Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 11
Theoretical and Spectral Investigation of Some Schiff Bases
Preethi B1,2, Sathiyaseelan M3*, Prabakaran K3, Kutti Rani S1, Sivashanmugam G4 and Anbu Srinivasan P4
1Department of Chemistry, B.S. Abdur Rahman Crescent Institute of Science and Technology, Chennai, India
2Department of Chemistry, Anand Institute of Higher Technology, Chennai, India
3Department of Chemistry, Annai College of Arts & Science, Kovilacheri Tamil Nadu, India
4Department of Chemistry, A.V.C College, Mayiladuthurai, Tamil Nadu, India
- *Corresponding Author:
- Sathiyaseelan M
Department of Chemistry
Annai College of Arts & Science
Kovilacheri Tamil Nadu, India
Abstract
ABSTRACT Complex (experimental and computational) investigation of the spectral and conformational analysis of N1,N2-bis(-3-phenylallylidene)ethane-1,2-diamine based schiff bases. Assignments were made in accordance with the calculated and experimental spectra. The experimental geometry is compared with the results of theoretical calculations.
Keywords
Schiff base, N1,N2-bis(-3-phenylallylidene)ethane-1,2-diamine, Spectra and theoretical studies, Conformational analysis.
Introduction
Schiff bases are well-known demanding area of research due to their simple synthesis, adaptability and different range of applications. These types of chemical ligands are wide use in food industry, dye industry analytical chemistry, catalysis, fungicidal, agrochemical and biological activities [1-5], DNA cleavage activity [6], biomimetic enzyme models [7-9], tumor growth inhibitors, and ability to reversibly binding oxygen [10], liquid crystal [11,12]. Lastly, we expected to find how far experimental findings are reflected in theoretical predictions and what kind of information can be extracted from spectroscopic investigations. However, the usefulness of electronic spectroscopy in studies of tautomeric phenomena of this group of compounds can be studied [13-16]. The tools of computational chemistry have been used to investigate the chemical behavior and different reactivity parameterized of synthetic derivatives [17,18]. Chemical synthesis reactions required optimized structures of their chemical species and their transition states of synthesis reaction. The structural properties are elementary keys to understand the chemical reactivity during the potential energy surface calculations [19,20].
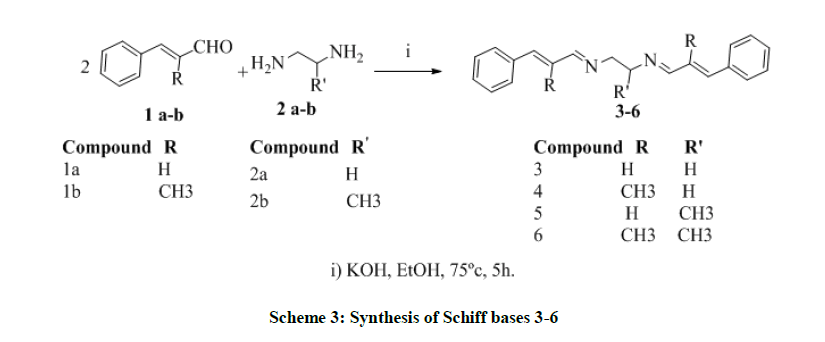
In present work, synthesis reaction for Schiff's base compound, using theaddition product of different aldehyde and amine components and confirm the possible conformations and structure by theoretical and spectral studies.
Material and Methods
Synthesis of N1, N2-Bis(3-phenylallylidene)ethane-1,2-diamine and its derivatives (3-6)
Trans-cinnamaldehyde 1a-b (0.02 mol) was mixed withethylenediamine 2a-b (0.01 mol) in ethanol and catalytic amount of Potassium hydroxide (0.01 mol, 0.39 g) was added and allowed to reflux at 75ºC for 5 h. The progress of the reaction was monitored by using TLC-technique. After completion of the reaction, the condensed material was then poured into ice-cold water; the precipitate was filtered and dried, then purified with column chromatography in petroleum ether elution.
Preparation of N1, N2-Bis(3-phenylallylidene)ethane-1,2-diamine (3)
Yield 2.42 g (85%) Brown semisolid, IR (KBr): 3410, 3028, 2923, 2858, 1632, 1450 cm-1; 1H-NMR (500 MHz, CDCl3): 8.05 (d, 2H, C9 & C14-Hα), 7.46 (m, 4H, C2, C6, C18 & C22-Hortho), 7.36 (m, 4H, C3, C5, C19 & C21-Hmeta), 7.26 (m, 2H, C4 & C20-H para), 6.93 (d, 4H, C7 & C16-Hγ), 6.89 (d, 4H, C8 & C15-Hβ), 3.85(d, 4H, C11 & C12-H); 13C-NMR (125 MHz, CDCl3): 164.2, 141.8, 135.7, 129.1, 128.1, 127.2, 61.70. Mass m/z: 288 Hz.
Preparation of N1, N2-Bis(2-methyl-3-phenylallylidene)ethane-1,2-diamine (4)
Yield 2.39 g (76%) Brown semisolid, IR (KBr): 3420, 3026, 2917, 2855, 1621, 1452 cm-1; 1H-NMR (500 MHz, CDCl3): 7.98 (d, 2H, C9& C14-Hα), 7.37 (m, 4H, C2, C6, C18 & C22-Hortho), 7.36 (m, 4H, C3, C5, C19 & C21-Hmeta), 7.26 (s, 2H, C4& C20-H para), 6.78 (s, 4H, C7 & C16-Hγ), 3.87 (s, 4H, C11& C12-H), 2.13 (d, 6H,J=1.24Hz, C23& C24-H); 13C-NMR (125 MHz, CDCl3): 167.6, 139.0, 138.9, 136.9, 129.4, 128.3, 127.6, 61.5, 13.2. Mass m/z: 317 Hz.
