Review Article - Der Pharma Chemica ( 2024) Volume 16, Issue 2
Triazole Benzene Sulfonamides as Leads for Human Carbonic Anhydrase IX Inhibition: Updates on Research Progress
Papichettypalle Gopinath*Papichettypalle Gopinath, Department of Pharmaceutical Chemistry, GITAM School of Pharmacy, GITAM University, Hyderabad, India, Email: gopi.pharma@gmail.com
Received: 07-Feb-2024, Manuscript No. Dpc-24-127165; Editor assigned: 12-Feb-2024, Pre QC No. Dpc-24-127165 (PQ); Reviewed: 26-Feb-2024, QC No. Dpc-24-127165; Revised: 01-Apr-2024, Manuscript No. Dpc-24-127165 (R); Published: 29-Apr-2024, DOI: 10.4172/0975-413X.16.2.274-282
Abstract
Introduction: Triazole moiety is considered as the most important heterocyclic therapeutic agent in medicinal chemistry. Identifying a lead compound or potential drug, with the requisite physical and biological characteristics from a library of known bioactive molecules is the first step in the traditional drug development process which involves identifying the protein target associated with the disease. This severely restricts the search space from the outset and makes the task of discovering new drugs (or novel chemical structures) very challenging.
Areas covered: This review discusses triazole derivatives possessing anticancer activity (hCA inhibition) and also illustrates the recent updates on triazole moiety for hCA inhibition research emphasizing the structure activity relationship aspects.
Conclusion: However, as the cellular and molecular causes of numerous diseases are better understood, more opportunities for logical therapeutic development broaden. Small molecule inhibitors of hCA IX interactions can now be found through screenings using large virtual chemical libraries and a series of novel compounds from research literature. In this context, the literature's triazole benzene sulfonamide derivatives have been thoroughly studied for structure-activity relationship and reported here for future design and development of leads.
Keywords
hCA IX; Benzene sulfonamides; Heterocyclic derivatives; Enzyme inhibition; Structure-activity relationship
Introduction
Cancer and metabolism
A group of abnormal cells having uncontrollable growth upon violating normal rules of cell division can be defined as cancer. Both intrinsic and extrinsic factors initiate and progress the cancer which include tobacco, chemicals, radiation and inherited mutations cause abnormal cellular functioning and proliferation. For metabolism and energy supply, cancer cells utilize glycolysis instead of oxidative phosphorylation. This aerobic glycolysis also called as ‘warburg effect’ occurs in variety of tumors. Many studies report genetic factors such as tumor suppression and oncogenes, micro environmental factors such as acidosis and hypoxia control cancer cell glycolytic metabolism. The signals that modulate the tumor microenvironment include apoptotic evasion, angiogenesis promotion and reactivation of energy metabolism, metastasis and immune system avoidance [1].
Carbonic anhydrase IX structure and functionalities
Human CA_IX belongs to mammalian α-CA’s having zinc bound to three histidines in the catalytic site. In 1992, this CA isoforms was initially discovered as MN protein in human cervical carcinoma HeLA cell line by Pasterokova, et al. using monoclonal antibody M75 and in later years by renal cancer cell line G250. The structure of mature CA IX consisting of proteoglycan domain at N-terminal, a conserved catalytic location, a hydrophobic transmembrane spanning helix and a short C-terminal intra cytoplasmic tail. The proteoglycan domain differentiates CA IX from other isoforms with respect to the cytoplasmic tail having three phosphorylation sites regulating signaling. CA IX binds competitively to β-catenin and modulating cell-cell adhesion by competing with E-cadherin and therefore supports cell motility. CA IX mediates various physiological and pathological responses varying from homeostasis, movement of CO2/HCO3 - amid lungs and tissues, biosynthetic reactions involving lipogenesis, gluconeogenesis, ureagenesis, followed by tumorogenesis and resorption of bone. In normal conditions, CA IX is located in gastro intestinal tract epithelia and its specific over-expression in hypoxia potentiated the CA IX as endogenous hypoxia biomarker [2].
The glycolytic switch producing acidic environment lowers the intracellular pH altering membrane stability and metabolism. Generally carbonic anhydrases convert CO2 to bicarbonate (CO2 + H2O→HCO3-+H+) at zinc catalytic site promoting tumor cell survival by maintaining favourable microenvironment. The extracellular produced bicarbonate by CA IX is transported into cytosol using NaHCO3 transporters (NB cascade) and anion exchangers. The transported bicarbonate acts as a buffer and gets convert back to water by CA II intracellular. The protons that are produced by CA IX remain at the cell surface and increase extracellular acidity. Thus intracellular bicarbonate levels make slightly alkaline environment. The H+ ions generated by CA IX affect the extracellular matrix and alters cell motility also.
