: C, 48.69; H, 4.83; N, 5.16; O, 17.69, S, 23.63; observed: C, 47.99; H, 4.71; N, 5.16; O, 17.65; S, 23.41; m/e = 271.06 (found), 271.44(calc.).
(3-Heptyl-4-oxo-2-thioxo-thiazolidin-5-ylidene)-acetic acid methyl ester (1e)
Yield (0.23g, 85%), thick oil, IR (cm-1) 1702(C=O), 1618 (C=C), 1212 and1323(S=C); 1H NMR: δ=0.87(3H triplet, terminal CH3), 1.27- 1.70(10 H -CH2 multiplet), 3.8(N- CH2triplet),4.07(3H,singlet-OCH3),6.82(vinylicH);13CNMR:δ=198,165,136,50.5,28.3,27.4,29.7,32.5,23.1,14.0;% elemental analysis for C13H19NO3S2,Calculated:C,51.80; H, 6.35; N, 4.65; O, 15.89, S, 21.28; observed: C, 51.70; H, 6.27; N, 4.06; O, 15.65;S,21.41; m/e = 301.06 (found), 301.42 (calc.).
2-[4-oxo-2-thioxo-3-(phenyl)- thiazolidine-5-yliden] acetic acid methyl ester (1f)
Yield (0.245 g, 78%), thick oil; IR (cm-1) 1721(C=O), 1618 (C=C), 1213 and1329(S=C); 1H NMR: δ= 6.90, 7.09, 7.28 (5H benzene ring protons), 7.10 (singlet, vinylic H), 3.74(3HsingletOCH3);13CNMR:δ=193,136,165.5,50.5,128.7,120.3,128.7,124.1,120.4; % elemental analysis for C12H9NO3S2, Calculated: C, 51.59; H, 3.24; N, 4.99, O, 17.18, S, 22.98; Observed: C, 51.49; H, 3.27; N, 5.01; O, 17.05; S, 22.61; m/e = 279.07(found), 279.33 (calc.).
2- [4-oxo-2-thioxo-3-(3-Chloro-benzyl)- 1,3 thiazolidin-5-ylidene]-acetic acid methyl ester (1g)
Yield (0.264, 80%), thick oil; IR (cm-1) 1702(O=C), 1618 (C=C), 1212 and1323(S=C); 1H NMR: δ= 7.08, 6.94, 4.22 (Singlet), 7.07, 6.20 (vinylic H), 3.76 (OCH3); 13CNMR:198,136,165.0,166.8,133.6,125.7,129.8,143.8,133.6,50.5;% elemental analysis for C13H10NO3S2Cl, Calculated:C,47.63;H,3.08;N,4.26;O,14.64,S,19.56;Cl,10.82;Observed:C,47.48;H,3.02;N,4.16;O,14.55;S,19.61;Cl,10.68; m/e=326.97(found),327.80 (calc.).
[3-(2,4-Difluoro-phenyl)-4-oxo-2-thioxo-thiazolidin-5-ylidene]-acetic acid methyl ester (1h)
Yield (0.25 g, 80%), thick oil; IR (cm-1) 1702(O=C), 1618 (C=C), 1212 and 1323(S=C); 1H NMR:= 6.66, 6.72, 7.60, 6.82 (vinylic H), 3.76 (OCH3); 13C NMR: 193, 136, 165.1, 50.5, 159.3, 11.3, 102.3, 165.1; % elemental analysis for C12H7NO3S2F2, Calculated:C, 45.71; H, 2.24; N, 4.44; O, 15.22; S, 20.34; F, 12.05 Observed: C, 45.39; H, 2.20; N, 4.36; O, 15.05; S, 19.99; F, 12.01; m/e = 314.63 (found), 314.98 (calc.).
[3-(3-Ethoxy-propyl)-4-oxo-2-thioxo-thiazolidin-5-ylidene]-acetic acid methyl ester (1i)
Yield (0.245 g, 85%), thick oil; IR (cm-1) 1702(O=C), 1618 (C=C), 1212 and1323(S=C); 1H NMR δ= 1.11, 3.41, 3.37, 1.72, 2.96, 6.20 (vinylic H), 3.76 (OCH3); 13C NMRδ=198, 136, 165.0, 50.5, 166.5, 41.1, 29.0, 69.3, 67.1, 63.0, 14.7; % elemental analysis for C12H7NO3S2F2, Calculated: C, 45.66; H, 5.22; N, 4.84; O, 22.`92; S, 22.16; Observed: C, 45.48; H, 5.20; N, 4.56; O, 22.75; S, 22.03; m/e = 289.01 (found), 289.04 (calc.).
