Research Article - Der Pharma Chemica ( 2025) Volume 17, Issue 2
An Overview and in vitro Evaluation of Effervescent Tablets of Lornoxicam with Antacids
Aishwarya Hiremath*Aishwarya Hiremath, Department of Pharmaceutics, Rajiv Gandhi University of Health Sciences, Karnataka, India, Email: aishwaryashiremath1990@gmail.com
Received: 11-Dec-2023, Manuscript No. DPC-23-122513; Editor assigned: 14-Dec-2023, Pre QC No. DPC-23-122513; Reviewed: 29-Dec-2023, QC No. DPC-23-122513; Revised: 01-Feb-2024, Manuscript No. DPC-23-122513; Published: 11-Feb-2024, DOI: 10.4172/0975-413X.17.2.638-642
Abstract
Objective: The objective of this study was to mask the bitterness of lornoxicam by inclusion complex method using β cyclodextrin (β CD), to prepare lornoxicam effervescent tablets with antacids by wet granulation method using citric acid, tartaric acid with sodium bicarbonate as effervescent mixture, which is useful to neutralize the acid that may be occurred due to the co-administration of lornoxicam and to compare the in vitro drug release and Acid Neutralizing Capacity (ANC) of tablets of optimized batch with marketed products.
Methods: Authentication of lornoxicam was done by FTIR and DSC analysis. The spectral interference of the excipients at analytical wavelength of lornoxicam was studied using UV method. Prepared tablets of lornoxicam then subjected to evaluation of post compression parameters such as thickness and diameter, wetting time, weight variation, hardness, friability, disintegration, dissolution and Acid Neutralizing Capacity (ANC). Drug release profile and ANC of the tablets of optimized batch F-9 were compared with that of the marketed products, further studied for their reproducibility and stability.
Results: FTIR and DSC analysis revealed that the received drug was pure lornoxicam. The excipients were showing no absorbance at analytical wavelength of lornoxicam (289.20 nm). The tablets of all the batches were pass the post compression parameters. The tablets of optimized batch F-9 were able to produce release of 100% drug within 5 min with 12.65 ± 0.160 mEq of ANC. The tablets were found to be reproducible and stable after stability studies.
Conclusion: Effervescent tablets of lornoxicam with antacids can be efficiently and successfully formulated by wet granulation method with addition of superdisintegrant. The prepared tablets of lornoxicam with sodium saccharin can be chewed and alternatively used as fast dissolving tablets, as it undergoes disintegration within 40 seconds and increases patient compliance, in addition they were able to produce faster dissolution and better ANC than that of the marketed products.
Keywords
Effervescent tablets; Lornoxicam; Inclusion complex; Superdisintegrant; Effervescent mixture; Crosspovidone; Fast dissolving tablets
Introduction
The per oral dosage forms (tablet and capsule) have vast range of acceptance of 50-60% of total dosage forms because, these can be easily administered, manufactured, pact in nature and can be given in exact dose. Per oral dosage forms suffering from decelerated absorption and thus slow onset of action, this can be avoided by administrating the drug in liquid form but, several APIs have restricted level of stability in liquid form. Hence, effervescent tablets can be considered as a substituent dosage form.
In modern years Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) have acquired great attention in the treatment of inflammation and to supress the pain resulting from wound and infection. The important reason behind such an attention is that NSAIDs are does not produce narcotic effects like narcotic analgesics because of that, these are also called as non-narcotic analgesics. The common NSAIDs available in the market are salicylates, ibuprofen, naproxen, diclofenac, meloxicam, lornoxicam, etc.
The NSAIDs mainly causes Gastrointestinal Tract (GIT) toxicity. If NSAIDs are used for longer duration, in the treatment of chronic disease like arthritis will definitely causes the acidity followed by ulcers and porosity. Around the world, on a daily basis, more than 35 million people consume these drugs. Amongst them 30% of users may develop sufficient degree of GI toxicity. It has also been assumed that one third of the cost of treating arthritis patients relates to treatment of the side effects of NSAIDs. Conservative calculation estimated that, nearly 1, 07, 000 patients are hospitalised annually for NSAID related GI complexities like ulcers and porosity. Five to 15 percent of patients, who are taking NSAIDs for the treatment of rheumatoid arthritis, can be expected to discontinue the therapy within a six-month period of treatment, because of occurrence of new gastric and duodenal ulcers.