Different approaches to target carbonic anhydrase IX
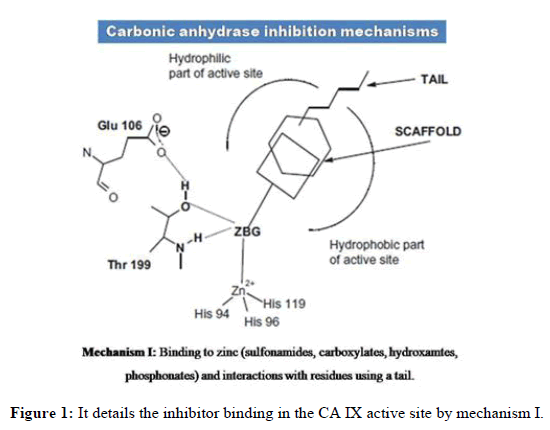
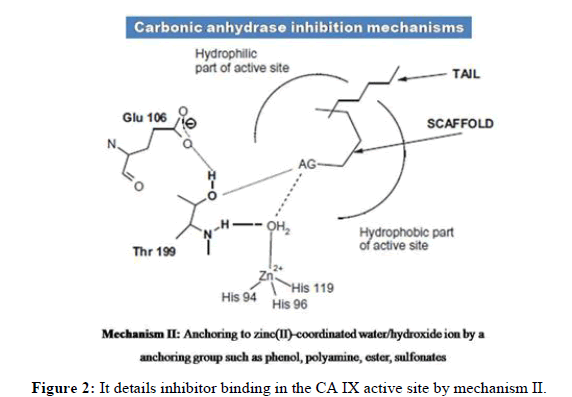
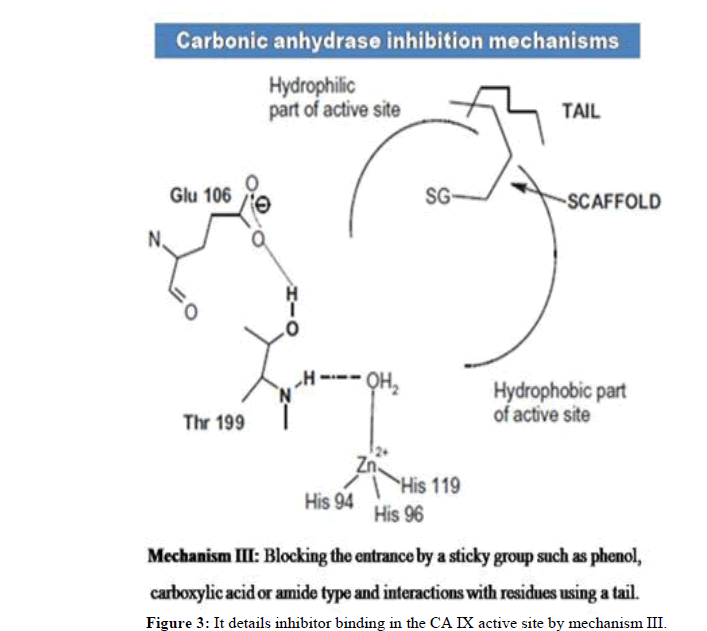
Over expression of CA IX in tumor tissues, its pH maintenance and its location on cell surface makes it an attractive target. Various CA IX inhibitors bind to zinc in the catalytic site and provide other interactions with favourable residues. Figures 1-3 details inhibitor binding in the CA IX active site by different methods. Usually compounds are to be in deprotonated form altering the zinc bound H2O molecule/OH- ion. Even in hypoxia, the tetrahedral coordination of zinc is not disturbed and this segment needs further research attention. Few CA IX inhibitors such as fluorescent labelled sulfonamides, coumarin derivatives and glycol-conjugates act like prodrugs and provide better understanding in binding. Bio reducible agents such as nitrogen mustards and nitroimidazoles acting as radio sensitizers or cytotoxins combine with CA IX inhibitors such as sulfonamides in hypoxia will be certainly a better combination where radio and chemo therapy constitutively can be an effective approach.
The CA IX inhibitors such as phenols, carboxylates, polyamines, 2-thiocoumarins and sulfocoumarins that anchor to zinc attached hydroxide ion were reported in literature. However, the carboxylic acid derivatives were shown to have binding residues outside the active site of CA IX. Also, coumarins show similar type of binding by occluding the entrance of the CA IX active site cavity. Fullerenes have shown to bind CA IX in an unspecific manner. Apart from chemical compounds, monoclonal antibodies such as girentuximab (cG250) have been targeting CA IX in renal cell carcinoma. However due to lack of efficacy, cG250 has failed in phase III clinical trials. On the other hand SLC-0111, an uriedo substituted benzene sulfonamide has entered phase IIb of clinical trials. Recent studies denote seleno-uriedo analogues of benzene sulfonamides have shown promising CA IX inhibitory activity. In this review, we focus on research work carried out on triazole based benzenesulfonamides as human carbonic anhydrase inhibitors. It is relevant to study the importance of triazole component interacting with CA active site residues. We present here a list of research papers focused on development of triazole benzenesulfonamides having nanomolar range of bioactivity towards hCA inhibition [3].