Preparation of N1, N2-Bis(3-phenylallylidene)propane-1,2-diamine (5)
Yield 2.09 g (76%) Brown semisolid, IR (KBr): 3419, 3028, 2922, 2849, 1635, 1449 cm-1; 1H-NMR (500 MHz, CDCl3): 8.03 (d, 1H, C14-Hα), 7.99(d, 1H, C9-Hα ), 7.47.43(d, 4H, Ar-C2, C6 C18 & C22-H)7.38-7.28 (m, 6H, Ar-C3, C4, C5,C19,C20 & C21-H), 6.88-6.91 (m, 8H, C8, C15 Hβ & C7 & C16-Hγ), 3.86-3.56 (m, 3H, C11& C12-H), 1.31 (d, 3H, J=6.15 Hz C23-H); 13C-NMR (125 MHz, CDCl3): 164.2, 162.2, 141.7, 136.3, 135.7, 130.5, 129.1, 128.7, 128.5, 128.1, 128.0, 127.2, 68.0, 66.3, 20.6. Mass m/z: 275 Hz.
Preparation of N1, N2-Bis(2-methyl-3-phenylallylidene)propane-1,2-diamine (6)
Yield 2.13 g (65%) Brown semisolid, IR (KBr): 3397, 3025, 2923, 2853, 1624, 1446 cm-1; 1H-NMR (500 MHz, CDCl3): 7.97 (d, 1H, J=3.92Hz C14-Hα), 7.92 (s, 1H,Hz C9-Hα), 7.60-7.77 (m, 4H, C2, C6, C18 & C22-Hortho), 7.39-7.35 (m, 4H, C3, C5, C19 & C21-Hmeta), 7.25 (s, 2H, C4 & C20-H para), 6.75 (d, 4H, C7 & C16-Hγ), 3.86-3.79 (m, 2H, C11-H), 3.75-3.59 (m, 1H, C12-H), 1.31(d, 3H, J=6.19 Hz, C23-H); 13C-NMR (125 MHz, CDCl3): 167.4, 165.4, 138.9, 138.6, 136.8, 136.7, 130.5, 130.4, 129.3, 128.5, 128.2, 128.0, 127.5, 67.7, 66.2, 20.5, 13.4, 13.2. Mass m/z: 332 Hz.
Theoretical studies
The molecular structures of Schiff bases 3-6 are optimized by HF and B3LYP with the 6-31G (d) basis set. The calculations are performed by using Gauss-View molecular visualization program and Gaussian 03 program package on personal computer.
Results and Discussion
Treatment of ethylenediamine and 1,2-diaminopropane with trans-cinnamaldehyde and trans-methylcinnamaldehyde (mol ratio 1: 2) yielded the corresponding Schiff bases 3-6.
IR, mass and high resolution 1H and 13C-NMR spectra of N1,N2-bis(3-phenylallylidene)-ethane-1,2-diamine and its derivatives (3-6) have been recorded and analyzed.
In IR Spectra the prominent peaks around 1600 cm-1 in the IR spectra are attributed to νC=N mode. The C=C stretching vibration of the aromatic ring appeared around 1500 and 1450 cm-1. The peaks around 750-650 cm-1 are attributed to aromatic C-H out of plane bending vibration. The peaks around 1350 cm-1 in the Schiff bases 5 and 6 are due to the symmetric bending vibration of methyl group. In 1H-NMR, The most downfield doublet centered at 8.05 ppm in the Schiff base 3 is assigned to azomethine protons [H(9) and H(14)]. The coupling constant extracted from this signal is found to be 7.71 Hz. In the 1H-1H COSY spectrum, this doublet exhibits cross peaks with the signals in the region 6.96-6.86 ppm. From this it is concluded that H(8) and H(15) [β-protons i.e., β with respect to azomethine nitrogen] resonate in this region (6.96-6.86 ppm). From the integral values and from the absence of extra correlation in 1H-1H COSY spectrum it is concluded that the γ protons [H(7) and H(16)] are also observed in the same region. Careful analysis of the signals in this region reveals that for γ-protons a doublet at 6.93 ppm with spacing 15.93 Hz is observed and for β protons a doublet of doublet at 6.89 ppm was observed. From the comparison of the chemical shifts of Schiff base 3 with those of N(1), N(2) bis(cinnamylidene) azine [21,22], it is found that the ortho-protons in the cinnamylidene ring resonate at 7.46 ppm (doublet with spacing 7.23 Hz) and for meta and para-protons of the cinnamylidene ring signals are observed at 7.36 and 7.26 ppm in 3. The upfield sharp singlet at 3.85 ppm, is assigned to methylene protons attached to the azomethine nitrogen [H(11) and H(12)].
13C-NMR spectrum shows an upfield signal at 61.70 ppm and this is assigned to the methylene carbon attached to azomethine nitrogen [C(11) and C(12)]. The most downfield signal at 164.2 ppm is obviously due to azomethine carbons [C(9) and C(14)]. From the correlation peaks observed in the 1H-13C COSY spectrum, it is found that β [C(8) and C(15)] and γ [C(7) and C(16)] carbons resonate at 128.8 and 141.8 ppm respectively. The less intense signal at 135.7 ppm is assigned to ipso carbons [C(1) and C(17)] of the phenyl ring of the cinnamylidene moiety. The remaining signals are due to aromatic carbons of the phenyl ring of the cinnamylidene moiety.