To treat such conditions physicians will prescribe an antacid tablet along with the NSAIDs like lornoxicam. To avoid intake of two tablets, it was thought to combine both NSAID and antacid, to formulate an effervescent tablet which contains both the actives. This study deals with formulation of a combined antacid and analgesic antacid effervescent tablet, this is useful to neutralise the acid that could be released due to co-administration of a lornoxicam. Lornoxicam is used as a model analgesic drug, the mixture of aluminium hydroxide and magnesium hydroxide is used as the model antacid, tartaric acid and citric acid in combination with sodium bicarbonate is being used as the effervescent mixture in 2:1:3.4 ratios to get good effervescence. This preparation can also be dispensed in the form of granules in unit sachets.
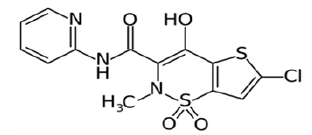
Lornoxicam is a new NSAID derived from oxicams, belongs to the Class II drug in BCS classification. Lornoxicam is an anti-inflammatory and analgesic drug acts by inhibiting the prostaglandin and thromboxane synthesis through the inhibition of both COX-1 and COX-2 which are responsible for the inflammation and elevated body temperature (Figure 1) [1].
Lornoxicam is extremely bitter in taste. Therefore, to provide this drug in a more convenient and patient compliant form, an effort was made in this study, to mask the bitter taste of lornoxicam and establish it in the form of effervescent tablet, it can even be chewed, in case of the inability to obtain the water. The lornoxicam inclusion complxes were prepared to mask the bitter taste of the drug, by adopting Lor: beta cyclodextrin (βCD) in 1:2 molar ratio using Polytron High Speed Mixer, Kinematic–Japan (Figure 2).
The effervescent tablet to be added to water just before administration and the drug dispersion or solution is to be drunk immediately. In presence of water, an interaction between tartar acid and citric acid will occur with alkali metal carbonates or bicarbonates which internally liberate CO2 in water so that the tablet quickly breaks off [2].
Antacids are widely used to relieve mild and frequent symptoms associated with acid- related disease. They act by neutralizing gastric acid and thus increasing gastric pH. The magnesium containing antacids may cause dose-related osmotic diarrhoea. But combining it with aluminium salts which can cause constipation when used alone can offset this side effect. The aluminium and magnesium hydroxide were both used with a substantial dosage 250 mg each in the proposed study, in conjunction, because the elevation of intragastric pH is dose-dependent and usually requires a substantial dose of antacids to raise the intragastric pH above 4 or 5.
Materials and Methods
Materials
Lornoxicam pure drug was received from Zydus Research Centre, Ahmedabad. Aluminium hydroxide, magnesium hydroxide, citric acid, tartaric acid, crospovidone, sodium hydroxide, and all of the other reagents and chemicals used were of pharmaceutical grade [3].
Method
Authentication of lornoxicam
Melting point: The melting point of lornoxicam (API, purified drug) was determined using a programmed melting point apparatus (DBK instruments, Mumbai), and the melting temperature of the drug was measured.
FTIR: FTIR studies were conducted using 8300 Spectrophotometer (Shimadzu, Kyoto, Japan) with potassium bromide pellet method. The source provided a continuous spectrum of radiation ranging between 4000 to 400 cm-1.
Differential scanning calorimetry: Lornoxicam was subjected differential scanning calorimetry analysis using DSC-60 (Shimadzu, Kyoto, Japan) calorimeter. The melting point of lornoxicam was recorded from the obtained DSC of lornoxicam and compared with the literature value.
Analytical method
In the range between 5 and 30 µg/ml, a series of lornoxicam solutions were prepared, where the drug obeys beer’s lamberts law. 10 µg/ml solution was scanned in UV visible spectrophotometer in the wavelength range of 200-400 nm using phosphate buffer solution, pH 7.4 as blank, to get absorption maxima of lornoxicam [4].
Spectral interference with analytical wavelength of lornoxicam
Since excipients were used along with the lornoxicam in the tablets, it was necessary to identify the interference of excipients with the analytical wavelength, 289.20 nm. The solutions of lornoxicam, antacids and other excipients were prepared at highest concentrations used in formulations. Spectral interference was studied by scanning the solutions in the UV region of 200 to 400 nm.