Literature Review
Research progress
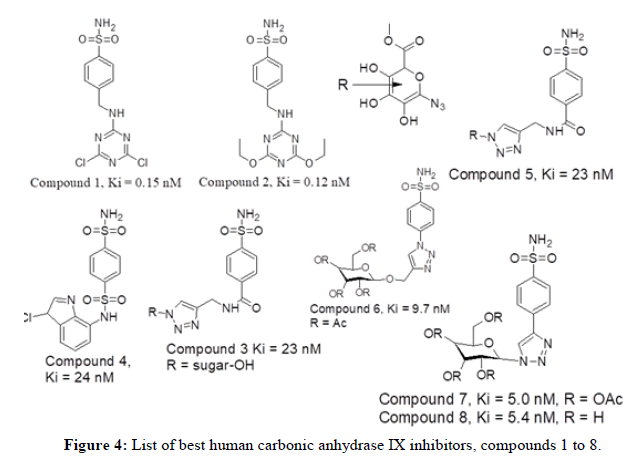
V. Garaj, et al., in 2004 reported novel dataset of nineteen benzenesulfonamide derivatives having triazine moieties by reaction of homosulfonilamide, sulfanilamide or 4-amino ethyl benzenesulfonamide fragments with cyanuric chloride. Among the synthesized series, compound 2 with ethyl substituent and compound 1 with dichloro substituent have shown better inhibition activity against hCAIX with 0.12 nM and 0.15 nM respectively compared to alkoxy derivatives and hydroxy triazines. Wilkinson, et al., in 2006 generated new series of twenty eight compounds by utilizing groups such as amide, ester and sugars thereby increasing hCA IX inhibition activity. Among the synthesized series, compound 3 and 4 (Indisulam) have shown better inhibitory action towards CA IX with Ki value of 23 nM and 24 nM respectively. The amide sugar-acetoxy compounds were more effective than sugar-hydroxy derivatives, apart from glucuronic acid derivatives. The only exceptions were the glucose and N-acetyl glucosamine tails, where the sugar triazole tail usually resulted in less inhibition than non-glycoconjugate parent scaffolds. In continuation to previous study Wilkinson, et al., in 2007 generated new series of thirty compounds by using glycoconjugate benzenesulfonamides. However, in comparison to previous studies the synthesized compounds do not produce incremental inhibition activity against hCA IX. Among the series, compound 5 have shown better hCA_IX inhibition activity with Ki value of 23 nM.
Further, Wilkinson, et al., in 2007 generated new eleven derivatives utilizing di-polar cyclo-addition reaction incorporating O-glycoside group linked to 1,2,3-triazole of benzenesulfonamides for better hCA IX inhibition. Among the series, compound 6 with Ki value of 9.7 nM have shown good hCA IX inhibition activity. Cu(I)-catalyzed 1,3-Dipolar cyclo-addition reaction of acylated O-propanyl glycosides with the azido scaffold yielded a library of ten benzenesulfonamides having triazole tethered O-glycoside tails. In continuance Wilkinson,et al., in 2008 developed a new series of seventeen glycosyl triazole benzene sulfonamide derivatives having acetyl and acetoxy moieties displaying better inhibition values. Among the series, the compounds 7 and 8 having α-D mannosyl triazole moiety have shown good hCA IX inhibition activity with value of 5 and 5.4 nM respectively. This study discovered that attaching a sugar tail to the hCA anchor pharmacophore allows for the generation of selective hCA IX inhibitors that outnumber the physiologically abundant hCA I and II isozyme inhibition [4].
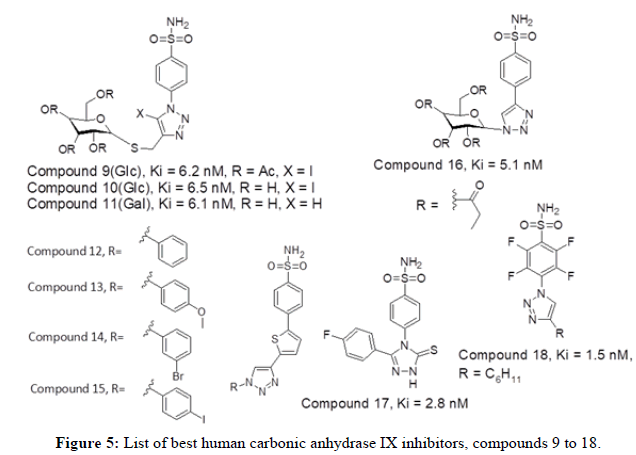
Figure 4 represents list of compounds 1 to 8 mentioned herewith from literature as human carbonic anhydrase IX inhibitors. In continuation to past studies, the novel series of twenty four compounds using sugar moieties attached to triazoles was reported targeting CA IX. The authors described a class of drugs targeting hypoxic tumor cell viability by inhibiting hCA IX and hCA XII using carbohydrate moieties. Among the series, the compound 9, 10 and 11 have shown best inhibitory activity of hCAIX with Ki value of 6.2 nM, 6.5 nM and 6.1 nM respectively. Triazoles carrying acetylated and deacetylated sugars along with S-glycosidic bridge provided the compounds 9 and 10. On the other hand, Raivis Zalubovskis, et al., in 2013 demonstrated the use of click chemistry to synthesize a series of fifteen compounds. The dataset of 2-thiophene sulfonamides with substituted aryl 1,2,3-triazolyl ring displayed human CA cytosolic and transmembrane inhibition activity. Out of which molecules 12, 13, 14 and 15 have shown good hCA IX inhibition values (Ki value of 7.2, 6.4, 10.9 and 5.4 nM respectively) [5].
