Abstract
Design, Efficient Synthesis and Antimicrobial Evaluation of Some Novel Pyrano[2, 3-b][1, 8]Naphthyridine and Pyrrolo [2,3-f][1,8] Naphth- yridine Derivatives
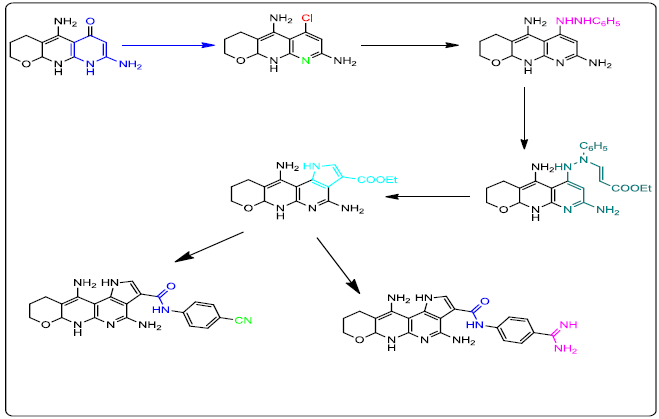
Author(s): Nadia Ali Ahmed Elkanzi*, Amira A Ghoneim and Rania B BakrReaction of Ethyl 5,7-diamino-3,4,8, 8a-tetrahydro-2H-pyrano[2, 3-b]pyridine-6-carboxylate (5) with acetonitrile and acetic acid afforded the corresponding 5,8-diamino-3,4,10,10a-tetrahydro-2H-pyrano[2,3-b][1,8]naphthyridin-6(9H)-one (6) which was reacted with phosphorus ox chloride to give crude 7. A solution of crude 7 was added to phenyl hydrazine to afford 6-(2-phenylhydrazinyl)-3,4,10,10a-tetrahydro-2Hpyrano[ 2,3-b][1,8]naphthyridine-5,8-diamine (8). Treatment of compound 8 with ethyl propiolate yielded compound 9 which was heated under reflux to produce the corresponding ethyl 4,11-diamino-1,6,6a,8,9,10-hexahydropyrano[2,3-b]pyrrolo[2,3-f][1,8]naphthyridine-3- carboxylate (10). Hydrolysis of the latter compound 10 with 10% sodium hydroxide afforded the acid derivative 11 which was reacted with 4- aminobenzamidinedihydrochloride (12), benzotriazol-1-yloxytris (pyrrolidino) phosphonium-hexafluorophosphate andN-ethyldiisopropylamine to afford the target compound 13. The formation of 4,11-diamino-N-(4-cyanophenyl)-1,6,6a,8,9,10-hexa-hydropyrano[2,3- b]pyrrolo[2,3-f][1,8]naphthyridine-3-carboxamide 15 was achieved by reaction of benzotriazol-1-yloxytris (pyrrolidino) phosphoniumhexafluorophosphate, 4-cyanoaniline (14) and N-ethyldiisopropylamine with the key intermediate (11). The structure of all the new compounds was demonstrated by elemental analysis, Infrared (IR), Proton Nuclear Magnetic Resonance (1H-NMR) and Carbon-13 Nuclear Magnetic Resonance (13C-NMR) spectra and mass spectra. Antimicrobial activity of these compounds 5-8, 10, 11, 13 and 15 was evaluated against Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa bacteria Aspergillus flavus and Candida albicans. 5,8- Diamino-3,4,10,10a-tetrahydro-2H-pyrano[2,3-b][1,8]naphthyridin-6(9H)-one (6) was found to be the most active against all the tested microorganisms. Furthermore, molecular modeling study had been performed on the target compound (6) to expect the mode of action of this candidate as a lead for designing other antimicrobial agents.
Select your language of interest to view the total content in your interested language
Google Scholar citation report
Citations : 15261
Der Pharma Chemica received 15261 citations as per Google Scholar report
Der Pharma Chemica peer review process verified at publons