The 1H-NMR spectrum of the Schiff base 4 reveals a sharp singlet at 7.98 ppm for azomethine protons. The upfield sharp singlet at 3.87 ppm is assigned to methylene protons attached to azomethine nitrogen. The spectrum also reveals a doublet with spacing 1.24 Hz at 2.13 ppm. The small spacing indicates the long range coupling observed with this proton and this is assigned to the methyl protons present in the side chain of the cinnamylidene moiety. From the comparison of chemical shifts of Schiff base 4 with those of Schiff base 3 it is predicted that the γ protons resonate at 6.78 ppm. The ortho and meta protons of the phenyl ring of the cinnamylidene moiety are observed at 7.37 and 7.36 ppm. The signal at 7.26 ppm is assigned to the para-proton of the phenyl ring of the cinnamylidene moiety. 1H-1H COSY spectrum, exhibits a weak cross peak between the methyl signal at 2.13 ppm with a signal at 6.78 ppm which corresponds to H(7) and H(16) [H(γ)] protons. The 13C NMR spectrum of the Schiff base 4 reveals signals at 61.5 and 13.2 ppm for the methylene carbons attached to azomethine nitrogen [C(11) and C(12)] and methyl carbons [C(23) and C(24)] present in the side chain respectively. The most downfield signal at 167.6 ppm is obviously due to azomethine carbons [C(9) and C(14)]. The signal at 139.0 ppm is assigned to the γ carbon [C(7) and C(16)] of the side chain moiety. The signal at 138.9 ppm is assigned to β carbons [C(8) and C(15)] The less intense signal at 136.9 ppm is due to ipsocarbons [C(1) and C(17)] of the phenyl rings of the cinnamylidene moiety. The remaining signals in the spectrum are due to the remaining aromatic carbons. This assignment is further confirmed from the results derived from 1H-13C COSY spectrum. The signal for azomethine protons (7.98 ppm) exhibits cross peak with the signal at 167.6 ppm, thus confirming the assignment of signal at 167.6 ppm to azomethine carbons. The signal centered at 6.78 ppm which is assigned to γ proton shows correlation with the signal at 139.0 ppm. From this it is confirmed that γ carbon signal was observed at 139.0 ppm. The upfield sharp singlet at 3.87 ppm exhibits cross peak with the signal at 61.5 ppm. From this it is confirmed that methylene carbons of the ethane moiety resonate at 61.5 ppm. The signal centered at 2.13 ppm, which is assigned to methyl protons shows correlation with the signal at 13.2 ppm, thus confirming the signal at 13.2 ppm to methyl carbons [C(23) and C(24)].
1H-NMR spectrum reveals the presence of two doublets at 1.31 and 1.37 ppm for methyl protons of 1,2-diaminopropyl moiety and four signals for azomethine protons in the region 8.0-8.4 ppm. This clearly shows that the Schiff base 5 exists in two isomeric forms. The signals of the azomethine protons and the methyl protons of the diaminopropyl moiety in the major isomer can be differentiated from the minor isomer based on intensities. However, for other protons overlapping of signals for the major and minor isomer occurs. In the major isomer, the doublets observed at 8.03 and 7.99 ppm are assigned to azomethine protons [H(9) and H(14)]. The coupling constants extracted from these signals are found to be 7.55 and 8.05 Hz respectively. The doublet at 1.31 ppm is assigned to the methyl protons of the 1,2-diaminopropyl moiety. The coupling constant extracted from this signal is found to be 6.15 Hz. Among the signals for azomethine protons at 8.03 and 7.99 ppm the one at higher frequency i.e. 8.03 ppm is assigned to H(14). This assignment is based on the following observations. Among the azomethine carbons C(14) is γ with respect to the CH3 group at C(12) whereas C(9) is δ. Generally γ carbon is expected to resonate at lower frequency [23]. Therefore, the signals for azomethine carbons are first assigned and from the 1H-13C COSY spectrum, the corresponding proton resonances are assigned. Obviously, the remaining azomethine proton signal at 7.99 ppm is due to H(9) only.
In the minor isomer, the azomethine protons resonate at 8.29 ppm (doublet) [H(14)] and 8.24 ppm (singlet) [H(9)]. The coupling constant extracted from the doublet centered at 8.29 ppm is found to be 3.60 Hz. The methyl protons are observed at 1.37 ppm. The coupling constant extracted from this signal is found to be 6.20 Hz. The 1H-NMR spectrum reveals overlapping of signals of the other protons of major isomer with the minor isomer. The signals in the region 3.86-3.56 ppm are due to methine and methylene protons of the 1,2-diaminopropyl moiety [N-CH and N-CH2]. The signals in the region 6.88-6.91 ppm are due to β [H(8) and H(15)] and γ [H(7) and H(16)] protons of the side chain of cinnamylidene moiety. The spectrum reveals a triplet at 1.31 ppm (two doublets overlapped with each other) and this is assigned to the methyl protons of 1,2-diaminopropyl moiety of the dimer of 5A. The signals of other protons in the dimer are however overlapped with the signals of the Schiff base 5. 13C-NMR spectrum of the Schiff base 5 reveals signals at 20.6 and 29.7 ppm for the methyl carbons of the 1,2-diaminopropyl moiety. The most downfield signals at 164.2, 162.3, 162.2 and 160.5 ppm are obviously due to azomethine carbons present in the major and minor isomer. The β-carbon signals are overlapped with the aromatic signals in the region 128.1-130.6 ppm. The signals at 141.8 and 141.7 ppm are assigned to γ carbons of the cinnamylidene moiety. The remaining signals in the region 137-127 ppm are due to aromatic carbons. The signals in the region 68-66 ppm are due to methine and methylene carbons of the 1,2-diaminopropyl moiety. The signals for the major isomer can be differentiated from the minor isomer based on intensities. This assignment is further confirmed from the results derived from 1H-13C COSY spectrum. The signals for azomethine protons centered at 8.03 