Poulsen, SA, in 2013 reported a prodrug intiative towards hCA inhibition. The series of twenty four glycoconjugates comprising three basic structural elements; a sugar moiety (glucose/galactose), 1,2,3-triazole moiety followed by benzenesulfonamides core. The sugar moiety provided a basic prodrug template, while the benzene sulfonamide group acts as core component. The hCA inhibitors act by means of sulfonamide anion which can coordinate with Zn2+ of the hCA active site by means of familiar endogenous reaction. The triazole ring acts as a biocompatible and non-labile bonding spacer connecting the sugar and benzene sulfonamide groups. Overall the compound 16 having galactose sugar moiety displayed good hCA IX inhibition activity with Ki value of 5.1 nM. CT Supuran, in 2014 reported a series of twenty one benzenesulfonamides bearing 1,2,4- triazole groups as best inhibitors of hCA_IX and XII. Among the series the compound 17 with flouro substituent has shown good hCA IX inhibition activity with a Ki value of 2.8 nM. The compound 17 has a ten-fold increase in hCA IX inhibition compared to acetazolamide. Both electronwithdrawing and electron-donating groups have shown potential bioactivity [6].
Mario Sechi reported in 2014, twenty two CA inhibitors having benzene sulfonamides and tetra fluoro benzene sulfonamides were produced by click chemistry. The azides (containing the sulfomoyl ZN2+ binding group) were combined with various alkynes along with nano sized metallo copper as a catalyst to produce a series of compounds. Among the series, the compound 18 have shown good CAIX activity with Ki value of 1.5 nM. Sulfonamides having low nanomolar to sub-nanomolar range show inhibition constants spanning from 1.5 to 38.9 nM towards hCA IX. The molecule 18 has a clenched nanopolar cyclohexyl ring that was identified in a hydrophobic pocket bordered by residues P202, V135, F131 and L204 in hCA II and has a bioactivity of 41.3 nM. Figure 5 represents list of compounds 9 to 18 mentioned herewith from literature as human carbonic anhydrase IX inhibitors. In 2014, Poulsen SA, et al., reported cyclic secondary sulfonamides having excellent suppression of cancer related CA enzymes. According to the researchers the addition of seven substituents to the 1,2,3-triazole ring of saccharin, which were studied for orientations in the CA IX active site and further determining CA IX binding and selectivity. The compound 19 have shown good CAIX inhibitory activity with Ki value of 8.6 nM. The researcher’s findings have shown that the cyclic secondary sulfonamide motif has the potential to be used to produce effective CA IX inhibitors [7].
Discussion
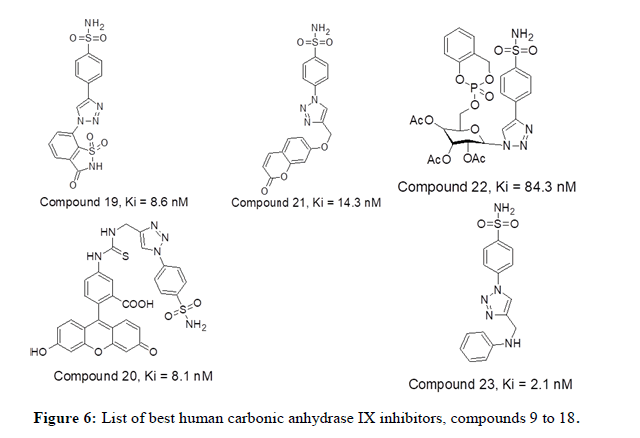
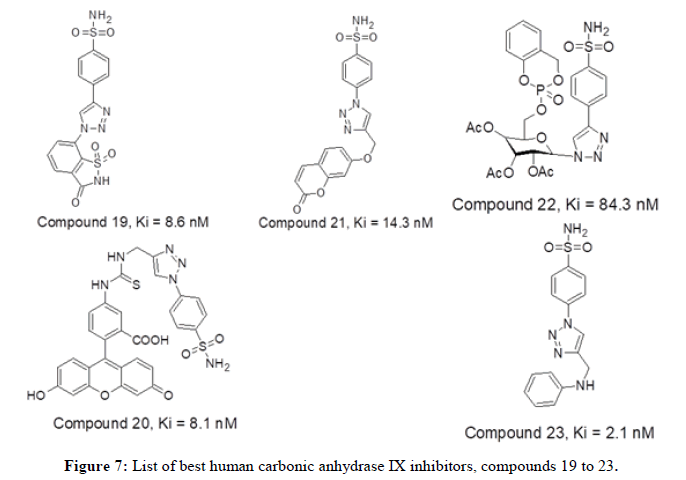