[3-(3,5-Bis-trifluoromethyl-phenyl)-4-oxo-2-thioxo-thiazolidin-5-ylidene]-acetic acid methyl ester (1j)
Yield (0.356 g, 85%), white powder melts at 86-88 0C; IR (cm-1) 1702(O=C), 1618 (C=C), 1212 and 1323 (S=C); 1H NMR: δ= 7.38, 7.83, 6.82 (4 H aromatic), 3.76 (vinylicH);C14H7NO3S2F6(MW=415);13CNMRδ=193,136,165.0,50.5,163.8,120.5,119.6,117,7,131.5, % elemental analysis for C14H7NO3S2F6, Calculated: C, 40.49; H, 1.70; N, 3.37; O, 11.56;S,15.44;F,27.45;Observed:C,40.38;H,1.68;N,3.26;O,11.57;S,15.33;F,27.32;m/e = 414.72 (found), 414.97 (calc.).
[3-(2-Fluoro-benzyl)-4-oxo-2-thioxo-thiazolidin-5-ylidene]-acetic acid methyl ester (1k)
Yield(0.24g,82%),thick oil; IR(cm-1)1702(O=C),1618(C=C),1212and1323(S=C); 1H NMR δ= 6.91, 7.05, 6.88, 4.22, 6.20 (vinylic H), 3.76 (OCH3); 13C NMR δ=198,136,165.1, 50.3, 128.7, 123,3, 129.4,160.7, 166.8, 115.3; % elemental analysis for C13H10NO3S2F, Calculated: C, 50.15; H, 3.24; N, 4.50; O, 15.42; S, 20.60; F, 6.09 Observed:C, 50.01; H, 3.20; N, 4.49; O, 15.25; S, 20.23; F, 5.99; m/e = 310.92 (found), 311.00 (calc.).
(3-Dodecyl-4-oxo-2-thioxo-thiazolidin-5-ylidene)-acetic acid methyl ester (1l)
Yield (0.28 g, 80%), white powder melts at 92-94 0C; IR (cm-1) 1702(O=C), 1618 (C=C), 1212 and 1323 (S=C); 1H NMR: δ = 0.96, 1.33, 1.29, 1.55, 2.96, 6.20 (vinylic H), 3.76(OCH3); 13C NMR: δ = 198, 136, 165.0, 50.5, 28.3, 27.4, 30.0, 30.3, 30.0, 23.1, 14.0; % elemental analysis for C18H29NO3S2, Calculated: C, 58.19; H, 7.87; N, 3.77; O, 12.`92; S,17.26; Observed: C, 58.01; H, 7.68; N, 3.66; O, 12.75; S, 17.03; m/e = 371.02 (found), 371.15 (calc.).
[3-(2-Ethyl-butyl)-4-oxo-2-thioxo-thiazolidin-5-ylidene]-acetic acid methyl ester (1m)
Yield(0.234g,84%),thick oil;IR(cm-1)1702(O=C),1618(C=C),1212 and 1323(S=C);1HNMR:δ=0.96,1.29,2.10,6.20(vinylicH),3.76(OCH3);13CNMRδ=198,135,165.0,50.5,136.9,33.5,24.6,11.5;% elemental analysis for C12H17NO3S2,Calculated:C,50.15;H,5.96;N,4.79;O,16.69;S,22.31; Observed:C,50.03;H,5.85;N,4.86;O,16.67;S,22.23; m/e = 286.78 (found), 287.06 (calc.).
[3-(2,3-Dichloro-benzyl)-4-oxo-2-thioxo-thiazolidin-5-ylidene]-acetic acid methyl ester (1n)
Yield (0.303g, 83%, thick oil; IR (cm-1) 1702 (O=C), 1618 (C=C), 1323, 1212(S=C);1HNMRδ=7.02,6.96,6.89,4.22,6.20(vinylicH),3.76(OCH3);13CNMRδ=198,136,165.0, 50.5, 134.0, 128.3, 127.8, 126.6, 144.2, 166.6; % elemental analysis for C13H9NO3S2Cl2, Calculated: C, 43.10; H, 2.50; N, 3.87; O, 13.25; S, 17.70; Cl,19.57;Observed:C,42.98;H,2.41;N,3.79;O,13.04;S,17.53;m/e=360.18(found),360.94(calc.).
(3-Decyl-4-oxo-2-thioxo-thiazolidin-5-ylidene)-acetic acid methyl ester (1o)
Yield (0.302 g, 81%), white powder melts at 91-93 0C; IR (cm-1) 1702(O=C), 1618 (C=C),1212 and 1323(S=C);1HNMR:=0.96,1.33,1.29(multiplet),2.98,6.82(vinylicH),3.76 (OCH3); 13C NMR δ= 166.5, 165.0, 45.0, 28.3, 198, 136, 30.0, 30.3, 32.5, 14.0, 50.5;%elemental analysis for C16H25NO3S2, Calculated: C, 55.94; H, 7.34; N, 4.07; O, 14.01; S, 18.67;Observed:C,55.69;H,7.23;N,4.06;O,13.74;S,18.53;m/e=342.89(found),343.12 (calc.).
