Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 3
Ultrasound Mediated Hydroboration of Alkenes, Alkynes Employing: N,NDimethylaniline Borane (DMAB)
Venkatesan Jayakumar1* and Mohammed Fakruddin Ali Ahamed2
1Department of Chemistry, DP International, Accra, GHANA
2Department of Chemistry, FIP, Kadapa, Andhra Pradesh, India
Abstract
The current investigation highlights the efficiency of N,N-Dimethylaniline.borane (DMAB) as a hydroboration reagent towards various alkenes, alkynes, enyne and unsaturated ester under sonication condition, the organoboranes were formed in very good yields with short time. Besides these properties it displays excellent control of regiochemistry, chemo-selectivity compared to hydroboration experiments using THF.borane. The combination of borane species from amine.borane with ultrasound irradiation provides a convenient, rapid method for hydroboration of unsaturated molecules.
Keywords
DMAB, THF.Borane, Sonication, Hydroboration, Chemo selectivity
Introduction
The notable contribution of hydroboration-oxidation and development of borane reagents made by Brown and Subba Rao sparked turbulent interest in the field of organoboron chemistry [1-3]. Hydroboration reaction is the addition of a B–H bond across an unsaturated C–C bond, results in the formation of various organoboranes, many boron reagents favor this process [1-5]. The role of boron reagents is vital in the field of organic synthesis and medicinal chemistry, amine.borane (AB) adducts are an important electrophilic boron reagent and these complexes have gained wide applications in synthetic organic chemistry as well as in various industrial applications [6-12]. Recently the utility of amine.borane complexes was reported for storing hydrogen in fuel cells, based on its stability and high gravimetric content of hydrogen [13]. Xiaodong Shi, et al. recently achieved the first catalytic hydroboration of alkynes with propargylamine.borane carbonitriles, which is accomplished with triazole- AuI complexes, via formation of cyclic amine.borane [14]. Preparation of first soluble polyaminoboranes was reported by Stubbs et al., through metal free hydrogen transfer from amine.boranes [15]. Itsuno et al. successfully concocted polyaminoboranes via dehydro polymerization of primary amine.boranes [16]. Repo et al. first accomplishes the dihydrogen activation of amine.borane complex through frustrated Lewis pairs (FLPs) [17].
In many instances the scope of amine.borane complexes in organic synthesis was restricted because of its stability, lack of reactivity towards functional group [18]. Amines as borane carrier which offer several advantages but in the case of pyridine.borane, triethylamine.borane harsh conditions like high reaction temperature, either acetic acid as solvent or mineral acid, and lewis acid (ZnCl2) are required to display the reducing property [19-21]. One set of amine.borane complexes derived from dialkyl anilines and N,N-dialkyl amines is significantly more reactive than most amine.boranes [22-27]. The effect of steric and electronic properties of these new amine influences their reducing property [28,29]. The main drawback of these dialkyl aniline derivatives is that their synthesis, it involves the alkylation of aniline or mono alkyl aniline, which is laborious and time consuming.
Instead of aiming to prepare a new reactive amine.borane complex, we choose the novel technique like microwave irradiation, sonication to activate moderately reactive amine.borane complexes for hydroboration and reduction of functional groups. We encouraged by the previous results of DMAB under microwave irradiation [30,31] and further expand its utility under sonication, so we demonstrated the simple and facile hydroboration of various alkenes, alkynes and diene, en-yne with DMAB activated insitu by ultrasound irradiation. On the other hand no report was available on the use of N, N-Dimethyl aniline.Borane (DMAB) as a hydroboration reagent for unsaturated systems under sonication condition as compared to N, N-Diethyl aniline.Borane [19-21,30,31]. It has been well known that the activation of various chemical reactions by ultrasound, is not only enhances the selectivity and product yield but also shorten the reaction time and minimize the undesired side products [32]. Furthermore, with the ease of recovery and recycling of N,N-Dimethyl aniline after the reaction makes amine.borane complex as an environmentally benign reagent.
Materials and Methods
All chemicals were purchased from Fluka and Aldrich. Melting point of compounds were measured using a differential scanning calorimeter (Shimadzu DSC-50) and are uncorrected. Liquid substrates were distilled prior to use. All the NMR spectra were recorded on Bruker AVANCE spectrometer operating at 400 MHz for 1H-NMR and 100 MHz for 13C-NMR. The compounds were dissolved in CDCl3, CD3OD and DMSO and the chemical shifts were referenced to TMS. Coupling constants were calculated in hertz (Hz). IR spectra were recorded on FTIR Shimadzu spectrometer. The mass spectra were recorded on EI-Shimadzu-GC-MS spectrometer. Elemental analyses were measured on a HERAEUS (CHNO, Rapid) analyzer.