Carta F, et al., in 2015 demonstrated that fluorescent sulfonamides having 1,2,3-triazole moiety have shown a nanomolar potency towards hCA IX inhibition. Compound 20 has shown selective inhibition of hCA_IX with Ki value of 8.1 nM. Further, the study encouraged the role of hCA IX isozyme as hypoxic marker and further development of novel inhibitors are needed in this regard. Further extending the studies in identification of triazole tail role in hCA IX inhibition Nocentini Alessio has explored a series of fourteen 7-substituted coumarins having aryl triazole moieties. All the fourteen compounds have shown selectivity towards hCA IX thus establishing a relationship with conformation of tail moiety, rotational aspects and hydrogen bond donor or acceptor ratio. Compound 21 having para sulfonamide moiety and triazole linked to coumarin by oxygen bridge displays good CAIX inhibition activity displaying Ki value of 14.3 nM.Gregory M, et al., in 2015 reported phosphate chemical probes intended for CA suppression. Out of five compounds tested for hCA inhibition, compound 22 with masking groups cyclosaligenyl phosphate moiety, 3x acetyl groups on sugar that have been attached to 1,2,3-triazole showcasing good CA inhibiton activity with Ki value of 84.3 nM. CycloSal acts as proinhibitor with excellent membrane permeability. The cyclosaligenyl phosphate moiety which is neutral and membrane-permeable gets hydrolyzed within the cell releasing the monophosphate intracellularly. CT Supuran, in 2016 reported thirteen compounds acting as very competent inhibitors for the cancer associated isoforms hCA_IX. The compound 23 having an inhibition constant Ki value of 2.1 nM denoted better bioactivity. However selectivity is not achieved as the tail group does not favor interactions [8].
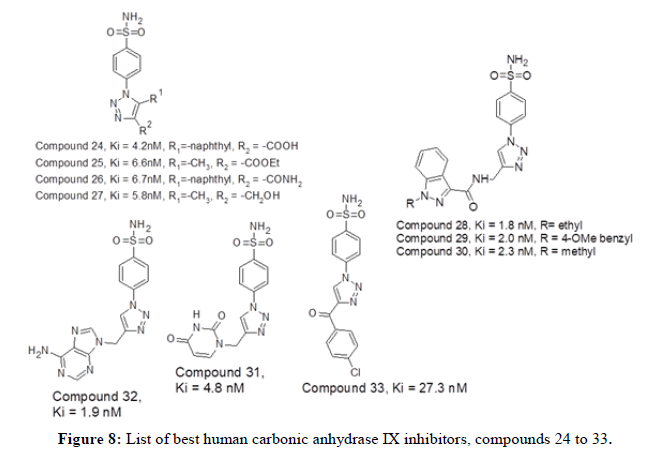
Figure 6 represents list of compounds 19 to 23 mentioned herewith from literature as human carbonic anhydrase IX inhibitors. P. K. Sharma et al in 2017 reported a new set of twenty unique compounds of 4-functionalised 1,2,3-triazole derivatives. Among the series, the molecule 24 with the 2- naphthyl substituent have shown good CA IX inhibitory action with Ki=4.2 nM. Other compounds such as 25, 26 and 27 having -CH3, 4-C6H4- OCH3 and 4-C6H4-OCH3 substituents respectively have shown good inhibitory activities against CA IX with Ki values 6.6, 6.8 and 5.8 nM respectively. Arifuddin Mohammed in 2017 published a novel series of twenty one sulfocoumarin/4-sulfomoyl phenyl/coumarin carrying indazole- 3-carboxamide moieties has been synthesized and tested in-vitro for their inhibitory capacity against four relevant hCAs (the cytosolic and transmebrane). The compounds 28, 29 and 30 in this series has shown better CA_IX inhibitory action with value of 1.8, 2.0 and 2.3 nM respectively. CT Supuran, et al., in 2017 reported synthesis of seven benzenesulfonamides with purine and pyrimidine moieties that can inhibit CA I, II, IX and IV. Among these the compound 31 and 32 have shown good hCA IX inhibition activity with Ki value of 4.8 and 1.9 nM respectively. CT Supuran, et al., in 2018 reported a series of twenty 1,2,3-triazoles substituted benzene sulfonamides as good human CA isoform inhibitors. Among the series, the compounds displayed their inhibition potencies in range of 27.3 nM to 0.36 μM compared to acetazolamide (Ki=25 nM). The compound 33 have shown good CA IX inhibition activity with Ki value of 27.3 nM [9].