Results and Discussions
The reaction was designed as given in scheme I. Firstly we reacted 3-trifluoromethyl benzyl amine with CS2 and but-2-ynedioic acid dimethyl ester in DMSO with constant stirring. Compound thus formed was separated purified and identified by spectral and physical data. IR spectrum of 1a showed absorption band at 1720 cm-1, unsaturation at 1682 cm-1, C=S at 1340 and 1185 cm-1. Proton spectra of 1a showed 3 sharp singlet corresponding to OCH3 group (∂ = 3.9), methylene protons (∂ = 5.32) and vinylic proton at (∂ = 6.85). Aromaticity was reflected in NMR spectrum.
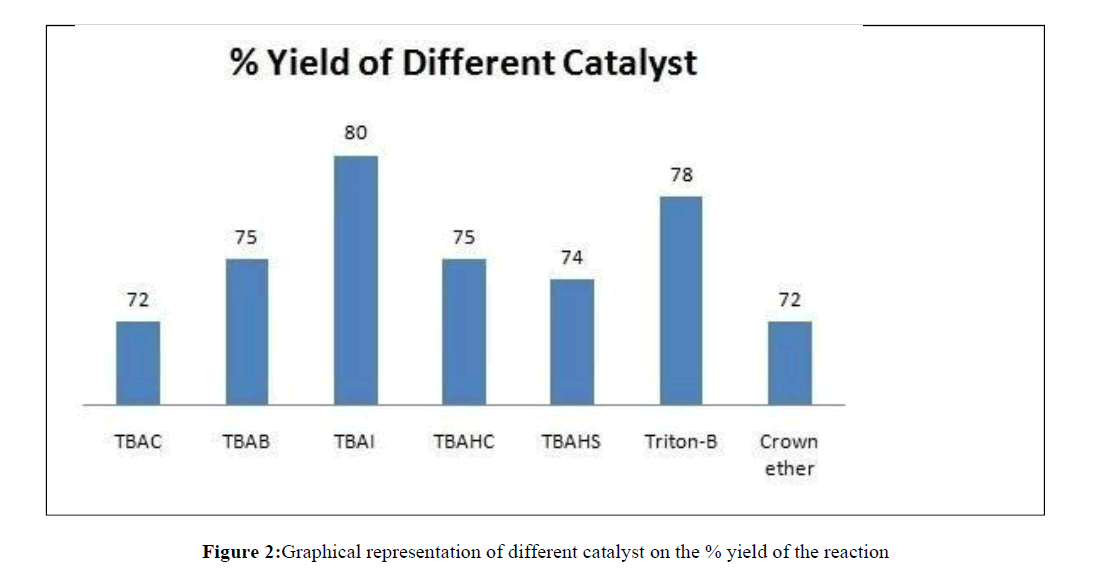
Once the product was obtained and characterized, we undertook the affordability of product with various phase transfer catalyst by reacting 3-trifluoromethyl benzyl amine with But-2- ynedioic acid dimethyl ester and carbon disulphide in different phase transfer catalyst like tetra n-butyl ammonium chloride (TBAC), tetra-n-butyl ammonium bromide (TBAB), tetra- n-butyl ammonium iodide (TBAI), tetra-n-butyl ammonium hydrogen carbonate (TBAHC), tetra-n-butyl ammonium hydrogen sulphate (TBAHS), Triton-B, Crown ether (18 crown 6) etc. It was found that tetra-n-butyl ammonium iodide (TBAI) served the best in getting high yield of the desired 3- substituted- 4-oxo-2-thioxo-thiazolidin-5-ylidene)-methyl ethanoate (Table 1, Figure 2).
| S. No. | Phase transfer catalyst | Time in Hrs. | % Yield |
|---|---|---|---|
| 1 | TBAC | 3.0 | 72 |
| 2 | TBAB | 3.0 | 75 |
| 3 | TBAI | 2.5 | 80 |
| 4 | TBAHC | 2.7 | 75 |
| 5 | TBAHS | 2.8 | 74 |
| 6 | Triton-B | 2.8 | 78 |
| 7 | Crown ether (18 Crown 6) | 3.0 | 72 |
Table 1: Effect of Phase transfer catalyst on time and yield
Thus we find that TBAI is the most suitable phase transfer catalyst in this reaction. Once the phase transfer catalyst was fixed we undertook the optimization in the solvent. For this synthesized compound 1a in different solvents like dimethylformamide, Dimethyl sulphaoxide (DMSO), chloroform, dichloromethane, methanol, benzene, toluene, n-hexane, n-heptanes and it was observed that DMSO was most efficient at room temperature (Table 2).
| S. No. | Solvent | Time in hr | % Yield |
|---|---|---|---|
| 1 | Dimethylformamide, | 3.0 | 50 |
| 2 | Dimethyl sulphaoxide (DMSO) | 2.5 | 80 |
| 3 | Chloroform | 3.5 | 40 |
| 4 | Dichloromethane | 3.2 | 50 |
| 5 | Methanol | 3.6 | 35 |
| 6 | Benzene | 4.0 | 38 |
| 7 | Toluene | 4.2 | 40 |
| 8 | n-hexane | 4.8 | 45 |
| 9 | n-heptane | 4.6 | 42 |
Table 2: Effect of solvent on time yield of the reaction
After optimizing the process we synthesized several compounds using different amines. Various 10, 20, 30 phenyl, benzylic and cyclic amines were considered for obtaining library of compounds given in Table 3.