Formulation development
Preparation of lornoxicam - cyclodextrin inclusion complexes
Lornoxicam drug is highly bitter in taste, hence to mask the bitter taste of the drug inclusion complexes were prepared. The complexes were prepared taking lornoxicam and β-cyclodextrin in 1:2 molar ratio. Based on the saturation solubility study, 80%v/v ethanol was selected as the solvent for complexation, which shown highest solubility of lornoxicam.
Microwave method: Precisely weighed amounts of lornoxicam (8 mg, molecular weight of 371.82) and β-CD (49 mg, molecular weight of 2269.97) at the molar ratio of 1:2 were taken in ethanol 80% (v/v) at 60 °C. After that the solution was kept in microwave at 60°C for 90 seconds. The solvent was evaporated under oven and the collected residue was pulverized and sized through sieve no. 60.
Co-evaporation method: Precisely weighed amounts of lornoxicam and β-CD at the molar ratio of 1:2 were taken in ethanol 80% (v/v) and stirred by using magnetic stirrer at 60°C. The resultant solution was dried in an oven, pulverized and sized.
Mixing at high speed (15000 rpm): Precisely weighed amounts of lornoxicam and β-CD at the molar ratio of 1:2 were taken in ethanol 80% (v/v) at 60°C and mixed with a high speed (15000 rpm) by using Polytron, Kinematic mixer for 8 minutes. The solution was dried in an oven at 60°C for 4 hours17 and further 1 hour at 80°C for the complete evaporation of ethanol.
Physical mixture: Accurately weighed amounts of lornoxicam at the molar ratio of 1:2 were blended by simple mixing method in a vial for 30 min. This mixture was used further for comparision purpose.
Content estimation of lornoxicamin inclusion complexes: Eight mg equivalent weight of complexes and physical mixture were placed in 100 ml phosphate buffer, 7.4 Ph. At 289.20 nm, assayed Spectrophotometrically for drug content [5].
Selection of dose of lornoxicam
The drug lornoxicam is available in the market as 4 mg, 8 mg, and 16 mg tablets. Lornoxicam injections are also available in the market. For the purpose of this study, lornoxicama NSAID drug was taken in the concentration of 8 mg. This medium dose was selected to avoid the excess utility of drug with 16 mg dose and also to meet the experimental sensitivity which may not be possible with 4 mg dose.
Selection of dose of antacids
The antacids available in the market were studied and compositions of widely used antacids are mentioned in the Table 1. It was observed that, the composition of antacids is not same. They used different antacids to get Acid Neutralizing Capacity (ANC) of 12.4 to 17.4 mEq. In the proposed formulation, antacid was planned to add with lornoxicam (NSAID). Hence it was aimed to get minimum ANC of 12.4 mEq [6].
| Name of antacid | Name of the company | Ingredients (mg) | |||||
|---|---|---|---|---|---|---|---|
| Al(OH)3 | Mg(OH)2 | Mg CO3 | Mg. Trisilicate | Mg.Al. Silicate | NaHCO3 | ||
| Digene | Abbott | 300 | 25 | - | - | 50 | - |
| Entasid | Cadila | 250 | 250 | - | - | 250 | - |
| Gelusil MPS | Pfizer | 250 | 250 | - | - | 50 | - |
| Almacarb | GSK | 325 | - | 50 | - | - | - |
| Embesil | Nicholas | 380 | 185 | - | - | - | - |
| Gaviscon | GSK | 100 | - | - | 25 | - | 170 |
| Practol | Zydus | 500 | - | - | - | - | - |
| PFT | Reckitt Piramal Ltd. | 400 | 400 | - | - | - | - |
| Reflux Forte | Dr. Reddy’s | 300 | 150 | - | - | - | - |
| Sebella | Wyeth | 426 | - | - | - | - | - |
Table 1: Data of compositions of marketed antacid tablets.
Wet granulation method
The wet granulation technique is used for the manufacturing of tablets by using polyvinyl alcohol as binding agent, the tablets so prepared were very hard and did not undergo disintegration in the limits stated by IP in spite of effervescent agents present in the tablets. Binder was changed to polyvinyl pyrolidone but the tablets posed the same problem. Finally the idea of using absolute alcohol as a binder was implemented just to increase the density of the tablet blend. The tablets passed for the disintegration test.
