Research Article - Der Pharma Chemica ( 2018) Volume 10, Issue 1
Electrodes Preparation of Cyproheptadine Hydrochloride Imprinted Polymer Based on PVC Matrix Membrane
Yehya Kamal Al-Bayati*
Department of Chemistry, College of Science, Baghdad University, Al-Jaderia, Baghdad, IRAQ
- Corresponding Author:
- Yehya Kamal Al-Bayati
Department of Chemistry, College of Science, Baghdad University, Al-Jaderia, Baghdad, IRAQ
Abstract
Four molecularly imprinted polymers (MIPs) of cyproheptadine hydrochloride (CPH) selective senses were prepared based on ion-pair formation using cyproheptadine hydrochloride as a template. Molecularly Imprinted Polymer (MIP) was prepared by using 2-Hydroxyethylmethacrylate (HEMA) as monomer, N, N-Ethylenebismethacrylamide (EBMAA) as cross linker, and benzoyl peroxide as initiator. NIPs prepared by using the same composition of MIPs except the template (CPH). The sensors were prepared using different plasticizers, Di-octyladipate (DOA), Diisobutylbutylmaleate (DIBM), 2-Nitrophenylpentadecylether (NPPE) and 3-Trimethyltrimellitata (TMTM). The potentiometric response of the sensors to cyproheptadine hydrochloride was tested and the selectivity coefficients of inorganic ions, sugars, amino acids, and drugs were studied. The best electrodes obtained were constructed with plasticized DOA and TMTM which have a linear response range from (1 × 10-1 1 × 10-4 and 1 × 10-1 5 × 10-4 M) with slope of 55.4 and 58.8 mV/decade, respectively. The electrodes were used for a period of 35 and 41 days and were tested with good results in synthetic and pharmaceutical CPH samples.
Keywords
Cyproheptadine hydrochloride, Molecularly imprinted polymers, Cyproheptadine hydrochloride determination
Introduction
Cyproheptadine Hydrochloride (CPH) his role in the treatment of cases as sedative a histamine antagonist with anticholinergic; CPH is also used as an appetite stimulant to assist weight gain. Chemical name is 4-(5H-Dibenzo [a, d] cyclohepten-5-ylidene)-1-methylpiperidine hydrochloride as shown in Figure 1 [1-3]. An estimate using per chloric acid as a titrant and crystal violet as indicator for indicated the end point [4]. Several methods applied for determined CPH in pharmaceutical formulations by Liquid Chromatography-Mass Spectrometry (LC-MS), Gas Liquid Chromatography (GLC) [5,6] and High Performance Liquid Chromatography (HPLC) [7-14]. Recently applied of visible spectrophotometric methods [15-22], and derivative UV-spectrophotometry for determination the assay of CPH in two-component systems.
In this work was application successfully method which selective extraction of the analyte, known as Molecularly Imprinted Polymer (MIP), which was applied for determine several drugs as ibuprofen [23], warfarin [24] and mebeverine hydrochloride [25].
Expermental Section
Chemicals and reagents
In this work two model of pH meter were used (WTW model, Germany) and (WTW model pH 720, Germany). Reference electrode saturated calomel electrode (Gallenkamp, USA) and silver/silver chloride as working electrode, with highest purity for using all chemical reagent: Polyvinyl Chloride (PVC) high molecular weight, Di-octyladipate (DOA) (97%), Di-isobutyl Butyl Maleate (DIBM) (99%), 2- Nitrophenylpentadecylether (NPPE) (99%) and 3-Trimethyltrimellitata (TMTM) (98%). 2-Hydroxyethylmethacrylate (HEMA) (99%), as monomer was used, N, N-Ethylene-bis-methacrylamide (EBMAA) as a cross-linker (99%) and benzoyl peroxide (78%). All chemicals including Methanol (MeOH) and Tetrahydrofuran (THF) were purchased from Fluka and Sigma Aldrich. The performance of synthesis electrodes based on the measuring its potential in the concentration range 10-6 to 10-1 M of CPH. Stock solution 10-2 M CPH was prepared by dissolving 0.1619 g in 50 ml methanol and a series of solutions were prepared gradual dilution of the stock solution.