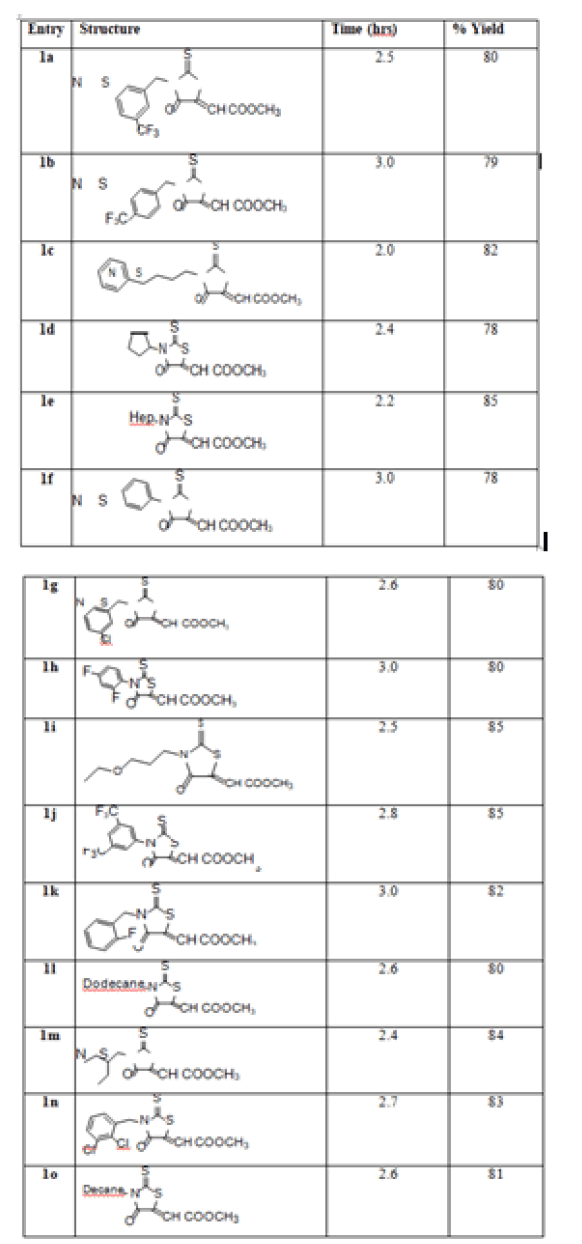
Table 3: Synthesized 3- substituted- (4-oxo-2-thioxo-thiazolidin-5-ylidene)-methyl ethanoate
We found that the reaction goes perfectly with the primary alkyl groups compared to secondary and tertiary alkyl groups which may be due to steric hinderance. At the same time it was also observed that electron releasing group bearing substituted aromatics and heterocylics have better yield and lesser time than the electron withdrawing groups attached to aromatic ring. Again it was found that if R2 was cyclic then also the yield was less compared to aliphatic R2. The spectral characterization of all products was confirmed through the standard data available for the existing compounds [28].
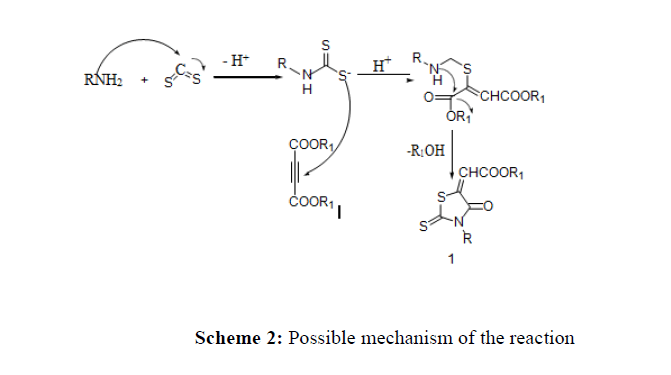
A probable path of the reaction is elucidated in (scheme 2). CS2 reacted with amine and formed alkylammonium carbothioate. The alkylammonium carbothioate formed attacked acetylenic ester to give an intermediate which cyclized losing R1OH formed compound 1 (Scheme 2)
Anticancer activity
Methods
The different human cancer cell lines were considered which includes HeLa (cervical cell line), Colon cancer cell line (HCT-15); and glioblastoma (Brain cancer cells) U87-MG. National Cell Center, Pune, India provided these cell lines. The culturing of cell lines was done in Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma-Aldrich, US) while culturing of HCT-15 cells was done in (RPMI, Sigma-Aldrich, US).
