Research Article - Der Pharma Chemica ( 2024) Volume 16, Issue 1
To Evaluate the Interaction of Zandopa® Powder and Parkitidin Tablet in Haloperidol Induced Catalepsy Model in Rat
Ravi A Vyas and Swati R Dhande*Swati R Dhande, Department of Pharmacology, Bharati Vidyapeeth College of Pharmacy, Navi Mumbai, India, Email: swati.dhande@bvcop.in
Received: 07-Dec-2023, Manuscript No. Dpc-23-122354; Editor assigned: 12-Dec-2023, Pre QC No. Dpc-23-122354 (PQ); Reviewed: 26-Dec-2023, QC No. Dpc-23-122354; Revised: 02-Jan-2024, Manuscript No. Dpc-23-122354 (R); Published: 23-Jan-2024, DOI: 10.4172/0975-413X.16.1.166-171
Abstract
Herb Drug Interaction (HDIs) is defined as an interaction between herbal medicinal products and allopathic drugs when administered concomitantly. HDIs alter the body’s function pharmacodynamically and pharmacokinetically. The study concerned on herb drug interaction of Zandopa® powder (Mucuna pruriens) and parkitidin tablet (Amantadine Hydrochloride) in haloperidol-induced catalepsy model in rats. The rats were divided into 5 groups namely group I vehicle control (1% Carboxy Methyl Cellulose (CMC)), group II disease control (1% Carboxy Methyl Cellulose (CMC), group III Zandopa® powder (775 mg/kg of body weight), group IV Parkitidin tablet (10 mg/kg of body weight) and group V combination (Zandopa® powder 775 mg/kg and Parkitidin tablet 10 mg/kg of body weight). Behavioral parameters i.e., muscle rigidity and locomotor activity and biochemical parameters i.e., (MDA, CAT and GSH) were evaluated. The result of the study combination group showed significant (p<0.05) improvement in fall off latency and locomotor activity when compared to the disease group. Biochemical parameters i.e., MDA level significant (p<0.05) decrease, GSH level significant (p<0.05) increase and CAT level showed non-significant effect when compared to the disease group, so it may be concluded that the combination group (Zandopa® powder 775 mg/kg and Parkitidin tablet 10 mg/kg of body weight) showed good additive effect and both can be given concomitantly.
Keywords
Zandopa® powder; Parkitidin; Catalepsy; Haloperidol
Introduction
Ayurveda is an ancient Indian, natural system of medicine practiced for over 5000 years. Ayurvedic treatment is the holistic treatment involving the use of body, mind and spirit in the prevention and treatment of illness. In Ayurveda, most of the disease concerning the nervous system is called ‘Vata’. Neurodegenerative disorders comprise a condition where nerve cells of various parts of the brain and spinal cord cells degenerate resulting in either functional loss or sensory dysfunction. It is also known as ‘Kampavata’ which is caused by an imbalance of Vata [1].
Herb-Drug Interaction (HDIs) is defined as an interaction between herbal medicinal products and allopathic drugs when administered concomitantly. These HDIs can be in terms of pharmacological effects i.e., an additive effect, synergetic effect or antagonist effect. HDIs are classified into two types like pharmacokinetic HDIs and pharmacodynamics HDIs [2]. Herbal products also have various chemical constituents which can cause changes pharmacodynamically and pharmacokinetically.
Parkinson’s Disease (PD) is the second most prevalent neurological disorder that is characterized by progressive neurodegeneration affecting partial loss of dopaminergic neurons within the substantia nigra parts and the presence of lewy bodies whose primary components include fibrillar synuclein [3]. The term Parkinsonism refers to a clinical syndrome comprising of a mixture of motor problems like bradykinesia, resting tremor and muscle rigidity, loss of postural reflexes and flexed posture [4]. Due to progressive neuronal degeneration, the need for the steady increase in the dose of levodopa is observed, but anti-parkinson drugs like carbidopa, entacapone which acts as catalase in levodopa activity whose dose should be reduced to minimize side effects such as dizziness, blurred vision and hallucination [5]. So, there is substantial scope to avail of the modalities of ayurvedic panchakarma therapies which are effective not only in preventing symptoms of PD but also in controlling the further progression of the disease.