Synthesis of imprinted polymer
In a 50 ml screw cap glass test tube (50 ml) was used, MIPs of (CPH) were produced by using a bulk polymerization method. The template (CPH) of 3 mmol (0.9716 g) was dissolved in a thick walled glass tube filled with methanol (18 ml). A functional monomer HEMA 9 mmol (1.1712 g), cross-linker EBMAA 27 mmol and correspondingly initiator (BPO) 0.32 mmol (0.0775 g) were then put in the tube to be mixed with the solution. In an ultrasonic water bath for a period of 35 min, the nitrogen purged the mixture. After removing the glass tube from the ultrasonic water bath, keeping nitrogen flow, it have been sealed then put in 45°C water bath to permit starting the reaction which continued 24 h.
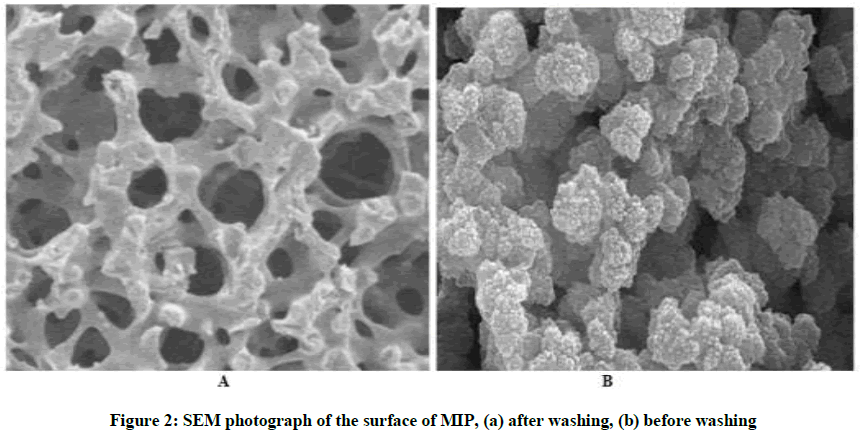
To remove the template molecule from the polymer matrix, the particles were soxhlet extraction with MeOH/acetonitrile (90/10, v/v) until the template removed completely. Finally, the residual acetonitrile was removed by washed with pure methanol and dried at 55°C. The resulting bulk rigid polymer was crushed and sieved; the particles which have size 125 μm were collected and used for synthesis the sensing membrane of the electrodes, and the same way was applied for prepared the Non-Imprinted Polymer (NIP) but without the template drug. A Scanning Electron Microscopy (SEM) was useful for information about size of the MIP particles. The morphology of MIP and NIP membranes for cyproheptadine hydrochloride before and after washing is showed by electron microscope in Figure 2. A porous on the surface (Figure 2a) about 5 μm may indicate the binding sides to the polymer. Figure 2b shows clear holes about 10 μm in sizes have been obtained and which were removed by soxhlet extraction.
Electrode membrane preparation and measurements
In order to prepare of the membrane it requiring by mixing 0.17 g PVC, 0.36 g of the plasticizer (DOA, DIBM, NPPE and TMTM) and 0.04 g of the MIP. After good stirring, 7-8 ml of THF was added as solvent and in order for obtained homogenization solution, the mixture was transferred onto a petri dish of 5 cm in diameter, a mixture leaving at room temperature for 24 h to allowed the THF for evaporate. All measurements were done at room temperature using pH meter (WTW model, Germany) and (WTW model pH 720, Germany). Reference electrode saturated calomel electrode (Gallenkamp, USA) and silver/silver chloride as working electrode, for obtain a familiarity ion all the sensors were soaking in 10-1 M cyproheptadine hydrochloride solution for 24 h.
Preparation of pharmaceutical Sample
Thirty tablets were accurately weighed using a pestle and mortar to grind to obtain a fine powder. Transferred an equivalent to 50 mg of the powder into 100 ml volumetric flask and diluted to 50 ml with same solvent and stirred for 15 min. The solution was filtered by using Whatman filter paper, and transferred into a 100 ml volumetric flask to get concentration of 1.0 × 10-3 M cyproheptadine hydrochloride.