Measurement of anticancer activity
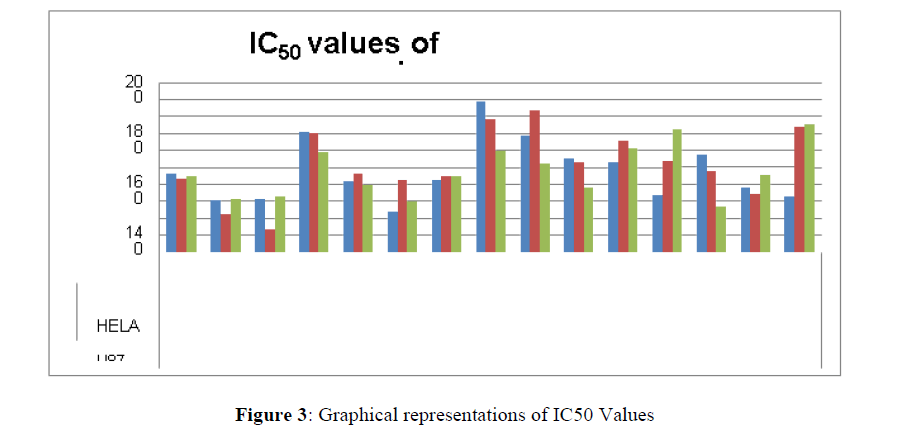
Cancerous cells of were placed in 3 × 104 cells in a well with 100 μL of DMEM for HeLa and U87MG and RPMI for HCT-15 cells] with 10% fetal bovine serum (FBS) in a 96- well tissue culture plate which were then made to grow for 72 hours at 37°C, in a CO2 incubator maintaining relative humidity of 90% and 5% CO2. Now cancer cells were incubated with 100, 50, 25 and 12.5 μM concentrations of various synthesized trithiocarbobates prepared in DMEM/RPMI i.e. for 24 hr. Further, the cancer cells also incubated with 100 μL of MTT dye (0.5 mg/ml) in CO2 incubator for next 4 hr. In the bottom of the well, the cells produced formazan of purple colour. Now the culture medium was taken out of the well. Following this, each well was filled with 100 μL of DMSO. The formazan crystals in each well were dissolved which gave purple solution. Following this Labsystems Multiskan EX ELISA reader was used to measure absorbance at 570 nm for 96 well-plates against a reagent blank. IC50 for 50% inhibition of each drug was calculated in micromolar concentration. Simultaneously MTT assay was done in triplicate in three independent experiments (Figure 3) (Tables 4 & 5).