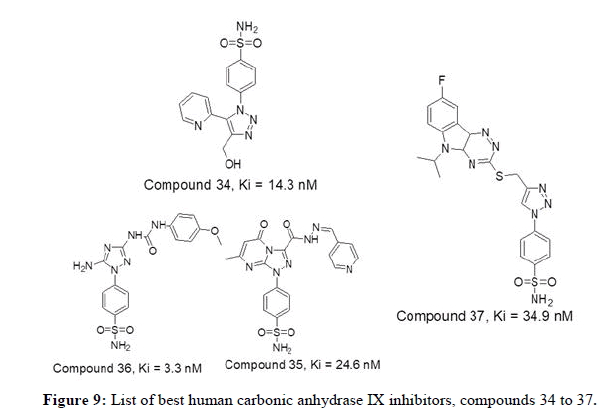
Figure 7 represents list of compounds 24 to 33 mentioned herewith from literature as human carbonic anhydrase IX inhibitors. PK Sharma, et al., reported a dataset of thirty new 4-functionalised 1,5-diaryl 1,2,3-triazoles bearing benzene sulfonamide group were synthesized and tested for their inhibitory activity against human CA isoforms. In comparison to the reference drug acetazolamide (Ki value of 25.8 nM). The compound 34 with 2-pyridyl substituent has shown good inhibition activity with Ki value of 14.3 nM against hCA IX. The study also shows that the compounds bearing 4- fluoro phenyl moiety were least effective inhibitors of tumour related isoform hCA_IX as compared to 4-methylphenyl moiety. Wagdy M.Eldehna, et al., in 2020 reported a explorative series of twenty six triazole-pyrimidine based benzenesulfonamides and evaluated against hCA I, II, IX and XII isozymes. Human CA IX and XII were well inhibited with Ki values in the range of 3.3nM to 85 nM and 4.4nM to 105 nM respectively. The compounds 35 and 36 were found to be the selective hCA_IX inhibitors. Mohammed Arifuddin, et al., in 2020 reported a series of fifteen 1,2,3-triazole benzene sulfonamide bearing triazino indole hybrids for evaluation of carbonic anhydrase inhibition. The results exhibited Ki values ranging from 7.7 nM to 41.3 nM against hCA II and hCA IX and much higher inhibition scores against hCA_I and XIII. Compound 37 has displayed inhibition score of 34.9 nM towards hCA_IX [10].
Figure 8 represents list of compounds 34 to 37 mentioned herewith from literature as human carbonic anhydrase IX inhibitors.
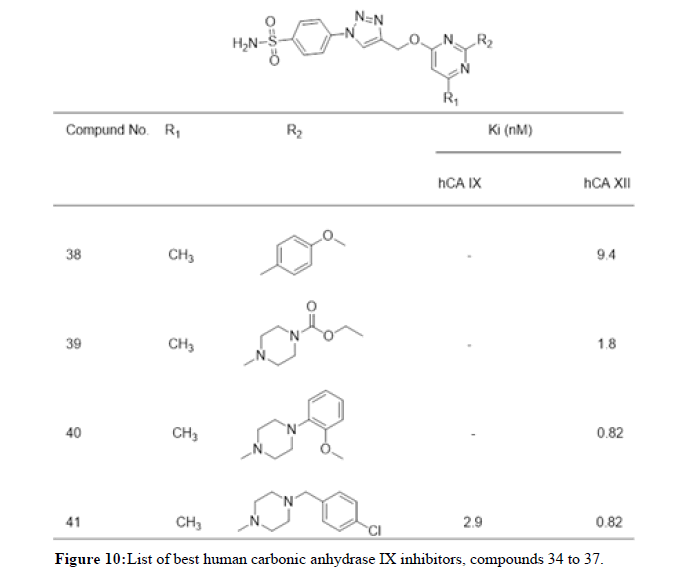
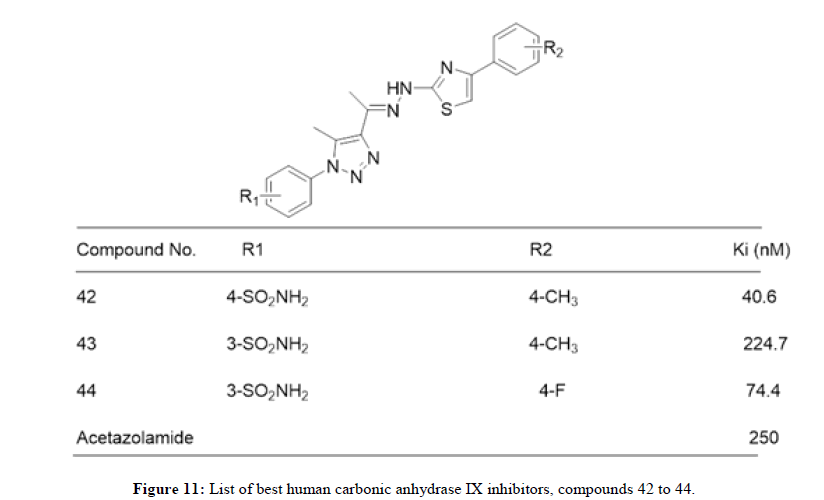
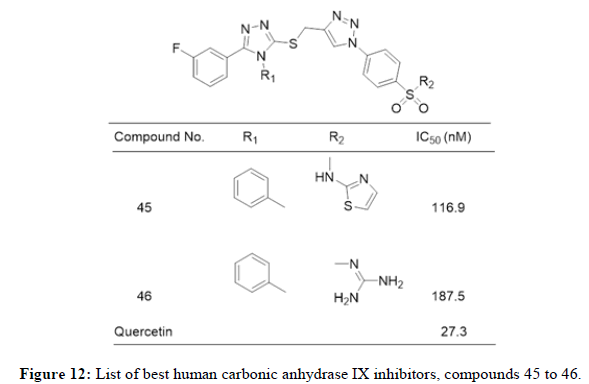
Shoaib Manzoor, et al., in 2021 reported 28 Triazole-sulfonamide bearing pyrimidine derivatives evaluated against human Carbonic Anhydrase (hCA) isoforms, such as hCA I, II, IX and XII, using a stopped flow CO2 hydrase assay. Most of these compounds exhibited significant inhibitory activity against hCA II and weak inhibitory activity against hCA I. Some compounds displayed moderate to excellent inhibitory activity against tumor-related hCAs IX and XII. Compounds 38 (Ki=.4 nM), 39 (Ki=1.8 nM) and 40 (Ki=0.82 nM) exhibited excellent inhibitory activity and selectivity profile against hCAs XII over IX. 41 displayed promising inhibitory activity and selectivity profile against both tumor-related hCAs