Lornoxicam complex, aluminium hydroxide gel, citric acid, magnesium hydroxide, tartaric acid, crosspovidone, sodium bicarbonate, sodium saccharin, and microcrystalline cellulose were passed through sieve #60. Required amount of all the above ingredients were weighed accurately and mixed in a polybag by geometric addition method for 20 minutes, manually and added peppermint flavour. Absolute alcohol is used to granulate the powder mix. The dough mass sifted through sieve (8 #), the granules dried using conventional hot air oven at 60°C. The sieves of size #20/#40 were used to sift the dried grnules, lubricated with talc (2%w/w). The compaction of powder mixture was done on 10 stations rotary compression machine (Rimek, Nanikadi) using 12.5 mm hexagonal shaped punch.
Design of formulations: Development of the formulation in the proposed study was mainly based on the different concentrations of effervescent mixture followed by randomly selected method of decreasing the concentration the effervescent mixture with same concentration of antacids and lornoxicam. Talc was utilised as glidant. MCC was utilised as a diluent to get the final weight of the tablet as 850 mg. Other excipients were chosen so as to get tablets with good physical properties. In total, seven different effervescent compositions (F3 to F9) were designed based on the observation made in first two formulations (F1 and F2), as shown in the Table 2.
Pre-compression parameters
Angle of Repose (θ)
The values of angle of repose for tablet mixtures were determined by funnel - method (Reposogram). The exactly weighed tablet blend was made flow freely through the funnel. The funnel was adjusted to a height, so that its tip just touches the peak of the powder cone. The diameter of the cone was determined, and angle of repose is designated by θ and given by equation.
θ = tan-1 (h/r)
Where,
h=the height of the powder cone and r = the radius of the powder cone.
Bulk density
Tapped Bulk Density (TBD) and Loose Bulk Density (LBD) of the tablet blends were calculated using bulk density apparatus-USP (Electrolab, India).
Procedure: Test sample powder having volume in between 40 to 45 ml was taken in a graduated measuring cylinder and initial/basic volume was measured (v1). At the beginning the cylinder was tapped for 500 times. Measured the tapped volume. The tapping was repeated an additional 750. Again the (v2) was measured to the nearest graduated unit. The tapping was repeated until the difference between two volumes was more than 2%.
The TBD and LBD were calculated in gm per ml using following formulae;
TBD=Weight of the powder/tapped volume of the packing
LBD=Weight of the powder/volume of the packing
Compressibility Index (Carr’s index)
The powder compressibility index was calculated by Carr’s compressibility index. Carr’s index (%) can be calculated by using the following formula:
Carr’s index (%)=[(TBD–LBD) x 100]/TBD
Hausner’s Ratio
The Hausner’s ratio for the powder was calculated by the following equation.
Hausner’s ratio=TBD/LBD
| Hausner’s ratio | Flow |
|---|---|
| 1.00-1.11 | good |
| 1.12-1.18 | excellent |
| 1.16-1.25 | fair |
| 1.26-1.34 | passable |
| 1.35-1.45 | poor |
| 1.46-1.59 | very poor |
| >1.60 | very very poor |
Table 2: Grading of the powders for their flow properties–Hausner’s ratio.
Post-compression parameters/Evaluation of lornoxicam tablets
Appearance
The tablets were inspected for the existence of cracks, depressions, pinholes etc., if any and uniformity of the color of the tablet.
Dimensions
10 tablets were selected randomly from each batch. Thickness and diameter of the tablets were measured three times using verniercalipers (Mitutuyo, Japan).
Weight uniformity test
20 tablets were individually weighed from each batch and the average weight was calculated from total weight of 20 tablets. The individual weights were compared to average weight. The percentage weight difference should be within the permitted limit (± 5.0%) as the tablets weigh 850 mg. Calculated for the percentage deviation using the following formula,
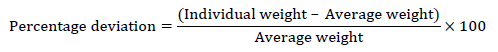
Hardness test
Hardness (diametric crushing strength) is a force required to break a tablet across the diameter. The strength of a tablet is determined by its hardness. The tablet should be stable to mechanical stress during handling and transportation. The hardness was determined by using Monsanto Hardness Tester (Sheetal Scientific Industries, Ahmedabad. The average of five determinations was calculated in terms kilograms per centimeter square and reported.
