Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 6
The Synthesis of Acyclic Beta Enaminones Based on Benzidine
Nabugomu Ronald Jowet*, Hao Haijun, Zhuang Jungpeng, Zhao Shisheng and Shi Yujian
Department of Organic Chemistry, College of Science Beijing University of Chemical Technology, Beijing, China
- *Corresponding Author:
- Nabugomu Ronald Jowet
Department of Organic Chemistry
College of Science Beijing University of Chemical Technology, Beijing, China
Abstract
(z)-4-((4-amino-[1,1’biphenyl]-4-yl)amino)pent-3-en-2-one,L3c1, (z)-5-((4’-amino-[1,1’biphenyl]-4-yl)amino)hept-4-en-3-one L3d1, (z)-3-((4’- amino-[1,1’biphenyl]-4-yl) amino)-1-phenylbut-2-en-1-one L3e1, were synthesized from the condensation of acyclic -1,3- betadiketone derivatives and Benzidine using ethanol. The presence of a linkage of two phenyl groups in the para position in relation to the amine group makes this particular class of enaminones have prospective added advantages and hence functionalization. The advantages offered by this method presented are use of mild, cheap, shorter reaction time for complete enamination and an aspect of green Chemistry.
Keywords
Enaminone, Acyclic betadiketone derivatives, Condensation reaction, Benzidine
Introduction
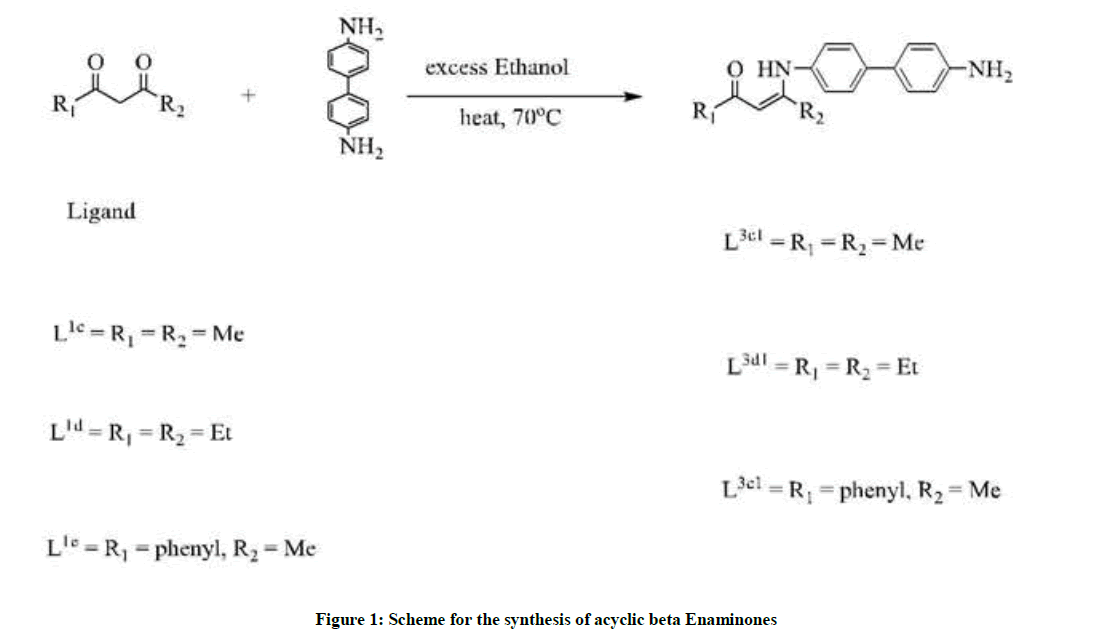
Enaminones are a class of nitrogen containing organic compounds that are well known for their great biological importance and as key precursors in organic synthesis [1], particularly, as starting materials for different heterocycles [2], antibacterial [3], anticonvulsant, anti-inflammatory [4], antitumour agents [5], and naturally occurring alkaloids [6]. Hence their synthesis has received great attention and a vast number of synthetic routes have been reported such as Lewis acids [7], P2O5/SiO2 [8], Cu-nanoparticles [9], and heteropoly acid [10]. In 1961, Martin [11] reported one of the most important ways to β-enaminone, viz the direct condensation of β-dicarbonyl compound with amine in refluxing aromatic hydrocarbon. In addition, β-enaminone has also been synthesized by using non-traditional methods [12]. While a lot has been reported about the synthesis of beta enaminones using aromatic amines [13-15], Benzidine has never been explored and used in the synthesis of beta enaminones as diamine functionality. The use and exploration of Benzidine in this synthesis is based on the fact that the linkage between two phenyl groups in the para position relative to the amine offers greater stability. In continuation with the non-use of Azeotropic removal of water by distillation method we reported earlier, we therefore wish to report herein the use of benzidine for the synthesis of acyclic beta enaminones. Secondly we report herein the use of ethanol as a relatively cheap, less toxic and readily available catalyst. The use of ethanol is an approach of green Chemistry [16-24].