IX (Ki=2.9 nM) as well as XII (Ki=0.82 nM) over hCA I and II. Figure 9 represents list of compounds 38 to 41 mentioned herewith from literature as human carbonic anhydrase IX inhibitors. Amit Kumar, et al., in 2022 synthesized a library of twenty-two arylthiazolylhydrazono-1,2,3-triazoles incorporating sulfanilamide and metanilamide moieties. These Compounds were studied on inhibitors of Human Carbonic Anhydrase (hCA) isoforms, such as hCA I, II, IX and XII, using a stopped flow CO2 hydrase assay. Three compounds 42, 43 and 44 (Ki of 40.6, 224.7 and 74.4 nM, respectively) appeared as better inhibitors than reference drug AAZ (Acetozolamide) (Ki=250 nM). Figure 10 represents list of compounds 42 to 44 mentioned herewith from literature as human carbonic anhydrase IX inhibitors. Mohamed Reda Aouad, et al., in 2022 reported 1,2,3 and 1,2,4- triazole hybrids bearing various sulfonamide appendages following pharmacophore hybridization strategy. Both 45 and 46 exhibited relatively moderate activity against the human CA II isoform (IC50=116.9 and 187.5 nM, respectively) relative to the reference Quercetin (IC50=27.3 nM). Figure 11 represents list of compounds 45 and 46 mentioned herewith from literature as human carbonic anhydrase IX inhibitors [11,12].
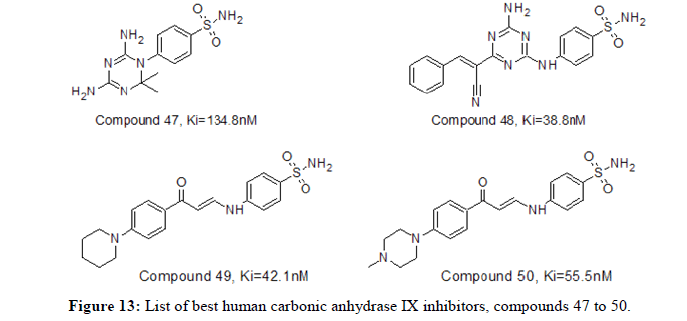
Abdelrahman I Zain‑Alabdeen, et al., in 2022 reported design and synthesis of two different benzenesulfonamides series acting as hCA IX inhibitors having rigid cyclic linkers (1,3,5-dihydrotriazines and 1, 3, 5-triazines). Compound 47 having 1, 3, 5-triazine efficiently inhibited hCA IX with Ki value of 134.8 nM. Cyanoethenyl group attached to the 1,3,5-triazine linker in compound 48, Ki value of 38.8 nM provided better inhibition of hCA IX. Insertion of 1, 3, 5-triazines, as cyclic linkers were found to be enhance the hCA IX inhibitory activity of substituted benzenesulfonamides. Heba Abdelrasheed Allam, et al., in 2023 reported the synthesis of aryl enaminone attached compounds (49 and 50) with sulphonamide functionalities for human carbonic anhydrase inhibition. Enaminone sulphonamide derivatives, 49 and 50 potently inhibited the target tumour-associated isoforms hCA IX (Ki=42.1 nM and 55.5 nM) and hCA XII (Ki=43.5 nM and 26.2 nM) respectively. It was found that N-methyl group promoted hCA XII inhibitory activity. Figure 12 represents list of compounds 47 to 50 mentioned herewith from literature as human carbonic anhydrase IX inhibitors [13].