Results and Discussion
Characterization
Fourier Transform Infra-Red (FTIR) spectra of CPH imprinted polymers MIP and NIP were recorded in the range of 400-4000 cm-1 by the KBr disk method (Table 1). The FTIR spectrum of CPH-MIP (HEMA) before template removal showed three variation absorption bands at 1562, 3061 and 1620 cm-1 for C=C stretching, C-H aromatic and C=N stretching group when comparing with the FTIR spectrum of CPH-MIP (HEMA) after template removal and CPH-NIP (HEMA) show disappearance the three bands which proved that CPH was removed from template.
| No. | Functional Group | CPH-MIP(HEMA) before template removal | CPH-MIP(HEMA)after template removal | CPH-NIP(HEMA) |
|---|---|---|---|---|
| 1 | OH str. (cm-1) | 3456 | 3484 | 3338 |
| 2 | N-H str. (cm-1) | 3572 | 3580 | 3460 |
| 3 | C-H aliphatic.(cm-1) | 2931 | 2971 | 2891 |
| 4 | C=O str.ester.(cm-1) | 1678 | 1723 | 1738 |
| 5 | C=O str.amide. (cm-1) | 1683 | 1745 | 1640 |
| 6 | C=C str. (cm-1) | 1562 | ---- | ---- |
| 7 | C-H aromatic.(cm-1) | 3061 | ----- | ----- |
| 8 | C=N str.(cm-1) | 1620 | ----- | ----- |
Table 1: The characteristic peaks of FTIR spectra for CPH-MIP and NIP using (HEMA) as a functional monomer
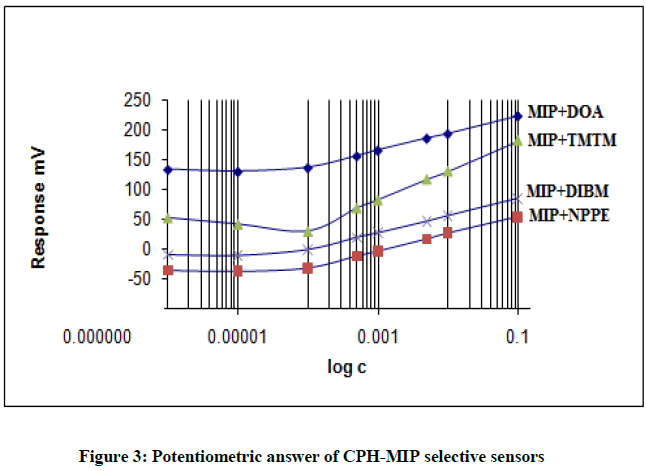
Four electrodes of different compositions were prepared using four different plasticizers DOA, DIBM, 2-NPPE and TMTM based upon the different viscosities. The calibration curve of the electrodes is shown in Figure 3. The results of electrode specification were obtained from the calibration curves are listed in Table 2.
From the Table 2 concluded that the best characteristics were obtained for the sensor with plasticizers DOA and TMTM which have a near- Nernstian in the range 1 × 10-1 – 5 × 10-4 M and 1 × 10-1 – 1 × 10-4 with a slopes of 55.4 and 58.8 mV/decade and a detection limit of 2.3 × 10-5 M and 1.3 × 10-5, respectively.