Mucuna pruriens Linn (Fabaceae) is a tropical legume that is native to Southern and Southeast Asian regions. It grows wildly in India. It is preferred as a medicinal plant, since the traditional ayurvedic era for diseases such as diabetes, male infertility, anti-epileptic and Parkinsonism. According to literature studies of Mucuna pruriens extract with some anti-Parkinson drugs such as carbidopa and benserazide have been performed. Mucuna pruriens has been mentioned to reverse neuroleptic induced catalepsy models in animals and found to be clinically useful against Parkinson’s disease [6-8]. Mucuna pruriens seed powder is marketed as a drug formulation under the brand name Zandopa® Powder (Formulation A). It is enriched with Mucuna pruriens that consist of natural levodopa. Formulation A is recommended in the treatment of Parkinsonism. The formulation contains 6-9% by weight natural levodopa [8].
Parkitidin tablet (Formulation B) is one of the formulations of Sun Pharma Limited, which contains Amantadine hydrochloride 100 mg used in the treatment of Parkinson's disease. Earlier studies show amantadine may have a direct or indirect effect on dopamine neurons, but recent studies have demonstrated that amantadine is a weak non-competitive N-Methyl-D-Aspartic Acid receptor antagonist (NMDA). It is indicated in the treatment of idiopathic Parkinson’s disease (paralysis agitans) and symptomatic Parkinsonism [9].
The formulation A contains levodopa which in CNS gets converted into dopamine and parkitidin being NMDA antagonist helps to increase dopamine release and prevents its uptake. Thus, formulation being a brain tonic and herbal formulation can be used consumed concomitantly with formulation B in management of Parkinson’s disease. Hence current study is focused on exploring pharmacodynamics interactions of the both the formulations in the rat model.
Materials and Methods
Drugs and Chemicals
• Haloperidol (Inj. Serenace), batch no. N07S20001, RPG life sciences, C1B-2528, G.I.D.C. estate, Ankleshwar, Distric, Bharuch, Gujrat.
• Zandopa® powder (Ayurvedic formulation) batch no. EO0010, Zandu Emami limited, survey no. 61/2P/1- Masat, Silvassa, D and NH
(U.T.). Amantadine Hydrochloride (Parkitidin tablet batch no. ESX1529, Sun Pharma laboratory ltd, Vill. Kokjhar, Mirza Palashbari
Road, district Kamrup, Assam.
• Hydrogen Peroxide (H2O2) research lab, Trichloroacetic Acid (TCA) Research lab, 5,5-Dithiobis-2-Nitrobenzoic acid (DTNB) research
lab, Thiobarbituric acid (TBA) research lab.
Animals
Healthy Sprague Dawley male rats (150 gm-180gm) were procured from Bombay veterinary college, Parel, Mumbai. The rats were housed in the animal house of Bharati Vidyapeeth’s college of pharmacy, Navi Mumbai at 23°C ± 2°C and 50%-65% humidity under a 12:12 hour light-dark cycle. The animals were fed with rat pellets and water ad libitum throughout the study. The study was approved by the Institute Animal Ethics Committee (Protocol number BVCP/IAEC/01/2020) and all the animal experiments were carried out according to CCSEA guidelines.
Haloperidol induced catalepsy model in rats
The male sprague dawley healthy rats (150 gm-180gm) were divided into 5 groups namely group I vehicle control (1% Carboxy Methyl Cellulose (CMC)), group II disease control (1% Carboxy Methyl Cellulose (CMC)). Group III formulation A (775 mg/kg of body weight). Group IV formulation B (10 mg/kg of body weight) and group V combination group (formulation A+formulation B) each group consisting of 6 animals. Catalepsy induced with haloperidol challenge (1 mg/kg i.p route) was given to all the animals except group I from the 1st day of the study till 15 days daily. The treatment groups were prophylactically treated 30 min prior haloperidol challenge for 15 days daily via oral route.
