Review Article - Der Pharma Chemica ( 2018) Volume 10, Issue 4
Topoisomerase an Ideal Target for Treatment of Cancer
Poonam P Patil*, Vishal M Sanklecha, Dr. Sunil J Aher and Musmade Deepak Sitaram
Department of Pharmaceutical Chemistry, SRES’s Sanjivani College of Pharmaceutical Education and Research, Kopargaon, MS, 423601, India
- *Corresponding Author:
- Poonam P Patil
Department of Pharmaceutical Chemistry
SRES’s Sanjivani College of Pharmaceutical Education and Research
Kopargaon, MS, 423601, India
Abstract
Topoisomerases are class of enzymes that alter the super coiling of double-stranded DNA. (In super coiling the DNA molecule coils up like a telephone cord, which shortens the molecule.) The Topoisomerases act by transiently cutting one or both strands of the DNA. Topoisomerases are enzymes that regulate the over winding or under winding of DNA. The winding problem of DNA arises due to the intertwined nature of its double-helical structure. During DNA replication and transcription, DNA becomes over wound ahead of a replication fork. If left unabated, this tension would eventually stop the ability of the enzymes involved in these processes to continue down the DNA strand. Topoisomerase inhibitors are agents designed to interfere with the action of topoisomerase enzymes (topoisomerase I and II), which are enzymes that control the changes in DNA structure by catalyzing the breaking and rejoining of the phosphodiester backbone of DNA strands during the normal cell cycle. The present review is an attempt made in the respect of highlighting the some important information of Topoisomerase enzyme and topoisomerase inhibitors and role of topoisomerase in cancer therapy.
Keywords
Topoisomerase, Topoisomerase inhibitors, Cancer, DNA
Introduction
Topoisomerase inhibitors are chemical compounds that block the action of topoisomerase (Topoisomerase I and II), which are enzymes that control the changes in DNA structure by catalyzing the breaking and rejoining of the phosphodiesterbackbone of DNA strands during the normal cell cycle. Topoisomerase I inhibitors are a new class of anticancer agents with a mechanism of action aimed at interrupting DNA replication in cancer cells, the result of which is cell death. There are several common and overlapping functions of topo I and topo II in maintaining DNA topology. Inhibition of one form of topo results in an increase in the activity of other topo [1,2]. DNA topoisomerases modulate the topological state of DNA by regulating supercoiling of the double-stranded DNA during replication, transcription, recombination and repair. Because topoisomerases play essential roles in cellular processes, they constitute a potential target for antitumour and antimicrobial drugs [1].
Topoisomerase I
Topoisomerase I acts by influencing a transient break (to scratch) of a solitary strand of DNA, catalyzing the section of DNA strands through each other and permitting arrival of the superhelical pressure. Topoisomerase I enzymes have been subdivided into type IA and type IB sub-families based on their reaction mechanism. Type I topoisomerases of the type IA sub-family shape covalent linkages to the 50 end of the DNA break, while type IB sub-family compounds frame covalent linkages to the 30 end of DNA break. Eukaryotic DNA topoisomerase I is appended to the 30 DNA end of the break site, and is in this way a sort IB topoisomerase. This compound is situated in ranges of dynamic RNA interpretation to discharge superhelical stretch produced amid mRNA union [3]. Escherichia coli sort IA topoisomerase, the primary topoisomerase found, was at first type ''u protein'' since it was decontaminated by ultracentrifugation that utilizations u as a parameter of angular velocity. Eukaryotic Top 1 is named type IB as a result of two key contrasts with E. coli Topo I. Initially, it unwinds both negative and positive supercoils, though E. coli Topo I just unwinds negative supercoils. Second, it divides DNA by shaping a tyrosyl-DNA covalent synergist middle of the road at the 30 end of the break, while E. coli Topo I shapes a 50-P-Y middle of the road. Eukaryotic type IA, named Top 3 [4].
Topoisomerase II
Topoisomerase 2 (Top2) is an essential enzyme responsible for manipulating DNA topology during replication, transcription, chromosome organization and chromosome segregation. It acts by nicking both strands of DNA and then passes another DNA molecule through the break. Type II enzymes cleave both DNA strands at a time to perform their catalytic functions [4]. Sort II topoisomerases cut the two strands of the DNA helix at the simultaneously in order to manage DNA tangles and supercoils. They utilize the hydrolysis of ATP, not at all like Type I topoisomerase. In this process, these enzymes change the connecting number of circular DNA by ± 2 [5].