| Electrode no. | I | II | III | IV |
|---|---|---|---|---|
| Type of Electrode | CPH- MIP + DOA | CPH- MIP +DIBM | CPH- MIP +NPPE | CPH- MIP + TMTM |
| Slope mV/decade |
55.4 | 52.3 | 49.4 | 58.8 |
| Detection limit (M) | 2.3×10-5 | 3.5×10-5 | 2.8×10-5 | 1.3×10-5 |
| Correlation coefficient | 0.9996 | 0.9987 | 0.9984 | 0.9990 |
| Linear range(M) | 1×10-1-5×10-4 | 1×10-1-5×10-4 | 1×10-1-1×10-4 | 1×10-1-1×10-4 |
| Life time(day) | ~35 | ~27 | ~15 | ~41 |
Table 2: Characteristic of CPH-MIP selective electrode by using (HEMA) as a functional monomers at different plasticizers
Effect of pH
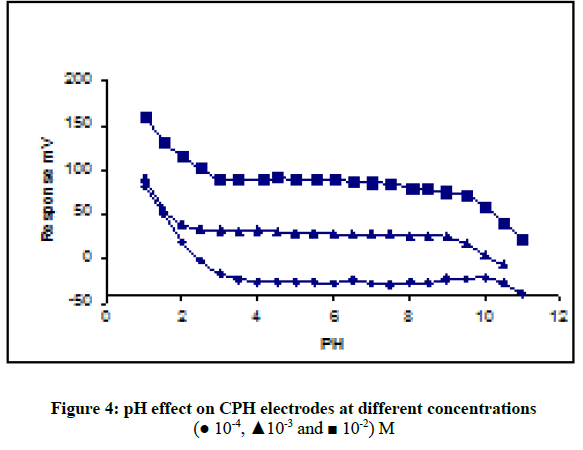
In order to know the suitable pH for measurement the potential of synthesis electrodes were tested over the pH range 2.5-11.0 for 1.0 × 10-2, 1.0 × 10-3 and 1.0 × 10-4 M CPH solutions, respectively. To control the acidic of solution by adding small volumes of (1.0 M HCl and 0.1 M NaOH) and the potentials were recorded. Figure 4 shows the PH was constant over the pH range 2.8-9.2 which considered suitable range of pH working for electrode, and deviation at pH less than 2.8, below this value the drug interfere with the hydrogen ion. But appearance at higher pH values, that potential decreases gradually less than 9.2 which can suggest to formation of free cyproheptadine hydrochloride base in the test solution.
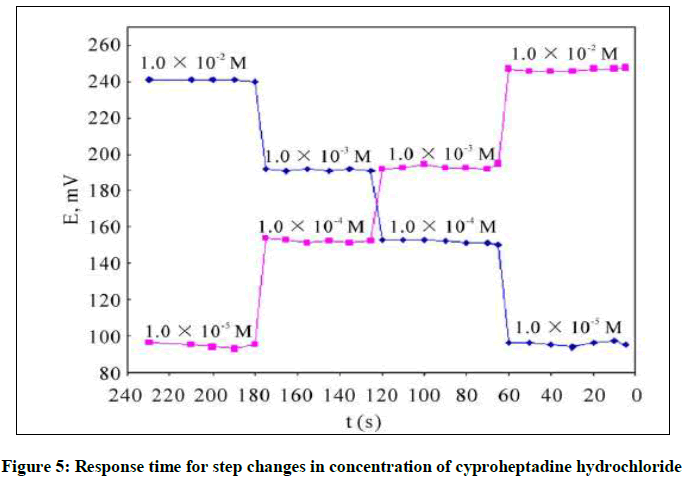
Response time and reversibility of the electrode
For analytical applications response time is an important role for evaluation for synthetic electrodes, which have relationship with life time and direct effect at the slope. Therefor the response time was recorded with varying the cyproheptadine hydrochloride concentration over the range 1.0 × 10−5 to 1.0 × 10−2 M. The potential arrived equilibrium state in about 5-8 sec as shown in Figure 5. Response time was recorded reversibility measurement initial sequence high-to-low (from 1.0 × 10−2 to 1.0 × 10−5 M) sample concentrations, although it did not have any significant both methods.
Effect of interfering ions
For determination the selectivity coefficient was determined by two methods, the first method was Separate Solution Method (SSM), which based on Nickolsky-Eisenman equation and the second method was matched potential method (MPM), which used when the analyte and/ or the interfering ion were not vassalage with the Nernst response or when the involved ions have unequal charges. Table 3 shows that the electrode response to the cyproheptadine hydrochloride more that the interfering ions that means the electrodes appears high selectivity to the CPH over interfering ions such as inorganic ions, sugars, amino acids, and drugs. Due the added excipients to pharmaceutical analysis it is important to test the selectivity. From the values of selectivity coefficient appearance no interfering obtained between and cyproheptadine hydrochloride and interfering ions.