Friability test
Roche friabilator (Electrolab, Mumbai) was used to measure the friability of the tablets. It is expressed in percents (%). Six tablet were initially weighed (w 1) and transfered into the Friabilator. The rotation was regulated at a speed of 25 rpm. After completion of 100 rotations (4 minutes), tablets from friabilators were taken and the same tablets collectively weighed again (w2). The percent Friability was calculated.
Friability = [(w1 – w2)/w1] × 100
Where, w1=weight of the tablets before test and w2 = weight of the tablets after test
Content uniformity test
Single tablet was powdered and shaken with 100 ml of phosphate buffer solution, pH 7.4 for 60 minutes and filtered. Further, dilutions were made, the absorbance of the resulting solution was measured. The experiment was performed three times and average was calculated. Acid Neutralizing Capacity of Tablet (ANC)
Here antacids were used along with a NSAID drug. Amount of hydrochloric acid neutralized by a tablet is called as the acid neutralizing capacity of that tablet and it expressed interms of miliequalant (mEq). This can be determined by a technique called back-titration. The ANC value of a formulation essentially should exceed five mEq per dose (minimal labeled dose) to be considered as an antacid.
Procedure: A single tablet was placed in 250ml beaker containing 70 ml water and mixed for 1 min on magnetic stirrer. Pipetted 30 ml of 1 N HCl and added to the test solution, which was prepared as per the above-mentioned procedure and kept with magnetic stirrer. Stirred for 15 min, after the addition of the acid, accurately timed, and titrated within 5 minutes. The extra HCl was titrated against 0.2 N NaOH to achieve a stable pH of 3.5. The ANC was determined. Calculated the amount of acid utilized and presented the results in terms of m Eq. of acid consumed per tablet tested.
Disintegration time
A single tablet was placed in a 250 ml beaker containing 200 ml of water at 25°C (20-30oC as per IP 2018). Numerous gas bubbles were evolved. Then the evolution of the gas around the tablet or its fragments had ceased. The time noted when the complete tablet gets disintegrated. Test done for 5 more tablets and average was determined.
In vitro drug release
In vitro release in the environment of gastrointestinal tract was determined for pure lornoxicma drug, lornoxicam complexes and all the developed formulations using the USP – type II, paddle type apparatus (Electrolab, Mumbai). 500 ml of phosphate buffer solution, pH 7.4 was taken as dissolution medium temperature and rpm were maintained at 37 ± 0.5 °C and 50 rpm respectively, 5 ml samples were withdrawn in 5 min interval upto 30 min, the aliquots were analyzed at 289.20 nm on UV spectrophotometer. The % drug release was determined using the standard curve equation and results shown in Figures 9-11.
Comparison of dissolution profile of optimized lornoxicam tablet with marketed lornoxicam 8 mg tablets
In vitro study of marketed formulation was carried out similar to the procedure given for in vitro drug release of lornoxicam from the developed formulations, in the environment of GI tract. All the conditions were kept maintained as earlier. The collected alequots were analyzed at 289.20 nm using dissolution medium as blank. The cumulative percentage drug release was calculated. Graph of cumulative percentage drug release vs. time (minutes) for all the tablets was plotted and shown in Figure 12.
Comparison of ANC of optimized formulation with marketed formulations
ANC of marketed formulations (Gelusil MPS and Digene) was carried out similar to the procedure given for ANC of the developed formulations of lornoxicam. This capacity can be determined by a technique called back-titration. Two different brands of antacid marketed products were taken for the study. A single antacid tablet was dissolved in excess of hydrochloric acid and then the excess acid was back titrated with standardized sodium hydroxide solution. ANC was calculated and average result of three trials was taken as ANC of the tablet which are given in Table 9.
Reproducibility
In order to verify batch to batch uniformity, tablets of the optimized batch F-9 were manufactured for the second time (reproducible batch). All the conditions were kept constant as earlier. The tablets were evaluated for dissolution and ANC as the rest of the parameters were proved to be reproducible and compared with tablets of the earlier batch as given in the Table 10.