Mechanism of action of DNA topoisomerase inhibitors
DNA topoisomerases are chemicals that manage DNA topology and are basic for the uprightness of the hereditary material during transcription, replication and recombination forms. Inhibitors of the mammalian chemicals are generally utilized antitumor medications. They stabilized topoisomerase- DNA cleavable buildings by preventing the DNA consigning venture of the synergist response, therefore bringing about DNA cleavage incitement. Investigations on the arrangement selectivity of DNA cleavage invigorated by synthetically inconsequential mixes set up that particular nucleotides flanking strand cuts are required for tranquilize activity. Also, structure- action relationship ponders have distinguished auxiliary determinants of medication succession specificities, along these lines in the long run permitting the outline of new operators focused at chose genomic locales. The underlying cell injury, i.e., the medication balanced out cleavable complex, is a reversible sub-atomic occasion; in any case, how it might prompt cell demise stays to be completely cleared up. A few research centers centered in past years on atomic and hereditary parts of medication initiated apoptosis. Irreversible twofold stranded DNA breaks, created from crashes between cleavable edifices also, propelling replication forks, were recommended to increment p53 protein levels, in this way activating the cell passing project [6].
Topoisomerase I inhibiters
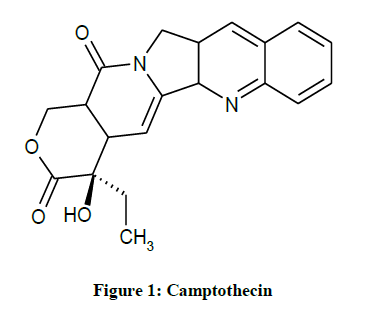
Camptothecin
IUPAC name-S)-4-ethyl-4-hydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b] quinoline-3,14-(4H,12H)-dione. Camptothecin (CPT) is a cytotoxic quinoline alkaloid which inhibits the DNA enzyme topoisomerase I (topo I). It was discovered in 1966 by M.E. Wall and M.C. Wani in systematic screening of natural products for anticancer drugs. It was isolated from the bark and stem of Camptotheca acuminata (Camptotheca, Happy tree), a tree native to China used as a cancer treatment in Traditional Chinese Medicine. CPT showed remarkable anticancer activity in preliminary clinical trials but also low solubility and (high) adverse drug reaction. Because of these disadvantages synthetic and medicinal chemists have developed numerous syntheses of CPT and various derivatives to increase the benefits of the chemical, with good results (Figure 1) [7].
Camptothecin is of little clinical use because of high systemic toxicity and very low solubility in aqueous media. Hence, along with investigations of the effect of this compound, its synthetic analogues with higher hydrophilicity and lower side effects are searched for [8,9].
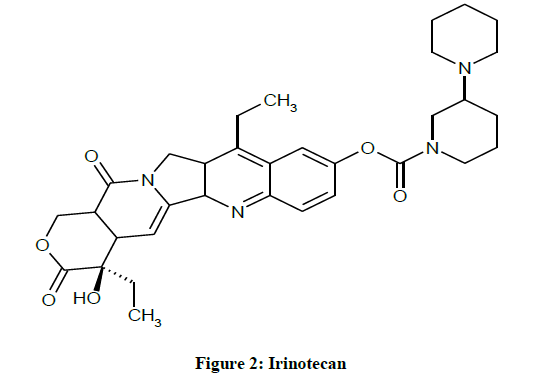
Iarinotecan
IUPAC name (S)-4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-3,14-dioxolH-pyrano[3’,4’:6,7]-indolizino[1,2-b]quinolin-9-yl- [1,4’bipiperidine]-1’-carboxylate (Figure 2).
It is also known as CPT-11. This is a water-soluble semisynthetic analog of CPT. It is metabolized mainly by the liver to 7-ethyl-10- hydroxycamptothecin. CPT-11 is approved for use in the United States for refractory metastatic colorectal cancer. It has a significant antitumor activity in 5-Fluorouracil (5-FU)-resistant colorectal cancer [10]. CPT-11 must undergo hydrolysis or de-esterification to form the active metabolite, SN-38 (Figure 3) [11].