General procedure for hydroboration –oxidation: example: 1-decene
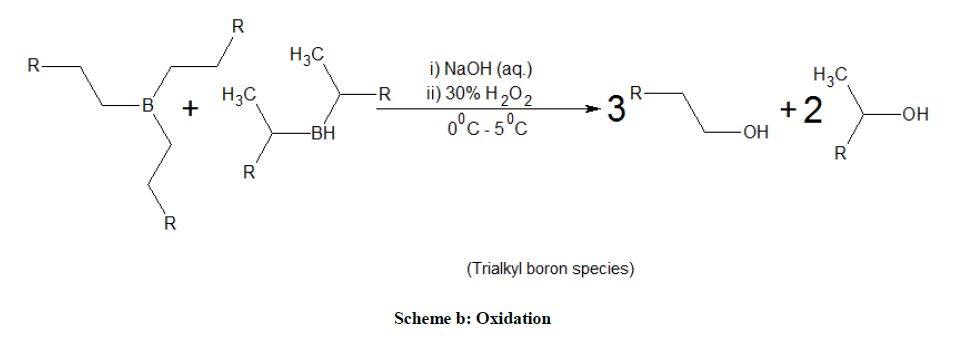
An oven dried, 50 ml three-necked round-bottom flask was kept under ultrasound bath further connected to a mercury bubbler by means of take-off adapter. Then, 1 ml 1-decene (6 mmol) was added followed by DMAB stock solution in dry THF (6 ml, 2 M solution) by syringe under nitrogen atmosphere. The contents were exposed to ultrasound (US) irradiation at 30°C for 20 min., reaction was monitored by TLC. Then, reaction mixture was made alkaline by the slow and careful addition of 2 ml of 20% sodium hydroxide. During the addition of alkaline sodium hydroxide solution mixture was cooled and followed by slow addition of 2 ml of 35% hydrogen peroxide. The progress of the oxidation reaction was monitored by TLC. Layers were separated and aqueous layer was extracted with diethyl ether (3 x 10 ml) organic layers are combined and solvent is removed by vacuum distillation. The hydroboration of other alkenes, alkyne, diene and en-yne were performed under similar way and followed the same workup procedure. The results were summarized in the Tables 1 and 2. In the case of unsaturated ester (ethyl undecenoate), selective hydroboration of double bond was observed than the tandem reduction – hydroboration (TRH).
| No. | Substrate | Stoich. | Time [min.] | Organoboron species | Product | Regioselectivity | Yield % |
|---|---|---|---|---|---|---|---|
| 1 | 1-Decene | 01:03 | 20 | R2BH & R3B | 1- & 2-Decanol | 1-Decanol (94%) 2-Decanol (6%) | 95% |
| 2 | Styrene* | 1:2 & 1:3 | 20 | R2BH & R3B | α- Phenyl ethanol & β- Phenyl ethanol | α- & β- is 15: 85 | 96% |
| 3 | Cyclohexene | 1:2 & 1:3 | 20 | R2BH | Cyclohexanol | Same, no difference | 90% |
| 4 | 1-Methyl cyclohexene | 01:02 | 22 | R2BH & RBH2 (less) | 2-Methyl Cyclo hexanol | 2-Methyl Cyclo hexanol (97%) | 90% |
| 5 | 2,3-dimethyl-2-butene# | 01:01 | 24 | RBH2 & R2BH (less) | Thexyl alcohol (2,3-dimethyl-2-butanol) | Same, no difference | 85% |
| 6 | Ethylidene cyclohexene† | 01:02 | 24 | R2BH | 1-Cyclohexyl ethanol | -------------------- | 95% |
| 7 | Ethyl-10- undecenoate†† | 01:03 | 25 | R3B & R2BH (less) | Ethyl-11-hydroxy undecanoate | -------------------- | 98% |
Styrene* the reported yield is for the mixture of α- phenyl ethanol & β-phenyl ethanol and % data is based on HPLC analysis; 2,3-dimethyl-2-butene# no difference in regioselectivity, same isomer formed; † Unable to get the regioselectivity data; †† reaction was performed with 1:3 stoichiometry ratio, unable to get the regioselectivity data.