and 7.99 ppm in the major isomer exhibit cross peaks with the signals at 162.2 and 164.2 ppm respectively. The signals for azomethine protons in the minor isomer (8.29 and 8.24 ppm) show cross peaks with the signals at 160.5 and 162.3 ppm. From the correlation peaks it is confirmed that azomethine carbons resonate at 164.2 and 162.2 ppm in the major isomer and 160.5 and 162.3 ppm in the minor isomer. The signals in the region 6.88-6.91 ppm which are assigned to β and γ protons show correlation with the signals at 141.8 and 141.7 ppm and the signals in the region 128.1-130.6 ppm. From this it is confirmed that γ carbons resonate at 141.8 and 141.7 ppm and β carbons resonate in the region 128.1-130.6 ppm. The signals in the region 3.86-3.56 ppm which are assigned to methine and methylene protons of the diaminopropyl moiety show correlation with the signals at 68.0, 67.9, 66.3 and 66.2 ppm. From these correlations it is established that methine carbons of the diaminopropyl moiety absorb at 68.0 ppm in the major isomer and 67.9 ppm in the minor isomer. The remaining two signals at 66.3 and 66.2 ppm are assigned to methylene carbons of the diaminopropyl moiety in the major and minor isomer respectively. The methyl carbons of the diaminopropyl moiety absorb at 20.6 ppm in the major isomer and 29.7 ppm in the minor isomer and this is confirmed by the cross peaks observed with the doublets at 1.31 (major) and 1.37 ppm (minor). Two doublets at 1.31 and 1.35 ppm for methyl protons of 1,2-diaminopropyl moiety and four signals for azomethine protons in the region 7.9-8.3 ppm are observed in the 1H-NMR spectrum. From this it is concluded that the Schiff base 6 also exists in two isomeric forms. Based on intensities the signals corresponding to major isomer can be differentiated from the minor isomer. The doublet at 7.97 ppm and a singlet at 7.92 ppm observed in the major isomer are assigned to azomethine protons. The coupling constant extracted from the doublet is found to be 3.92 Hz. The spectrum shows a doublet at 1.31 ppm with spacing 6.19 Hz for the methyl protons of 1,2-diaminopropyl moiety in the major isomer. However, the methyl protons of cinnamylidene moiety absorb at 2.12 ppm. Among the signals for azomethine protons at 7.97 and 7.92 ppm the one at higher frequency i.e., 7.97 ppm is assigned to H (14).
In the minor isomer the azomethine protons absorb at 8.28 (doublet, J=6.13 Hz) [H(14)] and 8.23 ppm (singlet) [H(9)] and methyl protons resonate at 1.35 ppm (JH,CH3 =6.34 Hz). The methyl protons of cinnamylidene moiety resonate at 2.09 ppm. From the comparison of the chemical shifts of 6 with those of 3-5 it is suggested that the γ protons resonate at 6.75 (major) and 6.71 ppm (minor). The signals in the region 3.59-3.75 ppm and 3.79-3.86 ppm are assigned to methine and methylene protons of diaminopropyl moiety in both the major and minor isomers. This assignment is based on the results obtained in 1H-1H COSY spectrum. In the 1H-1H COSY spectrum cross peaks are observed between the doublets at 1.31 and 1.35 ppm (methyl protons) with the multiplet in the region 3.59-3.75 ppm. From this it is confirmed that the methine protons resonate in the region 3.59-3.75 ppm. Obviously the other multiplet (3.79-3.86 ppm) is due to methylene protons. The spectrum reveals additional signals for the dimer of trans-α-methyl cinnamaldehyde in the region 2.1 ppm and aromatic regions. The downfield signals observed at 167.4 and 165.4 ppm (high intense signals) and at 162.4 and 160.5 ppm (less intense signals) in the 13C-NMR spectrum are assigned to azomethine carbons in the major and minor isomer respectively. The signals observed at 138.9 and 138.6 ppm are assigned to γ carbons in the major isomer. In the minor isomer γ carbons resonate at 139.0 and 138.6 ppm. The β carbons resonate at 136.7 and 136.8 ppm in the major isomer and at 136.9 and 137.0 ppm in the minor isomer. The high intense signals resonating at 66.2 and 67.7 ppm are assigned to the methylene and methine carbons of diaminopropyl moiety in the major isomer and in the minor isomer these carbons resonate at 66.5 and 67.5 ppm. The spectrum also reveals signals at 13.4 and 13.2 ppm. These signals are due to methyl carbons of the cinnamylidene moiety and both the major and minor isomers absorb in the same region. The signals at 20.5 and 20.4 ppm are obviously due to methyl carbons of the diaminopropyl moiety. These assignments were further confirmed from the results derived from 1H-13C COSY spectrum. The signals for azomethine protons centered at 7.97 and 7.92 ppm in the major isomer exhibit cross peaks with the signals at 165.4 and 167.4 ppm respectively. The signals for azomethine protons in the minor isomer (8.28 and 8.23 ppm) show cross peaks with the signals at 160.5 and 162.4 ppm respectively. From this it is confirmed that azomethine carbons resonate at 165.4 and 167.4 ppm in the major isomer and 160.5 and 162.4 ppm in the minor isomer. The signals at 6.75 and 6.71 ppm, which were assigned to γ–protons in the major and minor isomer show correlation with the signals in the region 138.6-139.0 ppm thus confirming the γ-carbon signals at 138.9 and 138.6 ppm in the major isomer and at 139.0 and 138.6 ppm in the minor isomer. The signals in the region 3.59-3.75 ppm and 3.79-3.86 ppm which were assigned to methine and methylene protons of diaminopropyl moiety exhibit correlation peaks with the signals in the region 67.7 to 66.2 ppm, thus confirming the methine and methylene carbon signals in the region 67.7-66.2 ppm. The signals at 20.5 (major) and 20.4 ppm (minor) are assigned to methyl carbons of diaminopropyl moiety based on the correlation peaks observed with the doublets at 1.31 and 1.35 ppm. The remaining signals in the region 13 ppm are due to methyl carbons of the cinnamylidene moiety.