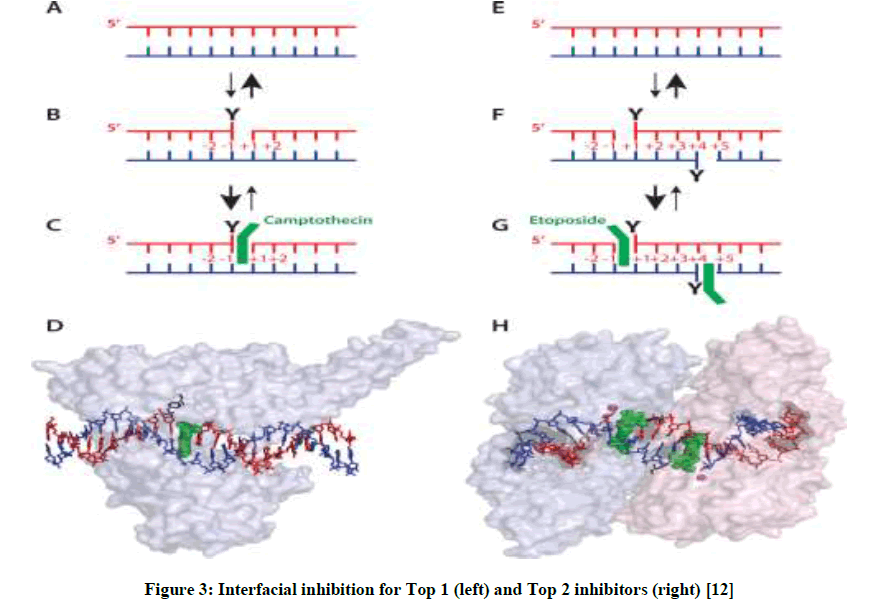
Figure 3: Interfacial inhibition for Top 1 (left) and Top 2 inhibitors (right) [12]
Topotecan
IUPAC name (S)-10[(dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1H-pyrano[3’,4’.6,7]indolizino[1,2-b]quinoline-3,145(4H,12H)-dione monohydrochloride. Topotecan is a water-soluble semisynthetic analog of CPT. Reduced albumin binding in vivo has been shown to be responsible for promoting its stability and activity in humans [1]. Topotecan (Hycamtin) is used to treat ovarian cancers and Small-Cell Lung Cancers (SCLC) (Figure 4) [13].
Pegamotecan
Pegamotecan, or CPT-di-20-O-ester of PEG (40-kDa) glycine (Gly), a pegylated CPT, was selected among various compounds for its biologic properties. Various amino acid spacers that conjugate CPT with PEG CPT were tested for antitumor activity, rates of hydrolysis and in vivo efficacy. Side effects include myelosuppression, cystitis, nausea, vomiting and diarrhea [14].
Indolocarbazoles
Indolocarbazoles is a non camptothecin derivative. Ther are the most advanced non-camptothecin Top 1 inhibitors in clinical development. The indolocarbazole derivative developed by Bristol Myers. It inhibit the generation of DNA supercoiling by DNA replication, transcription and chromatin remodeling [15].
Topoisomerase II inhibiters
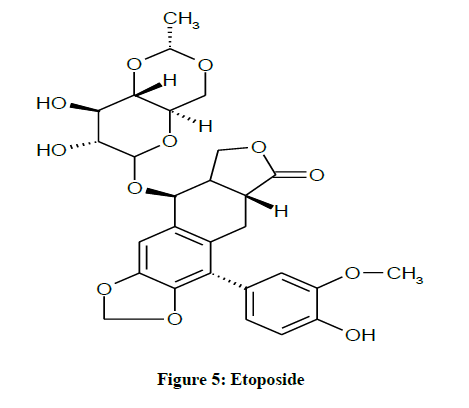
Etoposide
IUPAC name 4'-Demethyl-epipodophyllotoxin-9-[4,6-O-(R)-ethylidene-beta-D-glucopyranoside],4'-(Dihydrogen phosphate). Etoposide derives from podophyllotoxin, a toxin found in the American Mayapple. Etoposide is an important antineoplastic agent used against several types of tumors, including testicular and small cell lung cancers, lymphoma, leukemia, and Kaposi's sarcoma. In combination chemotherapy, it has been used successfully with cisplatin and several other antitumor drugs including cyclophosphamide, doxorubicin and vincristine [16]. Etoposide forms a ternary complex with DNA and the topoisomerase II enzyme Etoposide targets DNA topoisomerase II activities thus leading to the production of DNA breaks and eliciting a response that affects several aspects of cell metabolisms. Therefore, this causes errors in DNA synthesis and promotes apoptosis of the cancer (Figure 5) [17].