Behavioral parameters assessment
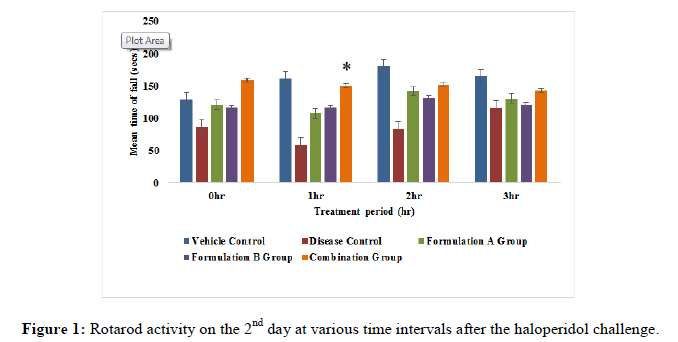
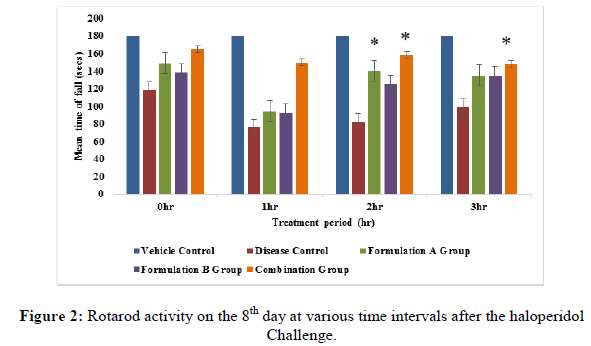
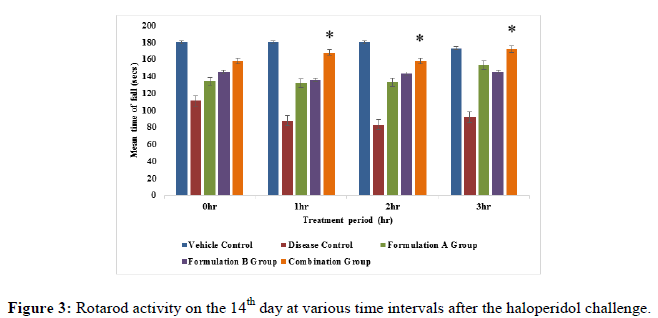
Rotarod activity: The animals were trained on the rotarod apparatus for 5 days prior to the study. Animals were placed on the rotating rod with a diameter of 7 cm (speed 12 rpm). Each rat was subjected to rotarod activity at an interval i.e., 0 hr, 1 hr, 2 hr and 3 hr on the 2nd, 8th and 14th day of the study. The cut-off time of 180 sec. was maintained throughout the experiment. The fall of time of animals was recorded [10-12].
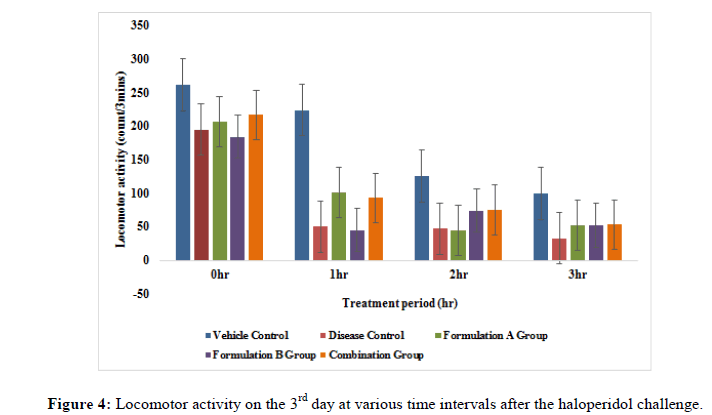
Locomotor activity: The activity cage was placed in a dark and light and sound attenuated and ventilated testing room. The individual animal was put into the activity cage and observed. Each rat was subjected to locomotor activity at an interval i.e., 0 hr, 1 hr, 2 hr and 3 hr on days 3rd, 9th and 15th day of the study. The values were expressed in terms of count recorded when the beam of light fell on the photocell and cut off the beam animal.
Biochemical parameters assessment
On the 15th day of study after behavioral parameters assessment, the animals were sacrificed by exposing them to an overdose of isoflurane in the euthanasia chamber. The brain was excised out immediately and rinsed with ice-cold isotonic saline solution. The 10% w/v brain tissue homogenate was centrifuged at 10000 rpm for 15 min in 0.1 M phosphate buffer. The supernatant was used for biochemical estimation of catalase, reduced glutathione and lipid peroxidation.
Lipid peroxidation (MDA level): To estimation of MDA level, the supernatant of the tissue homogenate was treated with Thiobarbituric Acid- Trichloroacetic Acid-Hydrochloric Acid (TBA-TCA-HCl) reagent. MDA quantitatively as an indirect indicator of lipid peroxidation. 1 mI of Aliquots of the supernatant were mixed with 3 ml of TBA reagent: 0.38% (w/w) TBA, 0.25 M Hydrochloric Acid (HCl) and Trichloroacetic Acid (TCA15%). The solution was shaken and placed for 15 min, followed by cooling on an ice bath. After cooling, the solution was centrifuged at 8000 rpm for 10min. The upper layer of solution was collected; pink color formation and absorption were taken on the spectrophotometer at 532 nm wavelength. The result was measured in nanomoles per mg of protein.