| Entry | HCT-15 | HELA | U87-MG |
|---|---|---|---|
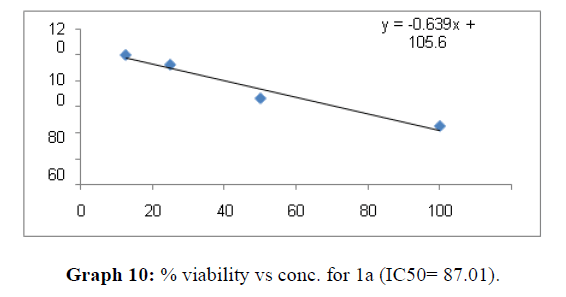
| 1a | 92.5 | 87.01 | 89.05 |
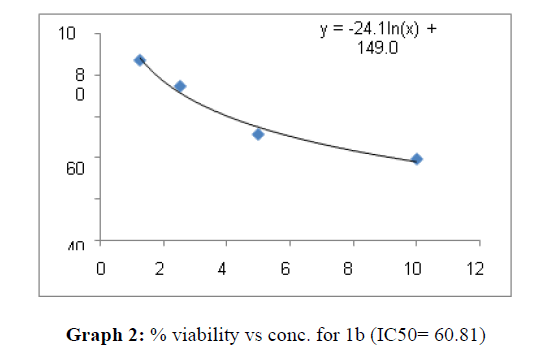
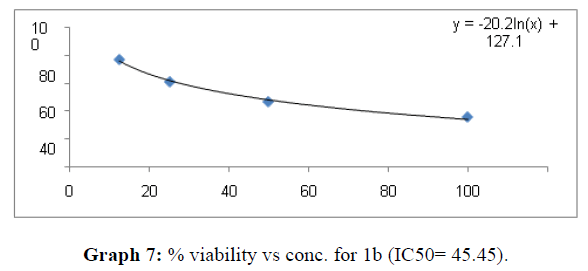
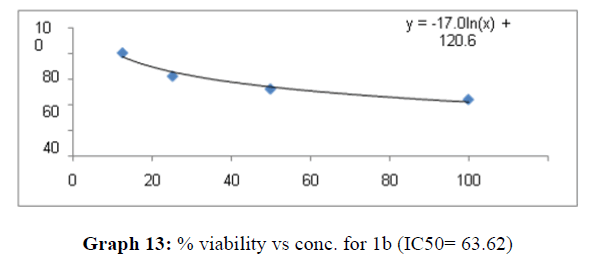
| 1b | 60.8 | 45.45 | 63.62 |
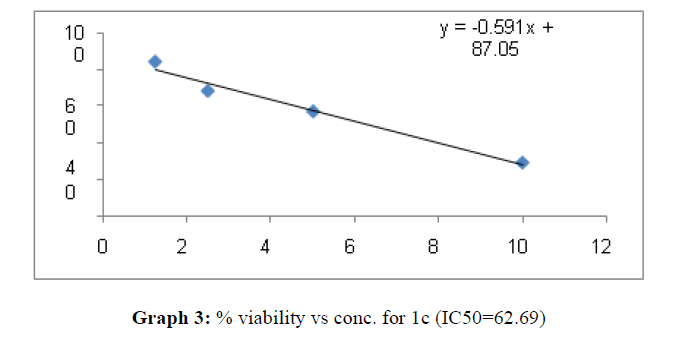
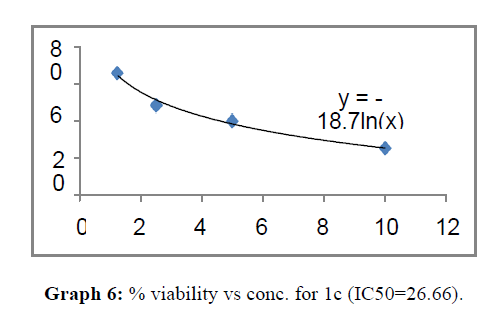
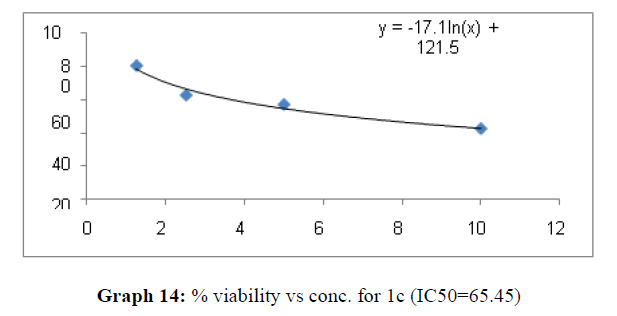
| 1c | 62.69 | 26.66 | 65.45 |
| 1d | 141.89 | 139.88 | 117.3 |
| 1e | 83.75 | 93.08 | 78.87 |
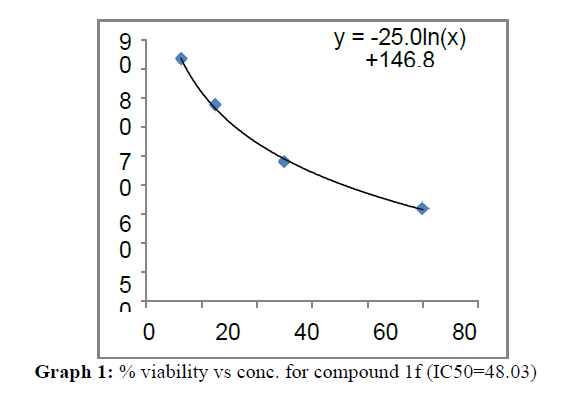
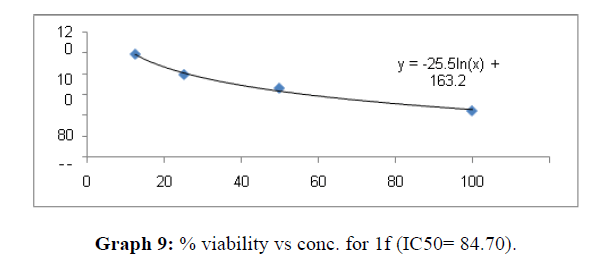
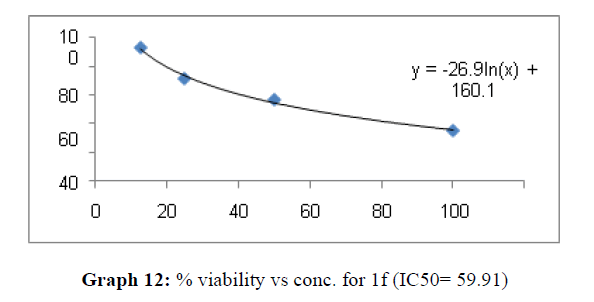
| 1f | 48.03 | 84.7 | 59.91 |
| 1g | 85.12 | 88.74 | 89.72 |
| 1h | 177.6 | 156.84 | 119.83 |
| 1i | 137.3 | 167.64 | 104.05 |
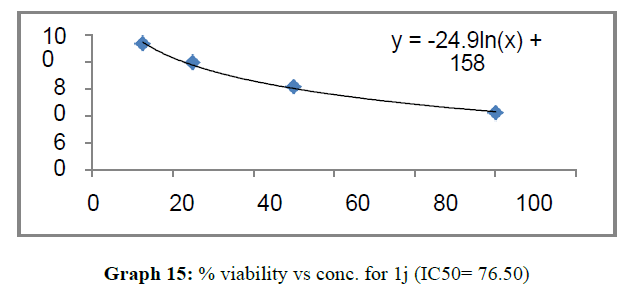
| 1j | 110.32 | 106.5 | 76.5 |
| 1k | 105.86 | 131.75 | 122.62 |
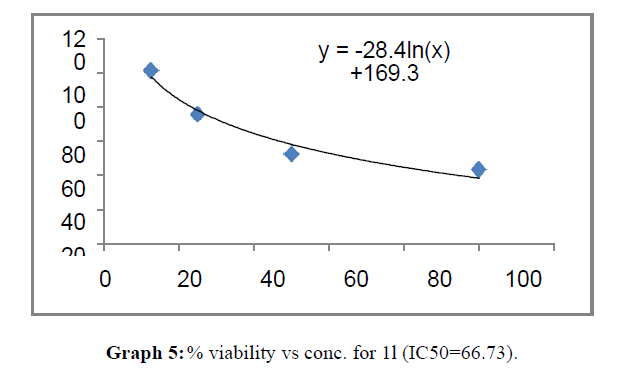
| 1l | 66.73 | 107.8 | 145.09 |
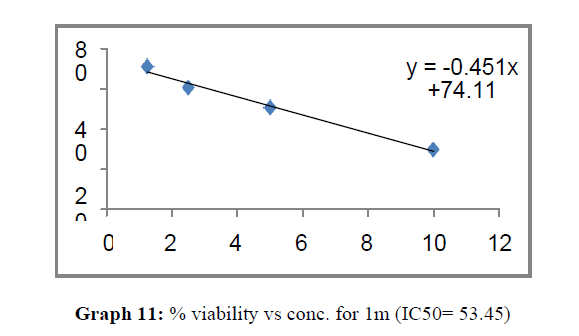
| 1m | 114.5 | 96.03 | 53.45 |
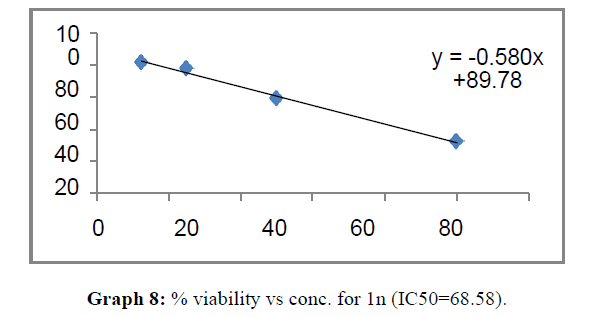
| 1n | 76.45 | 68.58 | 90.56 |