Conformation of schiff bases
Spectral studies
In the Schiff base 3 the azomethine proton is associated with the coupling of 7.71 Hz which is closer to the value observed in trans-cinnamaldehyde 1a [21]. There are two possible ways of attaching the ethylene moiety to the nitrogen (i) syn to azomethine proton, (ii) anti to azomethine proton.
Comparison of the chemical shifts of aldehydic proton in cinnamaldehyde (9.66 ppm) with the azomethine protons in the Schiff base 3 (8.05 ppm) reveals that conversion of the aldehyde to Schiff base shields –CH proton by 1.61 ppm. Such a large shielding magnitude suggests that the azomethine proton should be syn to the N-C(H2) bond in the Schiff base. This conclusion is based on the following observations.

Generally in Schiff bases and azines protons which are syn to N-C/N-N bond are expected to resonate at lower frequency relative to anti protons. For example in acetone azine [24] methyl protons which are syn to N-N bond (1.83 ppm) resonate at lower frequency compared to methyl protons anti to N-N bond (2.00 ppm) and the chemical shifts of antiprotons are closer to the values observed in acetone (2.09 ppm). Moreover, comparison of chemical shifts of aldehydic proton in salicylaldehyde (9.89 ppm) [21,22] with that of azomethine proton in the Schiff base (8.70 ppm) [25] reveals considerable shielding (-1.19 ppm) due to conversion of aldehyde to Schiff base. The crystal structure of the Schiff base reveals that the azomethine proton is syn to C-aryl bond only. Therefore, the favored conformation of Schiff base 3 is predicted to be the trans conformation as shown below.

One can visualize three possible conformations of the Schiff base 3 due to C-C rotation about –N-CH2-CH2-N moiety. The two gauche forms A and C are destabilized due to severe interaction between the substituent attached to azomethine nitrogen. Therefore, the stable conformation of the Schiff base is predicted to be the antiform. Similar comparison of the chemical shifts of azomethine protons in the Schiff bases 4-6 with those of aldehydic protons in trans-cinnamaldehyde (9.66 ppm) and trans-α-methylcinnamaldehyde [24] (9.56 ppm) reveals considerable shielding of CH protons due to conversion of aldehyde to Schiff bases. The shielding magnitude observed ware closer to the values observed in the Schiff base 3. Hence, it is reasonably concluded that the conformations of Schiff base 4 and major isomers of 5 and 6 are similar to the conformation of Schiff base 3. The minor isomers of the Schiff bases 5 and 6 probably exist in the gauche conformation. In 5 both the gauche conformations are expected to be of the same energy and hence equally populated. However in the Schiff base 6, the gauche conformation C′ is ruled out since in this confirmation two gauche interactions exists. Hence, the favored conformation of the minor isomer is predicted to be A′. The major isomer exists in conformation B′.
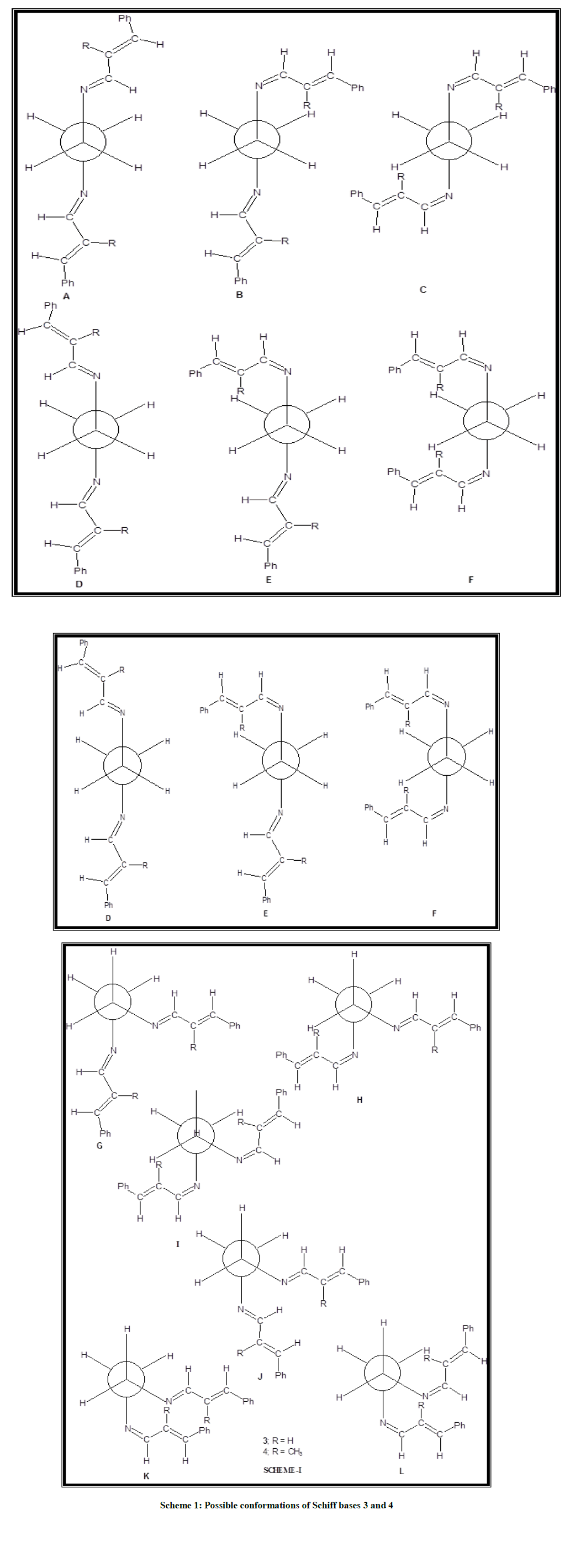
Theoretical studies of the Schiff bases 3 and 4
There are several conformations possible for the Schiff bases 3 and 4. Since, trans-cinnamaldehydes are used for synthesis the conformations derived from cis-cinnamaldehydes are ruled out in the present study. In conformations G-L in Scheme 1 the two C-N bonds are gauche with respect to each other, whereas in conformations A-F, the two C-N bonds are anti to each other. In the gauche conformation the torsional angle is close to 60°, whereas in the anti-conformation, the angle is close to 180°. In the conformations A-C the two C=N bonds are on opposite side to each other whereas in conformations D-F the two C=N bonds are on same side. Same trend is also seen in conformations G-I relative to J-L. Both the azomethine protons are syn to N-C(H2) bond in the conformations A, D, G and J i.e., syn-syn arrangement, whereas they are anti in conformations C, F, I and L i.e., anti-anti arrangement. In conformations B, E, H and K one of the azomethine protons is syn to N-C(H2) bond and the other azomethine proton is anti to N-C(H2) bond i.e., syn-anti arrangement. Density functional theory calculations available in Gaussian-03 package were carried out using the basis set B3LYP/6-31G [26,27]. The relative SCF energy values determined for the Schiff bases 3 and 4 are shown in Table 1. Tables 2 and 3 report the selective bond length, bond angle and torsional angles in the optimised structures 3A and 4A.