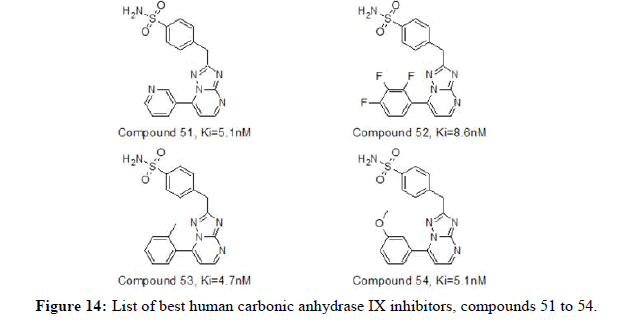
Romeo Romagnoli, et al., in 2023 reported a series of human carbonic anhydrase inhibitors based on the 1, 2, 4-triazole [1, 5-a] pyrimidine nucleus substituted with various groups at 7-position. The hCA IX was effectively inhibited with compounds 51, 52, 53 and 54 having Ki value of 5.1, 8.6, 4.7 and 5.1 nM, respectively. Similarly, the hCA XII was moderately inhibited with compounds 51 and 52 having Ki value of 8.8, 5.4 nM respectively. Compounds exhibited the best selectivity over off-target hCA II against hCA IX and hCA XII isoforms. Figure 13 represents list of compounds 51 to 54 mentioned herewith from literature as human carbonic anhydrase IX inhibitors [14,15].
Conclusion
Inhibitors of carbonic anhydrase are crucial in the hypoxia-related overexpression of important isozymes like hCA IX and XII. The development of benzene sulfonamide-containing heterocyclic compounds that act as effective hCA inhibitors is desperately needed. Due to its extensive range of therapeutic and pharmacological activities, triazole has become one of the benzene sulfonamide containing compounds that have acquired prominence in the drug discovery process. In an effort to discover a new anticancer agent with few side effects, researchers have been looking into triazoles and their derivatives bearing additional heterocycles. A better understanding of the pattern of substituents, such as electron withdrawing, electron donating, bicyclic, tricyclic and other heterocyclic rings on different positions of the triazole nucleus in regulating the interactive potential is provided by the detailed SAR of these reported triazole derivatives having Carbonic anhydrase inhibitory activity. Through structural alterations to the reported compounds, the study of these triazoles offers the opportunity to enhance their biological activity and drug like features. We firmly believe that a deeper comprehension of the SAR and cellular mechanisms behind the bioactivity of the triazole derivatives will aid in the thoughtful design of new small molecules. Advanced molecular modelling techniques like molecular dynamics simulations, X-ray diffraction analysis can reveal bonding and stability details of ligands thereby selective hCA IX inhibition can be achieved. In this context, we recommend exploring molecule conformation (eclipsed or staggered form), the bond distances for carbon-carbon and carbon-nitrogen between phenyl group and triazole moiety.
References
- DeBerardinis RJ, Chandel NS. Sci Adv. 2016; 2(5): p. 200-400.
[Crossref] [Google Scholar] [PubMed]
- Liberti MV, Locasale JW. Trends Biochem Sci. 2016; 41(3): p. 211-218.
[Crossref] [Google Scholar] [PubMed]
- Justus CR, Sanderlin EJ, Yang LV. Int J Mol Sci. 2015; 16(5): p. 11055-11086.
[Crossref] [Google Scholar] [PubMed]
- Hanahan D, Weinberg RA. Cell. 2000; 100(1): p. 57-70.
[Crossref] [Google Scholar] [PubMed]
- Nakamura J, Kitajima Y, Kai K, et al. Am J Pathol. 2011; 178(2): p. 515-524.
- Pastorekova S, Zavadova Z, Kostal M, et al. Virology. 1992; 187(2): p. 620-626.
[Crossref] [Google Scholar] [PubMed]
- Svastova E, Zilka N, Zatovicova M, et al. Exp Cell Res. 2003; 290(2): p. 332-345.
[Crossref] [Google Scholar] [PubMed]
- Supuran CT, Scozzafava A. Bioorg Med Chem. 2007; 15(13): p. 4336-4350.
[Crossref] [Google Scholar] [PubMed]
- Supuran CT. Nat Rev Drug Discov. 2008; 7(2): p. 168-181.
[Crossref] [Google Scholar] [PubMed]
- Pastorekova SI, Parkkila SE, Parkkila AK, et al. Gastroenterology. 1997; 112: p. 398-408.
[Crossref] [Google Scholar] [PubMed]
- Douglas CM, Bernstein JM, Ormston VE, et al. Clin Oncol. 2013; 25(1): p. 59-65.
[Crossref] [Google Scholar] [PubMed]
- He F, Deng X, Wen B, et al. Cancer Res. 2008; 68(20): p. 8597-8606.
[Crossref] [Google Scholar] [PubMed]
- Kim SJ, Shin HJ, Jung KY, et al. Jpn J Clin Oncol. 2007; 37(11): p. 812-819.
[Crossref] [Google Scholar] [PubMed]
- Olive PL, Aquino-Parsons C, MacPhail SH, et al. Cancer Research. 2001; 61(24): p. 8924-8929.
[Google Scholar] [PubMed]
- Stewart DJ, Nunez MI, Behrens C, et al. J Thorac Oncol. 2014; 9(5): p. 675-684.
[Crossref] [Google Scholar] [PubMed]