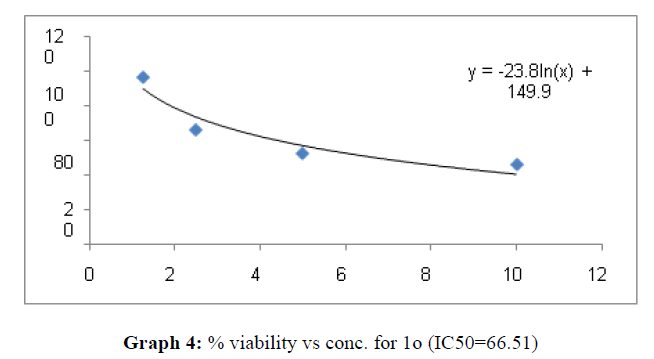
| 1o | 66.51 | 147.09 | 151.41 |
Table 4: Percentage viabilities at 100 μM concentration of the compounds
| 1a | 1b | 1c | 1d | 1e | 1f | 1g | 1h | 1i | 1j | 1k | 1l | 1m | 1n | 1o | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HCT-15 | 92.5 | 60.8 | 62.6 | 141 | 83.7 | 48 | 85.1 | 177 | 137 | 110 | 105 | 67 | 114 | 76.4 | 66.5 |
| 87 | 45.4 | 26.6 | 139 | 93 | 84.7 | 88.7 | 156 | 167 | 106 | 131 | 107 | 96 | 68.5 | 147 | |
| 89 | 63.6 | 65.4 | 117 | 78.8 | 59.9 | 89.7 | 119 | 104 | 77 | 122 | 145 | 53.4 | 90.5 | 151 |
Table 5: Results of anticancer activity
The percentage viability at 100μM concentration of the synthesized compounds is given in Table 4
The synthesized compounds in this class were assessed for cytotoxicity against 03 cancer cell lines i.e.HCT-15 (human colon cancer), U87-MG (Human glioblastoma) and Hela (human cervix cancer) using MTT assay.
On the treatment of the HCT-15 cancer cells with the synthesized compounds it was found that almost all the compounds are active against the cancer cells but the compound 1f, 1b, 1c, 1o, 1l have been most prominent for inhibition of HCT-15 cell proliferation.