| Conformers | SCF energy (Kcal/mol) | |
|---|---|---|
| 3 | 4 | |
| A | 0 | 0 |
| B | 3.31 | 8.35 |
| C | 6.41 | 16.29 |
| D | 0 | 0 |
| E | 3.29 | 8.35 |
| F | 6.42 | 17.43 |
| G | 3.53 | 3.23 |
| H | 7.67 | 12.93 |
| I | 11.68 | 23.5 |
| J | 1.02 | 1.01 |
| K | 4.25 | 10.03 |
Table 1: SCF energy values for the Schiff bases 3 and 4
| Bond length (Å) | Bond angle (°) | Torsional angle (°) | |||
|---|---|---|---|---|---|
| C1-C7 | 1.46 | C1-C7-C8 | 127.6 | C6-C1-C7-C8 | 179.9 |
| C7-C8 | 1.35 | C7-C8-C9 | 122.6 | C2-C1-C7-C8 | -0.1 |
| C8-C9 | 1.45 | C8-C9-N10 | 121.7 | C1-C7-C8-C9 | -179 |
| C9-C10 | 1.29 | C9-N10-C11 | 119.3 | C7-C8-C9-N10 | 179.8 |
| N10-C11 | 1.46 | N10-C11-C12 | 109.7 | C8-C9-N10-C11 | -179 |
| C11-C12 | 1.54 | C9-N10-C11-C12 | -126 | ||
| N10-C11-C12-N13 | -171 | ||||
Table 2: Selected geometric parameters of N1, N2-bis(3-phenylallylidene)ethane-1,2-diamine 3
| Bond length (Å) | Bond angle (°) | Torsional angle (°) | |||
|---|---|---|---|---|---|
| C1-C7 | 1.46 | C1-C7-C8 | 131 | C6-C1-C7-C8 | -159 |
| C7-C8 | 1.36 | C7-C8-C9 | 116.6 | C2-C1-C7-C8 | 21.5 |
| C8-C9 | 1.46 | C7-C8-C23 | 126.4 | C1-C7-C8-C9 | -179 |
| C8-C23 | 1.5 | C23-C8-C9 | 116.9 | C1-C7-C8-C23 | 1.59 |
| C9-C10 | 1.28 | C8-C9-N10 | 123 | C7-C8-C9-N10 | -178 |
| N10-C11 | 1.46 | C9-N10-C11 | 119.5 | C23-C8-C9-N10 | 179.7 |
| C11-C12 | 1.5 | N10-C11-C12 | 109.8 | C8-C9-N10-C11 | 0 |
| C9-N10-C11-C12 | -125 | ||||
| N10-C11-C12-N13 | -172 | ||||
| C11-C12-N13-C15 | -125 | ||||
| C12-N13-C14-C15 | 179.7 | ||||
| N13-C14-C15-C24 | 0 | ||||
| N13-C14-C15-C16 | -178 | ||||
Table 3: Selected geometric parameters of N1, N2-bis(2-methyl-3-phenylallylidene)-ethane-1,2-diamine 4
All the bond angles are closer to 120°, except N(10)-C(11)-C(12) or N(13)-C(12)-C(11) in the Schiff bases 3 and 4. Comparison of bond angles in Schiff base 3 with those of 4 reveals that introduction of methyl substituent in the cinnamylidene moiety influences the bond angle considerably. The torsional angles C(6)-C(1)-C(7)-C(8) (179.9°) and C(2)-C(1)-C(7)-C(8) (0.1°) reveals the coplanar nature of the phenyl ring with the conjugated side chain carbons C(7) and C(8) in the Schiff base 3. However, the corresponding bond angles in the Schiff base 4 (-159.0° and +21.5°) reveals that this planarity is deviated considerably due to the introduction of methyl substituent in the cinnamylidene moiety. However, the torsional angle N(10)-C(11)-C(12)-N(13) is not affected due to the introduction of CH3 substituent (171° in 3; 172° in 4). The other torsional angles are closer to 180° in both the Schiff bases 3 and 4 except C(9)-N(10)-C(11)-C(12)/C(11)-C(12)-N(13)-C(14) (126° in 3 and 125° in 4). These values suggest nearly coplanar nature of other moieties with the nearby nitrogen and the adjacent sp2 hybridized carbons except the ethylenic moiety. Thus, the theoretical study predicts the same conformations as the one derived from solution studies. Similar conformation has also been reported in a closely related N,N′-bis-(4-methoxybenzylidene)-ethylenediamine by Unaleroglue, et al. [28].