| Interfering ion | SSM | MPM |
|---|---|---|
| Li+ | 8.11×10-3 | 3.34×10-5 |
| Na+ | 5.12×10-3 | 6.74×10-5 |
| K+ | 2.76×10-3 | 5.41×10-5 |
| Cd2+ | 4.22×10-3 | 3.36×10-4 |
| Mg2+ | 5 . 29 ×10- 4 | 6.11×10-5 |
| CO2+ | 9.33×10-5 | 8.59×10-6 |
| Al3+ | 7.39×10-6 | 4.38×10-4 |
| Ce3+ | 1.25×10-4 | 5.61×10-4 |
| Fe3+ | 2.32×10-4 | 4.15×10-3 |
| D-Froctose | 2.44×10-2 | 1.76×10-3 |
| D-Galactose | 6.56×10-2 | 3.72×10-5 |
| Maltose | 4.77×10-4 | 5.82×10-2 |
| Sucrose | 9.34×10-2 | 3.29×10-4 |
| Gluctose | 2.76×10-3 | 7.32×10-3 |
| Captopril | 5.78×10-4 | 4.31×10-5 |
| Spiramycine | 2.66×10-2 | 1.77×10-2 |
| Ephidrine | 8.0×10-6 | 3.65×10-2 |
| Lidocaine | 8.0×10-6 | 8.26×10-3 |
Table 3: Selectivity coefficients of various interfering ions for (CPH-MIP (HEMA)+EBMAA+DOA) electrode
Sample analyses
To estimate the drug in pharmaceutical, first analysis had to be set from the experience for synthetic solutions of pure drug. Four potentiometric techniques were used for the estimation of cyproheptadine hydrochloride included, Direct (DM), Standard Addition (SAM), Multiple Standard Solution (MSAM) and Titration (TM) methods, respectively. Synthetic solutions of cyproheptadine hydrochloride were prepared at two different concentrations (10−3 and 10−4 M). The Percent Recovery (%RC), Relative Standard Deviation (%RSD) and Relative Error (%RE) were calculated and are listed in Table 4.
| Electrode No. | Concentrations (M) | ||||
|---|---|---|---|---|---|
| Sample | Measurements using different methods | ||||
| DM | SAM | MSAM | TM | ||
| CPH+HEMA + EBMAA +DOA (I) |
1×10-3 | 1.014×10-3 | 1.008×10-3 | 0.998×10-3 | 0.95×10-3 |
| RSD% | 2.38* | 1.12* | - | 1.38* | |
| RC% | 101.4 | 100.8 | 99.8 | 95 | |
| RE% | 1.4 | 0.8 | -0.2 | -5 | |
| 1×10-4 | 0.983×10-4 | 0.988×10-4 | 0.993×10-4 | 0.98×10-4 | |
| RSD% | 0.365* | 0.942* | - | 0.476* | |
| RC% | 98.32 | 98.8 | 99.3 | 98 | |
| RE% | -1.68 | -1.2 | -0.7 | 2 | |
| CPH–HEMA +EBMAA+ TMTM (IV) |
1×10-3 | 0.992×10-3 | 0.996×10-3 | 0.998×10-3 | 0.947×10-3 |
| RSD% | 2.26* | 1.08* | - | 1.37* | |
| RC% | 99.2 | 99.6 | 99.8 | 94.7 | |
| RE% | -0.8 | -0.4 | -0.2 | -5.3 | |
| 1×10-4 | 1.018×10-4 | 0.996×10-4 | 1.006×10-4 | 1.05×10-4 | |
| RSD% | 3.51* | 2.36* | - | 1.56* | |
| RC% | 101.8 | 99.6 | 100.6 | 10 5 | |
| RE% | 1.8 | -0.4 | 0.6 | 5 | |
Table 4: Determination of cyproheptadine hydrochloride ion samples by potentiometric technique
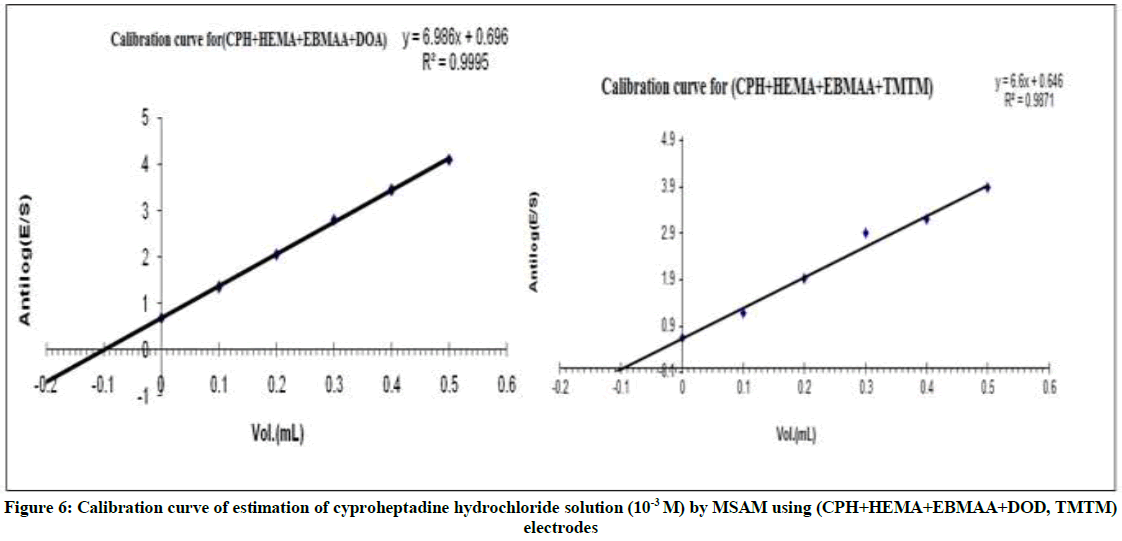