Results and Discussion
Three enaminones such as (z)-4-((4-amino-[1,1’biphenyl]-4-yl) amino)pent-3-en-2-one L3c1, (z)-5-((4’-amino-[1,1’biphenyl]-4-yl)amino)hept-4- en-3-one L3d1 and (z)-3-((4’-amino-[1,1’biphenyl]-4-yl) amino)-1-phenylbut-2-en-1-one L3e1 were synthesized. Structure L3c1 was readily established based on IR and 13C NMR that revealed the presence of one C-N 1261.72 band and 160.30 ppm signal; one C=C 1515.76 band and 97.61 ppm signal. Structure of L3d1 was readily established based on Infrared Radiation (IR) and Carbon-13 Nuclear Magnetic Resonance (13C-NMR) that revealed the presence of one C-N 1216.77 band and 166.08 ppm signal; one C=C 1555.82 band and 94.37 ppm signal. Structure of L3e1 was readily established based on IR and 13C-NMR that revealed the presence of one C-N 1191.98 band and 162.26 ppm signal; one C=C 1585.83 band and 94.31 ppm signal. The full assignment of the 13C-NMR data confirmed the structures of enaminones L3c1, L3d1 and L3e1, where the key signals at δ = 196.07, 200.13, 188.58 ppm assigned to carbon of the CO group respectively. The hydrogen spectrum showed two protons of amine group shown as a broad singlet peak at a shift of 3.74, 3.87, 3.73 ppm for L3c1, L3d1, L3e1 respectively. In addition, the hydrogen spectrum indicated the presence of a broad singlet signal at 12.54, 12.52, 13.14 ppm corresponding to the NH group for L3c1, L3d1, L3e1 respectively. The N–H resonance in all the ligands is broad due to the quadruple moment of the nitrogen atom. Overall the singlet peak for the proton on N-H in ligand L3e1 is far much less broad compared to all enaminone products synthesized. This is because this molecule is very asymmetric and large compared to the rest of the synthesized enaminones. All synthesized enaminones had a broad singlet peak of the hydrogen on the N-H more to the down field.
It was further noted that the synthesized enaminones were formed exclusively as the Z isomers as inferred from the downfield chemical shifts of their NH protons (ca.d=12.237–13.14 ppm), due to intramolecular hydrogen bonding with the carbonyl oxygen atoms. Further evidence for the Z configuration of the synthesized enaminone products L3c1– L3e1 was obtained from their Proton Nuclear Magnetic Resonance (1H-NMR) spectra, in which correlations were observed between their respective vinylic protons (ca. d=5.149–5.90 ppm). In all the ligand products the mass data matched the calculated values (Figure 1).
Experimental
All starting materials and reagents were commercially available and used without further purification. All the melting points were taken in an open capillary and are uncorrected. The progress of the reactions was monitored by Thin Layer Chromatography (TLC). IR spectra were recorded on Perkin-Elmer Fourier-transform infrared (FTIR) spectrophotometer in KBr disc. 1H-NMR spectra were recorded on mercury plus Varian spectrometer at 400 MHz in CDCl3 as a solvent and chemical shift values are recorded in units δ (ppm) relative to tetramethylsilane (Me4Si) as an internal standard. All HRMS were carried out at Beijing University of Chemical Technology.
General procedure
Synthesis of β-enaminones: A mixture of unimolar quantities of acyclic betadiketone derivatives, unimolar quantities of benzidine and ethanol (excess) were taken in a round bottomed flask. The reaction mixture homogenized with the help of magnetic stir bar and heated in an oil bath (70°C) for 10 h. The progress of reaction was monitored on TLC. After enamination was complete, ethanol was removed by rotatory evaporation and the crude solid was purified by column chromatography to afford the desired enaminone.