Mitoxantrone
Mitoxantrone is a type II topoisomerase inhibitor. Mitoxantrone binds to topoisomerase II resulting in cleavable complexes, it disturbs DNA synthesis and DNA repair in both healthy cells and tumor cells by intercalation between DNA bases, is signal for N-kappa B activation and induction of apoptosis [18,19].
Teniposide
Teniposide is an analogue of etoposide. Teniposide use has been limited primarily to the treatment of childhood lymphomas and leukemia’s and for treatment of CNS malignancies. Teniposide’s mechanism of action is similar to that of etoposide. Both drugs damage DNA by interaction with topoisomerase II to form cleavable complexes that prevent relegation of DNA leading to double-strand DNA breaks [20].
Fluoroquinolones
Fluoroquinolones are potent bactericidal drugs, the targets of which are the bacterial type II topoisomerases DNA gyrase and topoisomerase IV. They act by stabilizing the enzyme covalently bound to DNA in an intermediate drug-enzyme DNA complex. The resulting ternary complex is then processed into a so-called cleavage complex [21].
Conclusion
Here by the conclude that, various topoisomerase inhibitors drugs having Anticancer activity were study were highlighted and topoisomerases are the class of enzymes that alter the super coiling of double-stranded DNA. They regulate of DNA super coiling is essential to DNA transcription and replication, when the DNA helix must unwind to permit the proper function of the enzymatic machinery involved in these processes. They serve to maintain both the transcription and replication of DNA. Aside from topoisomerases I and II, there are more discovered Topoisomerases. In the present anticancer drugs with vast modification having glimmering market value. So the Topoisomerases Inhibitor having anticancer activity was studied here under.
References
- R.B. Ewesuedo, M.J. Ratain, The Oncologist., 1997, 2, 359-364.
- B.K. Sinha, Drugs., 1995, 49(1), 11-19.
- M.K. Goftar, N.A. Rayeni, S. Rasouli, Int. J. Adv. Biol. Biom. Res., 2014, 2(8), 2431-2436.
- Y. Pommier, E. Leo, H. Liang Zhang, C. Marchand, Chem. Biol., 2010, 17, 421-433.
- H. Yan, M. Tammaro, S. Liao, Genes., 2016, 7, 32.
- M. Binaschi, F. Zunino, G. Capranico, Stem Cells., 1995, 13, 369-379.
- Dhrmarajansriram, P. Yogeeswari, R. Thirumurugan, T. Ratan Bal, Natural Product Research., 2005, 19, 393-412.
- W.A. Denny, Expert Opin. Emerg. Drugs., 2004, 9(1), 105-133.
- L.G. Dezhenkova, V.B. Tsvetkov, A.A. Shtil, Rus. Chem. Rev.,2014, 83(1), 82-94.
- A.A. Zuma, D.P. Cavalcanti, M.C.P. Maiaa, W. de Souzaa, MC.M. Motta, Int. J. Antimicrob. Agents., 2011, 37, 449-456.
- M.L. Rothenberg, Ann. Oncol.,1997, 8, 837-855.
- Y. Pommier, Drugging Topoisomerases: Lessons and Challenges, the American Chemical Society Publication, Page no. A-N.
- Y. Pommier, Chem. Rev., 2009, 109, 2894-2902.
- J. Moukharskaya, C. Verschraegen, Hematol. Oncol. Clin. N. Am., 2012, 26, 507-525
- Y. Pommier, Nature Publishing Group., 2006, 6, 789-802.
- J.M.S. van Maanen, J. Retel, J. de Vries, H.M Pinedo, Journal of the National Cancer Institute, 1998, 80(19), 1526-1533.
- A. Montecucco, F. Zanetta, G. Biamonti, EXCLI. J., 2015, 14, 95-108.
- K.R. Hande, Update on Cancer Therapeutics., 2008, 3, 13-26.
- J.L. Nitiss, Nature Review Cancer., 2009, 9, 338-350.
- D.S. Thakur, Int. J. Pharm. Sci. Nanotechnol., 2011, 3(4), 1173-1181.
- P. Heisig, Mutagenesis., 2009, 24(6), 465-469.