Research Article - Der Pharma Chemica ( 2017) Volume 9, Issue 4
TG/DTA and Optical Studies on Nano ZrO2 Incorporated Polymer Electrolytes for Rechargeable Proton Batteries
Radha KP*Radha KP, Department of Physics, S.F.R. College for Women, Sivakasi 626123, Tamil Nadu, India,
Abstract
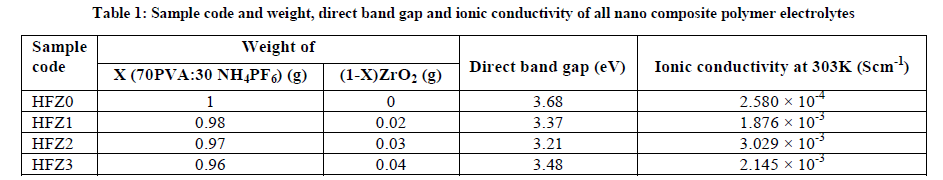
Nano composite polymer electrolytes based on poly (vinyl alcohol) PVA as host polymer, ammonium hexafluoro phosphate (NH4PF6) as salt and zirconium di oxide (ZrO2) as nano-filler have been prepared by Solution Casting Technique. Admittance analysis shows that the addition of the nano-filler ZrO2 the polymer electrolyte enhances the amorphous phases of polymer matrix which in turn increases the ionic conductivity. 0.03g ZrO2 added polymer electrolyte has maximum ionic conductivity 3.029 × 10-3 Scm-1 at ambient temperature. The weight loss for ZrO2 added an electrolyte is less compared to pure polymer electrolyte suggesting that the thermal stability of composite polymer electrolyte system has been improved due to the addition of the nano-filler ZrO2. The optical band gap decreases from 3.68eV of nano-filler undoped polymer electrolyte to 3.21 eV of nano-filler incorporated polymer electrolytes.
Keywords
Admittance, Thermal, Ultraviolet
Introduction
Most of the experimental research works are carried out towards development of proton conducting solid polymer electrolyte based on poly(vinyl alcohol) (PVA) doped with various ammonium salts like NH4 F, [1] NH4Cl, adipic acid [2] etc. PVA is a cost effective bio degradable synthetic polymer with good charge storage capacity excellent film forming capacity, donor dependent electrical and optical Properties etc. PVA is a semi crystalline material. The author Zhang et al. [3] reported that semi crystalline materials exhibit improvement in certain physical Properties due to crystal amorphous interfacial effect. The hydrogen bond present in PVA is an instrumental to proton conductivity in polymer electrolyte. PVA is well known to form complexes with ammonium salts. The conduction mechanism of proton conducting polymer electrolyte with ammonium salts have been investigated spectroscopically in the past decades [4]. Literature studies reveal that the incorporation of nano-filler is contemporary way of enhancing the ionic conductivity of the polymer electrolytes. In the present work, the nano-filler zirconium di oxide (ZrO2) is added to the polymer electrolyte PVA/NH4PF6. The prepared electrolytes are subjected to the electrical, thermal and optical studies.
Materials and Methods
Synthesis of polymer electrolyte
In the present work PVA with molecular weight 1,25,000 (AR grade Sd fine chem. make), ammonium hexafluoro phosphate (NH4PF6) purchased from Aldrich, USA and the nano filler ZrO2 from Aldrich USA of particle size 45 nm and dimethyl sulphoxide (DMSO) as solvent are used as starting material. Composite polymer electrolytes X (70PVA:30 NH4PF6): (1–X) ZrO2 (X=0, 0.02, 0.03 and 0.04 g) have been prepared by solution casting technique. Appropriate weights of PVA, NH4PF6 are dissolved in DMSO by using magnetic stirrer separately. Then these two solutions are mixed together and stirred well. The nano filler ZrO2 is suspended in this solution and then stirred well to get homogenous mixture. The mixture is then poured into glass petri dish and is allowed to evaporate the solvent in the vacuum oven at 80ºC for 5 days. Free standing nature of the electrolyte has obtained.
Characterization
Thermo gravimetric analysis (TG) has been studied using SDT Q600 V8.3 Build 101 at a heating rate of 20ºC/min in the range 0ºC to 800ºC in the atmosphere of Nitrogen. AC conductivity measurements have been carried out on PVA: NH4PF6:ZrO2 systems of uniform thickness having an area of 1 cm2. Polymer electrolytes have been sandwiched between two stainless steel (SS) electrodes applying a potential of 1V from 42 Hz to 1 MHz using HIOKI make LCZ meter (model 3532) interfaced to a computer. The conductivity has been calculated from complex impedance plots of measured impedance (Z) and phase angle (θ). The temperature of the cell has been controlled using a thermostat and electrical measurements of the polymer electrolytes have been carried out in the temperature range 303K–343K.The absorbance spectra have been measured using UV-2400 PC Series in the Wavelength Range 200 nm to 900 nm with medium scan speed of sampling interval 0.1.
Conclusion
Composite polymer electrolytes X (70PVA:30 NH4PF6): (1–X) ZrO2 (X=0, 0.02, 0.03 & 0.04. g) have been prepared by solution casting technique. The admittance plot shows a low frequency arc representing the series combination of the bulk resistance (Rb) and electrode capacitance Ce as shown in the equivalent circuit. TG/DTA analysis suggests that the thermal stability of composite Polymer electrolyte system has been improved due to the addition of the nano-filler ZrO2. The optical band gap decreases from 3.68eV for nano-filler undoped polymer electrolyte to 3.21 eV for 0.03 g of nano-filler incorporated polymer electrolytes.
Acknowledgements
The author K.P. Radha acknowledges the University Grants Commission for sanctioning the Minor Research Project.MRP 5975/15 SERO\UGC.
References
[1] K.P. Radha, S. Selvasekarapandian, S. Karthikeyan, M. Hema, Ionics., 2013, 19(10), 1437-1447.
[2] P. Mahalakshmi, S. Chitra, K.P. Radha, Int. J. Adv. Sci. Res., 2016, 1(1), 21-24.
[3] H. Zhang, J. Wang, Spectrochimica. Acta. part A., 2009, 71, 1927-1931.
[4] S. Chandra, S.A. Hashmi, G. Prasad, Solid. State. Ionics, 1990, 40-41, 651-654.
[5] E.M. Abdelrazek, I.S. Elashmawi, A. El-khodary, A. Yassin, Curr. Appl. Phys., 2010, 10(2), 607-613.
[6] J. Qiao, I. Fu, R. Lin, J. Ma, J. Liu, Polymer, 2010, 51(21), 4850-4859.
[7] N.F. Mott, E.A. Davis, Clarendon. Press, Oxford, 1979.
[8] J. Tauc, A. Menth, J. Non-Cryst. Solids., 1972, 8, 569.
[9] M.A. Hamed, S.H. Sabeeh, A.H. Sarkawt., ATST., 2006, 1(6), 16-20.
[10] P. Sharma, V. Sharma, S.C. Katyal, Chalcogenide. Letters., 2006, 3(10), 73 -79.