Synthesis of (z)-4-((4-amino-[1,1’biphenyl]-4-yl) amino)pent-3-en-2-one (L3c1): To a 250 ml round bottomed flask was added acetylacetone, L1c (2.00 g, 19.98 mmol), excess ethanol (50 ml) and 4,4’-Biphenyldiamine (3.68 g, 19.98 mmol). The mixture was heated at 70°C for 10 h. TLC was done to ascertain that the reaction was complete. The ethanol was removed by rotatory evaporation. The crude yellow solid was purified by column chromatography (P.E: E. A=1: 1) and dried in vacuo to afford a pale yellow solid. The product yield was (3.2 g, 60.2%). M.P. = 241°C; 1H-NMR (400 MHz, CDCl3, 25°C, δ (ppm)=12.54 (brs, H, NH), 7.42 (d, 2H, JH-H =8.0Hz, Ar-H); 7.37 (d, 2H, JH-H = 8.0Hz, Ar- H); 6.765 (dd, 4H, JH-H =8.0Hz, Ar-H), 5.22 (s, 1H, C-H), 3.74 (s, 2H, NH2), 2.14 (s, 3H, (CO)CH3), 2.06 (s, 3H, (=C)CH3); 13C-NMR(400 MHz, CDCl3, 25°C, δ (ppm)=196.07, 160.30, 145.99, 138.46,130.48, 127.79, 127.30, 124.90, 115.45, 97.61, 29.20, 19.95. ESI-MS m/z (%): calculated 267.15, found 267.1488 (M++H, 100); IR (KBr, cm-1): 3427.8(N-H), 2969.95 (w, =C-H), 1610.73 (m, C=O), 1515.76 (C=C), 1327.39 (w, C-N), 1261.72 (w, C-N).
Synthesis of (z)-5-((4’-amino-[1,1’biphenyl]-4-yl)amino)hept-4-en-3-one (L3d1): To a 250 ml round bottomed flask was added heptane-3,5- dione, L1d (2.00 g, 15.60 mmol), excess ethanol (50 ml) and 4,4’-Biphenyldiamine (2.87 g, 15.60 mmol). The mixture was heated at 70°C for 10 h. TLC was done to ascertain that the reaction was complete. The ethanol was removed by rotatory evaporation. The crude yellow solid was purified by column chromatography (P.E: E. A=1: 1) and dried in vacuo to afford a pale yellow solid. The product yield was (3.2 g, 60.2%). M.P. =246°C; 1H-NMR (400 MHz, CDCl3, 25°C, δ (ppm)=12.52 (brs, 1H, NH), 7.49 (d, 2H, JH-H = 8.0Hz, Ar-H); 7.39 (d, 2H, JH-H =8.0Hz, Ar- H), 7.13 (d, 2H, JH-H =8.0Hz, Ar-H), 6.75 (d, 2H, JH-H =8.0Hz, Ar-H), 5.23 (s,1H,C-H), 3.87 (brs, 2H, NH2), 2.37 (q, 4H, JH-H =8.0Hz, CH3CH2), 1.16 (t, 3H, JH-H =8.0Hz, CH2CH3), 1.093 (t, 3H, JH-H =6.0Hz, CH2CH3); 13C-NMR (400 MHz, CDCl3, 25°C, δ (ppm)=200.13, 166.08, 145.96, 138.55, 130.51, 127.79, 126.87, 125.35, 115.44, 94.37, 35.27, 25.23, 12.53, 9.97. ESI-MS m/z (%): calculated 295.18, found 295.1805 (M++H, 100); IR (KBr, cm-1): 3433.01 (br, s, N-H), 3035.61 (w, =C-H), 1610.51 (s, C=O), 1555.82 (m, C=C), 1308.99 (m, C-N), 1276.64 (m, C-N).
Synthesis of (z)-3-((4’-amino-[1,1’biphenyl]-4-yl) amino)-1-phenylbut-2-en-1-one (L3e1): To a 250 ml round bottomed flask was added phenylacetone, L1e (2.00 g, 12.33 mmol), excess ethanol (50 ml) and 4,4’-Biphenyldiamine (2.27 g, 12.33 mmol). The mixture was heated at 70°C for 10 h. TLC was done to ascertain that the reaction was complete. The ethanol was removed by rotatory evaporation. The crude yellow solid was purified by column chromatography (P.E: E. A=1: 1) and dried in vacuo to afford a pale yellow solid. The product yield was (3.2 g, 60.2%). M.P = 284°C; 1H-NMR (400 MHz, CDCl3, 25°C, δ (ppm)=13.14 (brs, H, NH), 7.93 (dd, 2H, JH-H = 8.0Hz,Ar-H); 7.52 (d, 2H, JH-H =8.0Hz,Ar-H), 7.41 (t, 3H, JH-H = 8.0Hz, Ar-H); 7.33 (d, 2H, JH-H =8.0Hz, Ar-H), 6.735 (dd, 4H, JH-H =8.0Hz, Ar-H), 5.90 (s, 1H, C-H), 3.73 (s, 2H, NH2), 2.18 (s, 3H, CH3); 13C-NMR (400 MHz, CDCl3, 25°C, δ (ppm)=188.58, 162.26, 146.05, 140.09, 138.66, 136.88, 130.90, 128.31, 127.82, 127.31, 127.09, 126.92, 124.92, 115.46, 94.31, 20.57. ESI-MS m/z (%): calculated 329.16, found 329.1653 (M++H, 100); IR (KBr, cm- 1): 3435.28 (br, s, N-H), 2993.34 (w, =C-H), 1608.72 (s, C=O), 1585.83 (s, C=C), 1305.10 (s, C-N), 1266.28 (s, C-N).