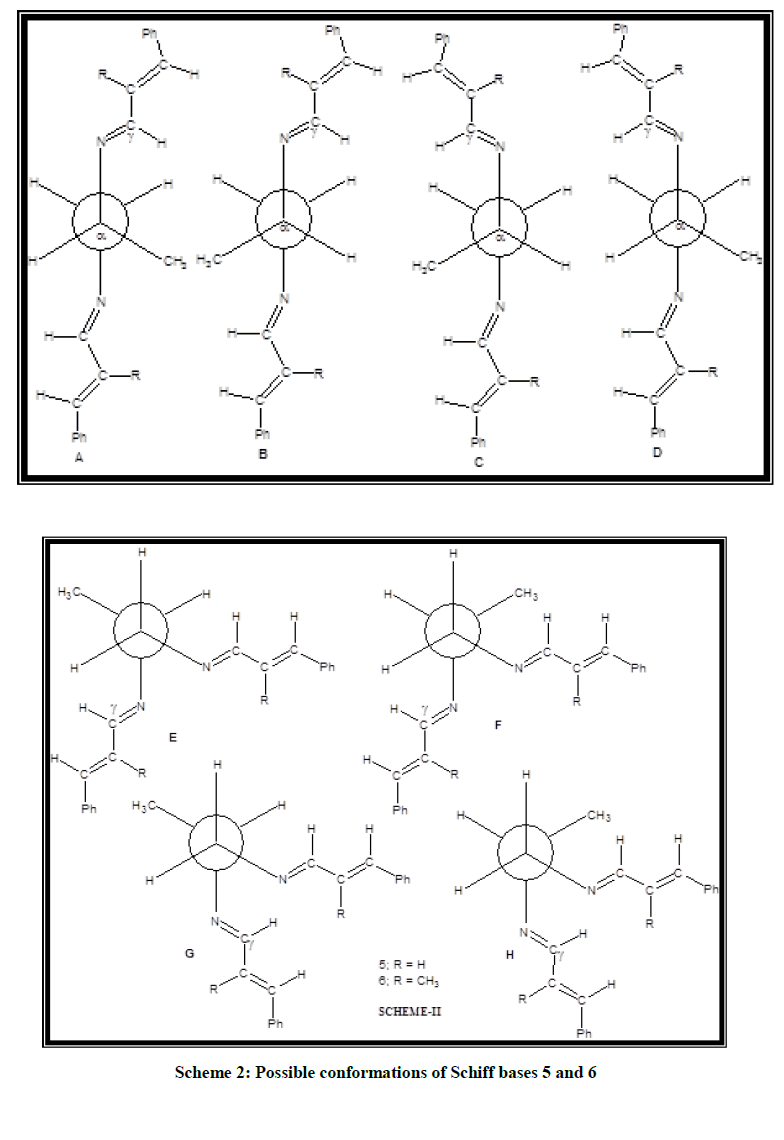
Theoretical studies of the Schiff bases 5 and 6
From the theoretical study of the conformations of the Schiff bases 3 and 4 it is inferred that conformations in which both the azomethine protons syn to N-C(H2) bond are more stable when compared to syn-anti and anti-anti arrangements. Therefore, for the Schiff bases 5 and 6 calculations were performed only for conformations in which both the azomethine protons are syn to N-C(H2) bond. The possible conformations for the Schiff bases 5 and 6 are shown in Scheme 2. Calculations were performed for all the conformations shown in Scheme 3 and the relative SCF energies calculated for the Schiff bases 5 and 6 are displayed in Table 4. For the Schiff base 5 theoretical calculations predict 5B or 5D as the minimum energy conformer and it is found to be the favored conformation of the major isomer. The optimized structure corresponding to 5B is similar to 5D. The argument given for Schiff bases 3 and 4 is applicable for the Schiff base 5 also. Therefore, in the favorable conformer of the major isomer of 5 (5B or 5D) the plane of the cinnamylidene moiety at one nitrogen is nearly perpendicular to the plane of the cinnamylidene moiety at the other nitrogen. The next favorable conformer is predicted to be 5G in which the two C=N bonds are gauche with respect to each other and methyl group is anti to azomethine nitrogen. It is seen from Table 4, that the favorable conformer for the major isomer of the Schiff base 6 is predicted to be 6B or 6D. The next favorable conformer (conformation of the minor isomer) is predicted to be 6A or 6C. Similar to the Schiff base 5 the optimized structure corresponding to 6B is similar to 6D and 6A is similar to 6C. In these conformers the plane of the cinnamylidene moiety at one nitrogen is nearly perpendicular to the plane of the cinnamylidene moiety at the other nitrogen. The conformation of the minor isomer of the Schiff base 6 (6A/6C) is different from the conformation of the minor isomer of the Schiff base 5. The gauche conformer of the Schiff base 6, i.e., 6G is expected to be of higher energy compared to the gauche conformer 5G due to severe interaction between the methyl group in the cinnamylidene moiety with the nearby azomethine proton at the other end and hence it is destabilized in the Schiff base 6.