Catalase (CAT level): The catalase activity was determined by the method of Aebi in 1974. Test solution contains 0.05 ml of tissue homogenate supernatant 1.95 ml of 50 mM phosphate buffer (pH 7.0) in the cuvette with a capacity of 3 ml,1 ml Hydrogen Peroxide (30 mM) (H2O2) was added and changes in absorbance were monitored for 15 sec. intervals at 240 nm total for 30 sec. The activity of H2O2 (0.071 mmol cm-1) was calculated as per mg of H2O2 oxidized micromoles per minute protein.
Reduced glutathione (GSH level): 1 ml of tissue homogenate was precipitated with 1 mL of 10% TCA to determine reduced glutathione levels. 4 ml phosphate solution and 0.5 mL 5,5-Dithiobis-(2-Nitrobenzoic Acid) (DTNB) reagent were added to an aliquot of the supernatant and absorbance was measured at 412 nm. The following values are in nM of reduced glutathione per mg of protein.
Statistical analysis
The data was presented as the average value with the standard error of the average (SEM). Statistical evaluation of data was performed by one-way Analysis of variance (ANOVA) (between disease control and treatment groups) followed by Dunnett’s multiple comparison test with significance level at p<0.05 using Graph-pad Prism version 9.0 (Graph Pad Software, Inc, CA, USA).
Results
Estimation of behavioral parameters
Rotarod activity: All the treatment groups were treated with formulation A group 775 mg/kg, formulation B group 10 mg/kg and formulation A 775 mg/kg+formulation B 10 mg/kg (combination group) along with haloperidol 1 mg/kg respectively. Animals treated with the formulation A+B group (775 mg/kg+10 mg/kg) showed significant (p<0.05) improvement in fall of latency when compared to the disease group on the 8th and 14th day of the study (Figures 1-3), but it did not show significant (p<0.05) improvement on 2nd day of the study. On the 8th day of the study, a significant (p<0.05) improvement was seen with the formulation A group and the formulation B group did not show significant improvement in fall of latency when compared to the disease group on 8th, 14th and 21st day of the study. This indicates that the combination group is significantly more active when compared to the formulation A group and formulation B group specifically on the 8th and 14th day of study.
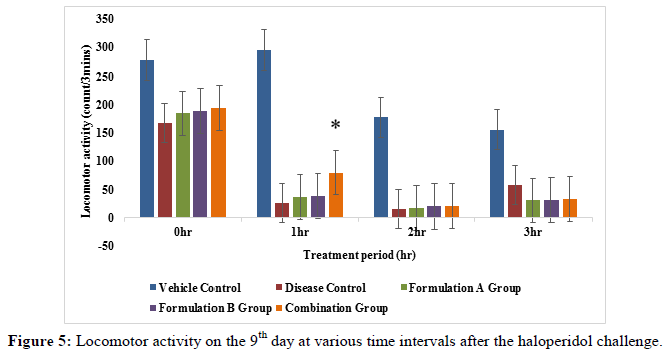
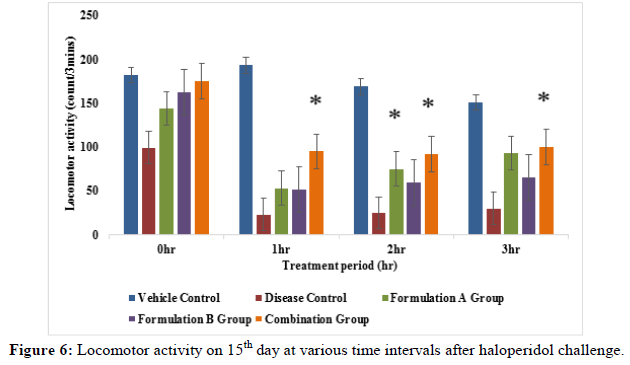
Locomotor activity: The locomotor activity was evaluated in treatment groups i.e., formulation A group, formulation B group and combination group in haloperidol induced rats. Total locomotor activity on the 9th day and 15th day of the study formulation A group and combination group showed significant (p<0.05) improvement when compared to the disease group. Formulation A group on the 3rd day of the study and formulation B group from 3rd to 15th day of the study did not show significant effects (p<0.05) as compared to disease group (Figures 4-6). The result of this study revealed that the combination group i.e., formulation A+B (775 mg/kg+10 mg/kg) showed a good additive effect when co-administered and henc indicates beneficial herb drug interaction.