Conclusion
(z)-4-((4-amino-[1,1’biphenyl]-4-yl)amino)pent-3-en-2-one L3c1, (z)-5-((4’-amino-[1,1’biphenyl]-4-yl)amino)hept-4-en-3-one L3d1, (z)-3-((4’- amino-[1,1’biphenyl]-4-yl) amino)-1-phenylbut-2-en-1-one L3e1 were synthesized as three new enaminone ligands based on Benzidine. While we are reporting for the first time the use of Benzidine in enaminone synthesis, it should be noted that the linkage of two phenyl groups in the para position in relation to the amine group makes this particular class of enaminones have several added advantages and prospective applications that have been reserved for the researchers’ interests. Besides that the presence of an amine group leaves room for further functionalization of our products. The remarkable advantages offered by this method presented are use of mild, cheap, an efficient and clean catalyst which favors a green Chemistry approach.
Acknowledgement
The authors thank Petrochemical Research Institute of Petrochina for financial support (Grant: PRIKY16018).
References
- D. Potin, F. Dumas, J. d’Angelo, J. Am. Chem. Soc., 1990, 112, 3483.
- G. Bartoli, C. Cimarelli, E. Marcantoni, G. Palmieri, M. Petrini, J. Org. Chem., 1994, 59, 5328.
- G. Palmieri, C. Cimmerelli, J. Org. Chem., 1996, 61, 5557.
- L.G. Beholz, R. Benovsky, D.L. Ward, N.S. Bata, J.R. Stille, J. Org. Chem., 1997, 62, 1033.
- H.M.C. Ferraz, F.L.C. Pereira, F.S. Leite, Tetrahedron., 1999, 55, 10915.
- O. David, J. Blot, C. Bellec, M.C. Fargeau-Bellassoued, G. Haviari, J. Celerier, P. Lhommet, G.J.C. Gramain, D. Garadette, J. Org. Chem., 1999, 64, 3122.
- C. Alan, . Spivey, R. Srikaran, C.M. Diaper, J. David, D. Turner, Org. Biomol. Chem., 2003, 1638.
- G.W. Wang, C.B. Miao, Green Chem., 2006, 8, 1080.
- M. Xia, B. Wu, G. Xiang, Synth. Commun., 2008, 38, 1268.
- Y.F. Wang, T. Izawa, S. Kobayashi, M. Ohno, J. Am. Chem. Soc., 1982, 104, 6465.
- J.P. Michael, C.B. Koning, G.D. Hosken, T.V. Stanbury, Tetrahedron., 2001, 57, 9635.
- D.L. Boger, T. Ishizaki, J.R.J. Wysocki, S.A. Munk, P.A. Kitos, O. Suntornwat, J. Am. Chem. Soc., 1989, 111, 6461.
- J.D. White, D.C. Ihle, Org. Lett., 2006, 8, 1081.
- R.R. Dalpozzo, A.D. Nino, M. Nardi, B.A. Procopio, Synthesis., 2006, 7, 1127.
- F. Epifano, S. Genovese, M. Curini, Tetrahedron Lett., 2007, 48, 2717.
- M.R. Mohammadizadeh, A. Hasaninejad, M. Bahramzadeh, Z.S. Khanjarlou, Synth. Commun., 2009, 39, 1152.
- M. Kidwai, S. Bhardwaj, N. Mishra, V. Bansal, A. Kumar, S. Mozumdar, Catal. Commun., 2009, 10, 1514.
- E. Rafiee, M. Joshaghani, S. Eavani, S. Roshidzadeh, Green Chem.,2008, 10, 982.
- D.F. Martin, G.A. Janusonis, B.B. Martin, J. Am. Chem. Soc., 1961, 83, 73.
- A.S.Y. Lee, R.Y. Cheng, Tetrahedron Lett., 1997, 38, 443
- C.A. Brandt, A.C.M.P. D’Silva, C.G. Pancote, C.L. Brito, M.A.B. da Silveria, Synthesis.,2004, 10, 1557.
- M. Nisar, I. Ali, M.R. Shah, A. Badshah, M. Qayum, M. Zia-Ul-Haq, U. Rashid / iful Islam. Molecules., 2013, 18, 1420-3049, 15182-15192.
- A.R. Gholap, J. Mol. Catal. A. Chem., 2006, 245 37-46.
- B. Sandip Rathod, K. Machhindra Lande, R. Balasaheb Arbad, B. Anil Gambhire, Arabian J. Chem., 2014, 7, 253-260.