These compounds have been found to have minimum IC50 values required for inhibition of the HCT-15 cancer cells. The decrease in cell viability with increase in concentration of compounds against HCT-15 cancer cells is represented in Graphs 1-15 below:
Now on consideration of the activity of the compounds of this series against the proliferation of HeLa (cervical cancer cells), it is found that all the compounds synthesized were active against reduction in cancer cell survival with increase in concentration of compounds. But the compounds 1c, 1b, 1n, 1f, 1a are more active against a specific number of HeLa cells. The percentage viability against concentration is shown in graphs below for compounds with highest activity.
Finally Considering the activity of the compound synthesized against the proliferation of the brain cancer cell U87-MG, the IC50 value and the % viability graphs show that almost all the synthesized compounds are active against cancer cells. Among them, the compounds 1m, 1f, 1b, 1c, 1j are most active in retarding the growth of cancer cells. The percentage viability and the concentration curve of the most active compounds of the series are given in the graph below:
Conclusion
Here in this communication we report single flask synthesis of rhodanines using Tetrabutyl ammonium iodide (TBAI), carbon disulfide at normal lab temperature which were found to have anticancer activities. The reaction was self occurring at normal lab temperature DMSO/ Tetrabutyl ammonium iodide (TBAI). The process here was simple, occurred at normal temperature, easier separation and higher yield with cheaper and less toxic chemicals. The synthesized compounds have shown very good in-vitro anticancer activity against cervical cancer, colon cancer and brain cancer. These biologically potent scaffolds can be developed further to effective drugs against various cancers.
Acknowledgment
The authors are thankful to Pro-Vice Chancellor, Amity University Uttar Pradesh, Lucknow Campus for providing research infrastructure in conduct of this research. This research has been supported by Department of Science & Technology, Govt. of India for providing financial support.
References
- Y Inamori, Y Okamoto and Y Takegawa. Biosci Biotechnol Biochem. 1998, 62: p. 1025-1027.
- MG Orchard, JC Neuss, CM Galley. Bioorg Med Chem Lett. 2004, 14: p. 3975-3978.
- P Mitra, AS Mitra, J Indian Chem Soc. 1984, 61: p. 77-78.
- SP Gupta, S Rani, J Indian Chem Soc. 1978, 55: p. 483-485.
- MG Orchard, JC Neuss, CM Galley. Bioorg Med Chem Lett. 2004,14(15): p. 3975-3978.
- W Sing, CL Lee, SL Yeo et al., Med Chem Lett. 2001, 11: p. 91-94.
- MG Orchard, JC Neuass, CMS Galley, et al., Bioorg Med Chem Lett. 2004, 14: p. 3975-3978.
- NS Cutshall, C O'Day C and M. Prezhdo, Bioorg Med Chem Lett 2005, 15(14): p. 3374-3379.
- EB Grant, D Guiadeen, EZ Baum, et al., Med Chem Lett. 2000, 10(19): p. 2179-2182.
- NJ Gaikwad and P Gautam. Indian J Heterocycl Chem. 2002, 12: p. 181-182.
- SK Shukla, SP Singh and LP Awasthi. Indian J Pharm Sci. 1982, 44: p. 153-155.
- TK Smith, BL Young, H Denton et al., Bioorg Med Chem Lett. 2009, 19(6): p. 1749-1752.
- L Wang, F Kong, CL Kokoski et al., Bioorg Med Chem Lett. 2008, 18(1): p. 236-240.
- BT Moorthy, S Ravi and M Srivastava, Bioorg Med Chem Lett. 2010, 20 (21): p. 6297-6301.
- NS Habib, SM Ridal, EAM Badaweyl et al., Eur J Med Chem. 1997, 32(9):p. 759-762.
- D Hardej, CR Ashby Jr, NS Khadtare et al., Eur J Med Chem. 2010, 45(12): p. 5827-5832.
- S Rajamaki, A Innitzer and C Falciani, Bioorg Med Chem Lett. 2009, 19(13): p. 3615-3618
- BB Lohray, V Bhushan, BB Rao, et al., Bioorg Med Chem Lett. 1997, 7:p. 785-788.
- S Gabillet, D Lecercle, O Loreau et al., Org Lett. 2007, 9: p. 3925-3927.
- A Alizadeh, F Movohedi, H Masrouri, et al., Synthesis. 2006, 3431.
- A Alizadeh, F Movahedi and AA Esmaili, Tetrahedron Lett., 2006, 47: p. 4469.
- J Liu, Y Zhou, Y Wu,et al., Tetrahedron Asym. 2008, 19: p. 832-837.
- D Wesselinova and I Dikova. Dokl Bolg Akad Nauk. 2003, 56: p. 89-94.
- Z Wang, H Shi and H Zheng, Youji Huaxue. 1994, 14: p. 190-194.
- K Das, D Panda and B Dash, J Indian Chem Soc. 1990, 67: p. 58-60.
- T Tomašić and LP Mašič, Curr Med Chem. 2009, 16: p. 1596-1629.
- A Alizadeh, S Rostmania, N Zohreh et al., Tetrahedron Letters. 2009, 50: p. 1533- 1535.
- N Srivastava, M Saxena. Asian J Chem. 2019, 31(1): p. 176-180.