Estimation of biochemical parameters
The administration of haloperidol induced oxidative stress is indicated by increased MDA levels and decreased CAT and GSH levels when compared to vehicle control animals. The treatment with formulation A, formulation B and combination group showed significant (p<0.05) decrease in MDA level compared to the disease control group. Similarly, daily administration of formulation i.e., (formulation A, formulation B and combination group show significant (P<0.05) increased the level of GSH in formulation A group and combination group as compared to the disease control group, while catalase level did not show significant (P<0.05) increased in treatment groups (Table 1).
| Groups | MDA | CAT | GSH |
|---|---|---|---|
| (µM/mg of protein) | (nM of H2O2 used/min/mg of protein) | (nM/mg of protein) | |
| Vehicle control | 0.3083 ± 0.00* | 24.34 ± 0.01 | 1.52 ± 0.10 |
| Disease control | 0.4218 ± 0.01 | 03.40 ± 0.00 | 0.62 ± 0.07 |
| Formulation A group | 0.6102 ± 0.00* | 05.32 ± 0.00 | 1.43 ± 0.07* |
| Formulation B group | 0.08283 ± 0.01* | 02.83 ± 0.00 | 0.49 ± 0.10 |
| Combination group | 0.1858 ± 0.01* | 10.21 ± 0.00 | 1.54 ± 0.37* |
Note: Value is expressed as mean SEM; n=6, where n is the number of animals per group. * Indicates there is significant difference with p<0.05 when compared with disease group one-way ANOVA followed by Dunnett’s multiple comparison test. Time in hours indicates recording of activity after haloperidol challenge. *P<0.05
Table 1: Effect of formulations on the level of lipid peroxidation (MDA), Catalase (CAT) and reduced Glutathione (GSH) in the brain tissue.
Discussion
Herbal medicine resurgence is the currently used remedy by people all over the world. People believe in its long term treatment since it is produced by plants and now has valid scientific information about it. Several interaction studies have been carried out indicating that herbal medicine can cause interactions with conventional drugs on concomitant use. Thus, in the present study; one such combination of herbal and conventional drug combination was selected to evaluate the pharmacodynamic interactions if any i.e., formulation A (Mucuna pruriens) and formulation B (Amantadine hydrochloride).
Parkinson’s is the second most prevalent neurological disorder that is characterized by progressive neurodegeneration affecting partial loss of dopaminergic neurons within the substantia nigra parts and the presence of lewy bodies whose primary components include fibrillar synuclein. The term Parkinsonism refers to a clinical syndrome comprising of a mixture of motor disturbances like bradykinesia, resting tremor and muscle rigidity, loss of postural reflexes and flexed posture. Haloperidol an antipsychotic drug, which blocks nonselective to the central dopamine receptor in the striatum, produces a behavioral state in the animals such as mice and rats in which they fail to correct externally imposed posture. This is referred to as catalepsy. Mucuna pruriens seeds contain L-DOPA as one of the constituents that help to reduce antiparkinsonian activity. The efficacy of Mucuna pruriens extracts has been shown significant on haloperidol induced catalepsy, whereas parkitidin tablets work as an adjuvant.
A similar study conducted on the neuroprotective effect of Spirulina fusiform and amantadine in the 6-OHDA induced Parkinsonism in rats shows that there is substantial evidence of generational interaction. The chemical constituent i e., L-DOPA, nicotine dehydrogenase, coenzyme Q-10 and adenine dinucleotide) have been proven to have positive benefits in Mucuna pruriens seed powder has also been discovered to have substances that are linked to Parkinson's disease. Haloperidol catalepsy is prevented by blocking L-DOPA sensitization.