Table 1: Hydroboration of Alkenes with N, N-Dimethyl aniline.borane (DMAB)
| No. | Substrate | Stoich. | Time [min.] | Organoboron species | Product | Regioselectivity | Yield % |
|---|---|---|---|---|---|---|---|
| 1 | Limonene | 1:2 & 1:3 | 25 | R2BH & R3B | p-Menth-1-en-9-ol & 5- (1-hydroxy propan-2-yl)-2-methyl cyclo hexanol | Both exocyclic & endocyclic bonds | 0.95 |
| 2 | C1,11-Diene (10E)-hexa deca-1,11-diene | 1:2, 1:3 & 1:9 | 22 | R2BH & R3B | (10E)-hexadec-10-en-1-ol & Hexa deca-1,10-diol | Mono & Di - alcohol | 0.9 |
| 3 | 1-Octyne | 1:2 & 1:3 | 22 | --------------- | 1-Octanol | 1-Octanol 100% | 0.95 |
| 4 | Diphenyl acetylene | 1:2 & 1:3 | 22 | --------------- | 1,2-Diphenyl ethanol | 1,2-Diphenyl ethanol (100%) | 0.95 |
| 5 | 1-Octen-4-yne | 0.04375 | 20 | R2BH & R3B | 4-Octyne-1-ol | Terminal double bond selectivity | 0.95 |
Table 2: Hydroboration of Alkynes & Diene with N, N-Dimethyl aniline.borane (DMAB)
Results and Discussion
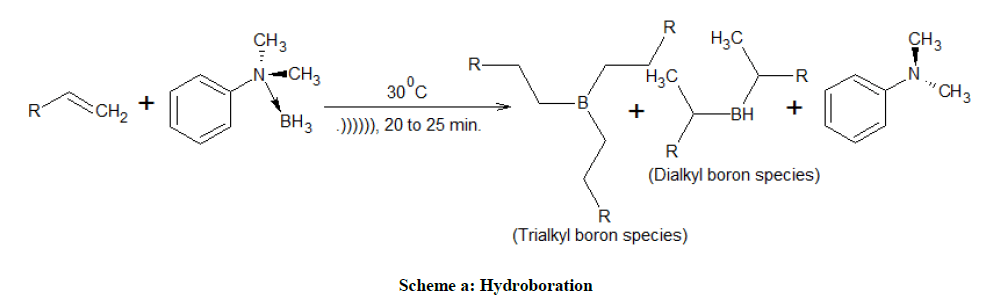
Hydroboration (Scheme a) of various alkenes with DMAB were carried out in dry THF under sonication condition and the reaction pathway was followed by 11B-NMR studies, for less substituted, simple olefins (Table 1; entry 1, 2, 3) the hydroboration was proceeded by trialkyl organoborane species (82 ppm). However the hydroboration of hindered olefins (Table 1; entry 4, 5, 6) were proceeded via mono (35 ppm) (or) dialkyl organo borane species (82 ppm) and confirmed by the signal in 11B-NMR. Regioselectivity is one of the prime concerns in hydroboration, less substituted double bond get more preference than substituted double bond (Table 1). In the case of C1,11-diene, terminal double bond get precedence over substituted double bond for hydroboration, further the oxidized product (Z)-11-alkene-1-ol which is an intermediate for the synthesis of pheromones.
Similarly, hydroboration-oxidation (Scheme b) of limonene (Table 2) is mainly yield 1-hydroxy limonene. It is conspicuous to mention that the regioselective hydroboration also depends on the ratio between alkene and amine.borane complex. For example, limonene was exclusively hydroborated at exocyclic double bond with 1:3 stoichiometric ratios of amine.borane and alkene. However, with excess of amine.borane complex 1:2, hydroboration of both double bonds were observed (Table 2). The formation of dialkyl boron species was also observed in the case of styrene, cyclohexene, 1-methyl cyclohexene and α-pinene etc. Alkynes undergo dihydroboration to give 1-alcohol in 95% yield. On the other hand, the selective hydroboration of double bond was observed in the presence of alkyne and ester.
In order to understand the efficiency of N,N-dimethyl aniline as borane carrier, we also studied the hydroboration of 1-decene with triethyl amine (TE) as borane carrier under sonication, to our surprise, the alkene was not at all hydroborated by TE.borane complex even after exposed to sonication 180 min. whereas it was completely hydroborated by DMAB in 20-25 min.
Conclusion
We have developed a simple procedure of hydroboration having quite a lot of advantages, such as mild reaction conditions, short reaction times, and good yields. The ease of recovery of amine after hydroboration-oxidation reaction and the possibility of recycling it makes the present method environmentally friendly process. Reagent DMNB has certain advantages over the currently available borane reagents such as THF.BH3 and Borane.dimethyl sulfide (BMS), pure DMAB is 1) quite concentrated (5.6 M), makes available all three hydrides for the hydroboration, 2) thermally stable and comfortable to handle and 3) environment friendly, not disagreeable odour. Studies on further applications and limitations of this methodology are undergoing and will be reported in due course.