Figure 6, show the relationship between antilog (E/S) and volume of the five addition of standard (CPH), due to taken several volumes results and measurements were repeated several times, this methods appear low %RE when used to determine the concentration of cyproheptadine hydrochloride solutions.
For potentiometric titration a 10-3 M N-bromo succinimide (NBS) was used as a titrant, a typical titration plot was shown in Figure 7.
For each method: %RC, %RSD and %RE were calculated and the results were listed in Table 4. The electrodes I and IV were proved to be useful in the potentiometric determination of cyproheptadine hydrochloride in pharmaceutical preparations by DM, SAM, MSAM and TM method and the data obtained for pharmaceutical samples were listed in Tables 5 and 6.
| Pharmaceutical | Germany | ||||||
|---|---|---|---|---|---|---|---|
| DM | SAM | MSA | TM (NBS) |
||||
| Concentration Prepared | 1×10-3 | 1×10-3 | 1×10-3 | 1×10-3 | |||
| Found | 1.028×10-3 | 1.023×10-3 | 1.020×10-3 | 0.950×10-3 | |||
| RC% | 102.8 | 102.3 | 102.0 | 95.0 | |||
| RE % | 2.8 | 2.3 | 2.0 | -5.0 | |||
| *RSD% | 0.642* | 0.127* | - | 1.521* | |||
| Fexperimental | 12.592 | 9.345 | - | 11.692 | |||
| F theoretical | 19.0 | ||||||
| Pharmaceutical | Turkey | ||||||
| DM | SAM | MSA | TM (NBS) |
||||
| Concentration Prepared | 1×10-3 | 1×10-3 | 1×10-3 | 1×10-3 | |||
| Found | 1.052×10-3 | 1.033×10-3 | 1.023×10-3 | 0.960×10-3 | |||
| RC % | 105.2 | 103.3 | 102.3 | 96.0 | |||
| RE % | 5.2 | 3.3 | 2.3 | -4 | |||
| *RSD% | 2.791* | 0.665* | - | 3.845* | |||
| Fexperimental | 13.672 | 11.835 | - | 14.257 | |||
| F theoretical | 19.0 | ||||||
Table 5: Sample analyses of pharmaceutical cyproheptadine hydrochloride using (CPH+HEMA+EBMAA+DOA) electrode
| Pharmaceutical | Germany | ||||
|---|---|---|---|---|---|
| DM | SAM | MSAM | TM (NBS) |
||
| Concentration Prepared | 1×10-3 | 1×10-3 | 1×10-3 | 1×10-3 | |
| Found | 1.041×10-3 | 0.987×10-3 | 0.981×10-3 | 0.51×10-3 | |
| RC % | 104.1 | 98.76 | 98.1 | 95.0 | |
| RE % | 4.1 | -1.24 | -1.9 | -5.0 | |
| *RSD% | 1.405* | 0.509* | - | 3.251* | |
| Fexperimental | 14.562 | 9.306 | - | 15.576 | |
| F theoretical | 19.0 | ||||
| Pharmaceutical | Turkey | ||||
| DM | SAM | MSAM | TM (NBS) |
||
| Concentration Prepared | 1×10-3 | 1×10-3 | 1×10-3 | 1×10-3 | |
| Found | 1.036×10-3 | 1.031×10-3 | 1.022×10-3 | 0.96×10-3 | |
| RC % | 103.6 | 103.1 | 102.2 | 96.0 | |
| RE % | 3.6 | 3.1 | 2.2 | -4.0 | |
| *RSD% | 1.735* | 0.310* | - | 3.818* | |
| Fexperimental | 15.143 | 12.062 | - | 16.493 | |
| F theoretical | 19.0 | ||||
Table 6: Sample analyses of pharmaceutical cyproheptadine hydrochloride using (CPH+HEMA+EBMAA+TMTM) electrode