In the present experimental study, two behavioral parameters were performed: Rotarod test and locomotor activity to examine haloperidol-induced PD in rats. The rats when pre-treated with formulation A, formulation B and combination of both the formulation A+B orally for 15 days. The significant increment in the locomotor activity and rotarod activity showed in formulation A treated group, Whereas the overall greatest neuroprotective impact was observed with the combination group. The combination group showed a significant increment in behavioral parameters. The etiology of Parkinson's disease is complicated by oxidative stress caused by the mitochondrial malfunction. The oxidative stress marker lipid peroxidation affects membrane function, distorts structural integrity and inactivates many membrane-bound enzymes found in all biological membranes. Even glutathione deficiency is a strong predictor of neuronal loss. Evidence suggests that an increase in MDA and a depletion of GSH and CAT in the brain cause neuronal loss (substantia nigra) and are essential variables in the etiology of Parkinson's disease.
Lipid peroxidation is created by attacking the double bonds of arachidonic acid and unsaturated fatty acid, creating lipid peroxyl radicals that trigger some additional attacks on other unsaturated fatty acids that can lead to the oxidative breakdown of polyunsaturated and unsaturated acids. The highest level of MDA was found in the disease control group whereas a significant decrease in the levels of MDA was found in formulation A and combination groups.
Catalase decomposes hydrogen peroxide into water and oxygen and plays an important role in the protection of tissue against oxidative damage. Depleted catalase activity suggests an increase in oxidative stress. Depleted catalase activity was observed in the disease control group when compared with the all-treatment groups.
The depletion of reduced glutathione in the substantia nigra in PD could be the result of neuronal loss due to oxidative stress of free radicals. A positive correlation has been found to exist between the level of neuronal loss and the reduction of glutathione. Reduction in the availability of reduced glutathione would impair the ability of neurons to neutralize hydrogen peroxide and increase the risk of free radical formation and lipid peroxidation. A reduction in GSH levels was evident in haloperidol treated disease control animals. After treatment with formulation A and combination of formulation significant increment in GSH level was found when compared with the disease group.
The combination of formulation A+B was proven to be quite efficient in lowering the symptoms of PD in rats in our investigations. The results of this study demonstrate that a combination of formulation A+B and can reduce oxidative stress and have neuroprotective properties.
Conclusion
Our study suggests that the activity of the haloperidol challenged rats in the formulation A group and combination group showed improvement in muscle coordination and locomotor activity, inhibited the extrapyramidal features and reversed the altered oxidative stress parameters. Hence it can be a good approach for the prevention of extrapyramidal side effects of neuroleptics and from the above findings, we can say that the combination group showed good additive effect and both can be given concomitantly it shows a new insight for further research in this field.
Acknowledgment
The authors are thankful to Dr. Vilasrao J kadam principal of Bharati Vidyapeeth’s college of pharmacy, CBD Belapur, Navi Mumbai for providing all facilities to complete project work. Special thanks to my batchmates Shifa Surti, Tahsin Attar, Shubham Kolge and Dnyaneshwar Patil for their valuable help.
Conflict of Interest
The study's authors affirm that there were no financial or commercial ties that might be seen as a potential conflict of interest throughout its execution.
References
- Ovallath S, Deepa P. Mov Disord. 2013; 28(5): p. 566-568.
[Crossref] [Google Scholar] [PubMed]
- Tsai HH, Lin HW, Lu YH, et al. PloS one. 2013; 8(5): p. 642-655.
- Makhoul K, Jankovic J. J Neurol Sci. 2021; 21(9): p.422-425.
- Trenkwalder C, Kuoppamaaki M, Vahteristo M, et al. Neurology. 2019; 92(13): p.1487-1496.
[Crossref] [Google Scholar] [PubMed]
- Maldonado RG. J Neurol S. 2018; 14(7): p. 30-33.
- Neta F, Da Costa I, Lima F, et al. Pharmacogn Rev. 2018; 12(23): p.55-57.
- Katzenschlager R, Evans A, Manson A, et al. J Neurol Neurosurg Psychiatr. 2004; 75(12): p. 1672-1677.
[Crossref] [Google Scholar] [PubMed]
- Stoof JC, Booij J, Drukarch B. Clin Neurol Neurosurg. 1992; 94(55): 4-6.
- Rout S, Rath B, Bhattamisra SK, Kumar A, Rath I, Panigrahy PK. J Herb Med Pharmacol. 2021; 10(4): p. 468-475.
- Aasim M, Baloch FS, Nadeem MA, et al. J Crop Sci. 2018: 18(6): p. 381-408.
- Chaudhary P, Dhande S. Neurology. 2020; 11(1): p. 9-17
- Hira S, Saleem U, Anwar F, et al. Front pharmacol. 2018; 8(4): p. 945-947.