| Conformers | SCF energy (Kcal/mol) | |
|---|---|---|
| 5 | 6 | |
| A | 1.76 | 1.75 |
| B | 0 | 0 |
| C | 1.76 | 1.75 |
| D | 0 | 0 |
| E | 6.29 | 5.84 |
| F | 3.53 | 3.14 |
| G | 0.93 | 3.33 |
| H | 3.31 | 3.25 |
Table 4: SCF energy values for Schiff bases 5 and 6
Conclusion
It is conclude that, Calculation reveals that the stable conformer is predicted to be either 3A or 3D. The optimised structure corresponding to 3A is similar to 3D. Table 1 inferred that the conformations in which both the azomethine protons syn to N-C(H2) bond i.e., syn-syn arrangement (3A, 3D, 3G and 3J) are more stable when compared to syn-anti (3B, 3E, 3H and 3K) and anti-anti arrangements (3C, 3F, 3I and 3L). The order of stability is syn-syn>syn-anti >anti-anti. For the Schiff base 4 also the stable conformers is predicted to be 4A or 4D similar to the Schiff base 3. The Schiff base 5 exists as an equilibrium mixture of conformer 5B/5D (major isomer) and 5G (minor isomer) only. The Schiff base 6 exists as an equilibrium mixture of conformations of 6B/6D (major isomer) and 6A/6C (minor isomer). Thus, the conformations of Schiff bases 3-5 predicted from theoretical calculations are in good agreement with the conformations predicted from spectral data. The conformation of the major isomer of Schiff base 6 predicted in solution is the same as that observed by theoretical calculation. However, for the minor isomer there is a discrepancy between the conformations predicted in solution (gauche conformation) and by theoretical studies (trans conformation).
Acknowledgement
The authors are thankful to Indian Institute of science, Bangalore for spectral assistance and B.S. Abdur Rahman Crescent Institute of Science and Technology for Sophisticated lab facility.
References
- N. Mahalakshima, R. Rajavel, Arab. J. Chem., 2014, 7, 509.
- M.V. Lokhandeh, M.R. Chaoudhary, Inter. J. Pharm. Sci. Res., 2014, 5(5), 1757.
- L. Li, Q. Guo, J. Dong, T. Xu, J. Li, J. Photochem. Photobiol., 2013, 56, 125.
- G. Geindy Mohamed, M.M. Omar, A. M. Hindy, Turk. J. Chem., 2006, 30, 361.
- T. Priya Devi, R.K. Hemakumar Singh, Rasayan. J. Chem., 2010, 3(2), 266.
- N. Shahabadi, S. Kashanian, F. Darabi, Eur. J. Med. Chem., 2010, 45, 4239.
- S.R. Collinson, D.E. Fenton, Coord. Chem. Rev., 1996, 19, 148.
- M.L.P. Santos, I.A. Bagatin, E.M. Pereira, A.M.C. Ferreira, J. Chem. Soc., Dalton Trans.,2001, 838.
- H.S. He, D.T. Puerta, S.M. Cohen, K.R. Rodgers, Inorg. Chem., 2005, 44, 7431.
- S. Park, V.K. Mathur, R.P. Planap, Polyhedron, 1998, 17, 325.
- F. Yuksel, D. Atilla, V. Ahsen, Polyhedron, 2007, 26, 4551.
- B.Y. Zhang, F.B. Meng, M. Tian, W.Q. Xiao, Teact. Funct. Polymer, 2005, 66, 551.
- H. Ebead, A.D. Roshal, A. Wróblewska, A.O. Doroshenko, Blazejowski, J. Spectrochim. Acta A,2007, 66, 1016.
- H. Ebead, R.F. Fandy, S.E. Zayed, E. Abd-Elshafi, S.A. Ibrahim, Can. J. Anal. Sci. Spectrosc., 2009,53(6), 274.
- H.M.A. Salman, Can. J. Anal. Sci. Spectrosc., 2000, 45(5&6), 117.
- H. Dal, Y. Süzen, E. Şahin, Spectrochim. Acta A.,2007, 67, 808
- A. Dearing, J. Computer-Aided Molec. Design,1988, 2, 179.
- P. Gund, D.C. Barry, J.M. Blaney, N.C. Cohen, J. Med. Chem., 1988, 31, 2230.
- A.R. Katritzky, Z. Wang, R. J. Offerman, J. Heterocyclic Chem., 1990, 27, 139.
- C.H. Zhengming, Y. Itan, J. Agr. Food Chem., 2000, 48, 5312.
- S. Vijayalakshmi, M.Phil. Thesis, Annamalai University, Tamil Nadu, india, 2008.
- SDBS web: http//riodbol.ibase.aist.go.jp/sdbs/
- H. Friebolin, Basic one and two-dimensional NMR spectroscopy, 5th (Edn.), New York, VHC Publishers, 2010, 1-442.
- E. Kolehmainen, K. Loihia, P. Manttari, Magn. Res. Chem.,1991, 29, 1109.
- H. Unver, E. Kendi, K. Guven, T.N. Durlu, Z. Naturforsch.,2002, 57b, 685.
- A. Frish, A.B. Nielson, A.J. Holder, Gaussview user manual, Pittsburg: Gaussian Inc., 2001.
- M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, V.G. Zakrzewski, J.A. Montgomery Jr., R.E. Stratmann, J.C. Burant, S. Dapprich, J.M. Millam, A.D. Daniels, K.N. Kudin, M.C. Strain, O. Farkas, J. Tomasa, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomeli, C. Adamo, S. Clifford, J. Ochterski, G.A. Peterson, P.Y. Ayala, Q. Cui, K. Morokuma, P. Salvador J.J. Dannenberg, D.K. Malick, A.D. Rabuck, K. Raghavachari, J.B. Poresman, J. Cioslowski, J.N. Ortiz, A. G. Babboul, B.B. Stefavov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R.L. Martin, D.J. Fox, T. Keeth, M.A. Allaham, C.Y. Peng, A. Nanayakkara, M.W. Wong, J.L. Anders, C. Gonzales, M. Challacombe, P.M. Gill, B. Johnson, W. Chen, M. Head-Gordon, E.S. Replogle, J.A. Peple, Gausiyen 98, Revision A. 9, Pittsburg, Pa: Gaussian IC., 2001.
- C. Unaleroglu, B. Temelli, T. Hokelek, J. Mol. Struct.,2001, 570, 91.