Acknowledgement
We express gratitude to our respected Director and Chairman of DPSI, ACCRA, GHANA, WEST AFRICA, for providing the facilities for completion of research work.
References
- H.C. Brown, B.C. Subba Rao, J. Am. Chem. Soc., 1956, 78 (11), 2582.
- H.C. Brown, B.C. Subba Rao, J. Am. Chem. Soc., 1959, 81 (24), 6423.
- H.C. Brown, B.C. Subba Rao, J. Am. Chem. Soc., 1960, 82 (3), 681.
- C.F. Lane, Aldrichimica Acta., 1973, 6, 51.
- H.C. Brown, L.T. Murray, Inorg. Chem., 1984, 23, 2746
- J.V.B. Kanth, Aldrichimica Acta., 2002, 35, 2.
- G.O. Mallory, J.B. Hajdu, (eds.) Electroless plating, Fundamentals and Applications, Am. electroplaters and surface finishers society, 1990, Orlando, Florida.
- A. Pelter, K. Smith, H.C. Brown, Borane reagents; Academic press, 1988, London.
- H.C. Brown, Organic synthesis via boranes vol.1; Aldrich chemical company, Inc.; 1997, Milwaukee, WI.
- M. Follet, Chem.Ind., 1986, 123.
- L.H. Long, In A Comprehensive Treatise on inorganic and theoretical chemistry, Longman, 1981, London, Supplement Vol.5, Part B1.
- A. Meller, Gmelin Handbook of Inorganic and Organometallic Chemistry: Vol. 3, 1, Springer: Berlin, 1992; 4th Supplement.
- C.F. Lane, N-B-H Survey, Contract # DE-FC36-05GO 15060 Northern Arizona University, 2006.
- Q. Wang, S.E. Motika, N.G. Akhmedov, L. Jeffrey. Petersen, X. Shi, Angewandte Chemie International Edit., 2014, 53 (21), 5418.
- N.E. Stubbs, A.P.M. Robertson, E.M. Leitao, Ian Manners; J Organometallic Chem, 2012, 84, 730, 15/4/2013, 25th ICOMC.
- S.Itsuno, T. Sawada, T. Hayashi, K. Ito; J Inorganic and Organometallic Polymers., 1994, 4, (4), 403.
- K. Chernichenko, A. Madara´sz, I. Pa´pai, M. Nieger, M. Leskela, T. Repo, Nature chemistry., 2013, 5, 718.
- R.O. Hutchins, K. Learn, B. Nazer, D. Pytiewski, A, Pelter, Organic preparations and procedures Int., 1984, 16(5), 335.
- R.P. Barnes, J.H. Graham, M.D. Taylor, J. org. chem.,1958, 23, 1561.
- P.G.M. Wuts, J.E. Cabaj, J.L. Havens, J. org. chem.,1994, 59, 6470.
- H.C. Kelly, M.B. Giusto, F.R. Marchelli, J. Am. chem. soc., 1994, 59, 6470.
- H.C. Brown, M. Zaidlewicz, P.V. Dalvi, Organometallics., 1998, 17, 4202.
- H.C. Brown, J.V.B. Kanth, M. Zaidlewicz, J. org. chem.,1998, 63, 5154.
- H.C. Brown, J.V.B. Kanth, P.V. Dalvi, M. Zaidlewicz, J. org. chem., 1999, 64, 6263.
- J.V.B. Kanth, M. Periasamy, J. chem. Soc., Chem. Commun.,1990, 1145.
- M. Periasamy, J.V.B. Kanth, C.K. Reddy, J. chem. Soc., Perkin Trans.,1, 1995, 427.
- M. Periasamy, J.V.B. Kanth, A.S.B. Prasad, Tetrahedron., 1994, 50, 6411.
- A. M. Salunkhe, E. R. Burkhardt, Tetrahedron Letters., 1997, 38, 1519.
- A. M. Salunkhe, E. R. Burkhardt, Tetrahedron Letters., 1997, 38, 1523.
- S.V. Jayakumar, K.A. Srinivas, G. Hiriyana, H.N. Pati, Rasayan J. Chem.,2008, 1(2), 326.
- S.V. Jayakumar, S.M. Krishna Ganesh, Orbital: Electron. J. Chem., 2014, 6(1), 56.
- M. Changa, K. Biswas, Der Pharma Chemica.,2016, 8 (18), 320.