Conclusion
Non-covalent molecularly imprinted polymer electrodes were prepared using cyproheptadine hydrochloride as a template and HEMA as monomer in different plasticizers: DOA, DIBM, NPPE and TMTM. Excellent results of MIP which show high sensitivity, reasonable selectivity, fast static response, long-term stability and applicability over a wide pH range were obtained by using electrode based on DOA and TMTM plasticizers. Good recoveries and low relative error were obtained for the determination of cyproheptadine hydrochloride in commercial pharmaceutical tablets comparing with the British Pharmacopoeia.
References
- S. C. Sweetma Martindale: the complete drug reference. Pharmaceutical Press, London, Electronic version (2007).
- A. C. Moffat Clarke's analysis of drugs and poisons. Pharmaceutical Press, London, Electronic version (2006)
- The United States Pharmacopoeia 30, the National Formulary 25, US Pharmacopeial Convention; Electronic version (2007)
- X. Feas, L. Ye, S. V. Hosseini, C. A. Fente and A. Cepeda, J. Pharm Biomed Anal 50 (2009) 1044.
- C. Yang, Q. Men Q, Yaowu Fenxi Zazhi, 11 (1991) 113.
- R. T. Sane, P. P. Karkhanis, P. G. Anaokar, Indian J. Pharm. Sci., 43 (1981) 111.
- A. D. Mao, B. F. Wang, Yaowu Fenxi Zazhi, 21 (2001) 60.
- P. A. Williams, Pharmacopeial Forum., 14 (1988) 3463.
- G. R. Rao, S. Raghuveer, Indian Drugs, 22 (1985) 377.
- G. W. Burrows, C. L. Alliger, J. Pharm. Sci., 72 (1983)1212.
- K. Basavaiah, V. S.Charan , U. Chandrashekar, P. Nagegowda and B. C. Somashekar,Bulgarian Chem. Comm., 36 (2004) 112.
- Z. Lingya, China. Pharmacis., 09 (2009).
- A. El-Gindy, F. El-Yazby, A. Mostafa and M. M. Maher, J. Pharm. Biomed. Anal., 35 (2004) 703.
- C. Ying, J. Yin and Z. Wengang, China Feed, 02 (2011)
- S. Adamski, Acta Pol. Pharm., 22 (1965) 311.
- J. Emmanuel and T. V. Yegnanarayan, Indian Drugs, 19 (1982) 505.
- R. T. Sane, U. M. Vaidya and V. G. Nayak, Indian Drugs 19 (1982) 398.
- C. N. Carducci, G. Barcic and A. Mascaro, SAFYBI 19 (1979) 1358.
- K. Basavaiah and V. S. Charan, Sci. Asia, 30 (2004) 163.
- K. Basavaiah and V. S. Charan, Turk. J. Chem., 26 (2002) 653.
- D. M. Shingbal and S. D. Naik, Indian Drugs, 18 (1981) 444.
- K. Basavaiah, Indian J. Chem. Technol., 13 (2006) 360.
- Y. K. Al-Bayati and F. I. Aljabari, Asian J. of Chem., 28 (2016) 1376.
- Y. K. Al-Bayati, K. H. Al-Saidi, and M. A. Hussain, Asian J. of Chem., 28 (2016) 1962.
- Y. K. Al-Bayati, and IK. H. Al Khafaji, Iraqi J. of Sci., 57 (2016) 2790.