Stability studies
The purpose of stability testing is to prove how the quality of a drug product changes with time under influence of different environmental factors such as temperature and humidity. In the current work stability study was conducted for the tablets optimized formulation F-9 at 40 ± 2°C and 75 ± 5% RH for 6 months.
The tablets were checked for post compression tests and compared with tablets which were evaluated immediately after manufacturing. The results were given in Table 12 and Figure 14.
Results and Discussion
Authentication of Lornoxicam
To check, weather the given drug sample was pure lornoxicam or not, authentication of the sample was done by 3 different tests as followed.
Melting Point (MP): lornoxicam exhibited MP of 223.67°C (n=3). This value matches with the literature value.
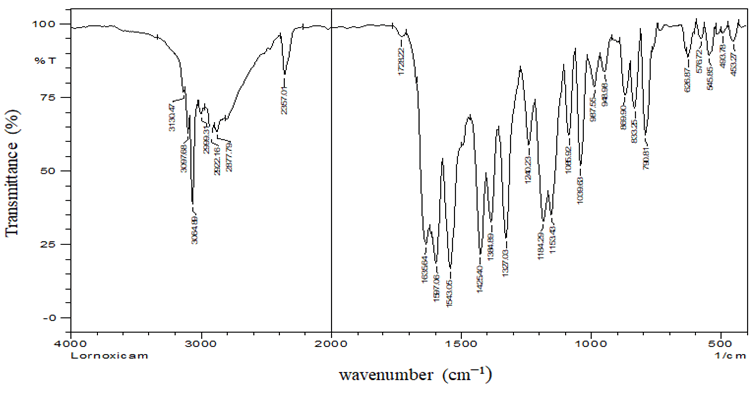
FTIR Study: FTIR studies were conducted on the pure drug lornoxicam using 8300 Spectrophotometer. The source provided a continuous spectrum of radiation ranging between 4000 and 400 cm-1. Intensities of absorption bands were expressed as % transmittance. The sample was prepared using the potassium bromide pellet method. The spectrum was obtained by placing the pellet in the IR chamber and the peak intensities were taken. The peaks found in the spectrum were compared with the data reported in the literature. FT-IR spectrum of pure lornoxicam is shown in the Figure 3 and peaks are listed in Table 3 [7].
| Functional groups | Principal Peaks (cm-1) | |
|---|---|---|
| Standard Peak Region | Standard drug | |
| N-H bend | 1510-1560 (m, s) | 1543.05 |
| -C-Hstretch | 3010-3100 (m) | 3064.89 |
| S=O | 1030-1060 | 1039.63 |
| C=O stretch | 1700-1600 (s, broad) | 1635.64 |
| C-Clstretch | 750-850 (s) | 790.81 |
| C=C stretch | 1450-1600 (m, s) | 1597.06 |
| -C-N | 1350-1000 | 1153.43 |
Table 3: The peak of functional groups observed in IR spectra of lornoxicam.
Since different peaks of the API, which are formed due to the presence of different functional groups, were fall within the standard peak region of the respective functional groups. So, the conclusion can be made that, the given API is lornoxicam only.
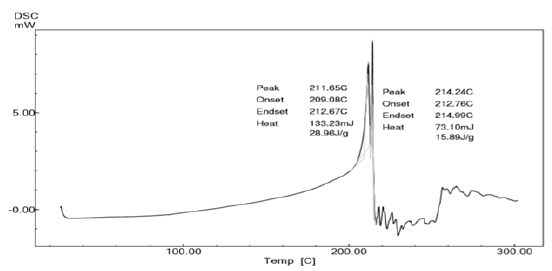
Differential Scanning Calorimetry(DSC) study: DSC analysis of pure drug was performed and the thermogram is shown in the Figure 4. Perusal to the Figure 4, it could be stated that the drug possessed polymorphism. The most stable form of the drug melted at 211.65°C. Later the liquefied drug had undergone polymorphic transition to give less stable or metastable form of lornoxicam. This form again undergone melting at 214.24°C followed by decomposition. These melting points are nearer to the melting point of lornoxicam reported in the literature [8].
By looking at the results of these three tests collectively, the conclusion can be made that, the given drug is pure lornoxicam.
Analytical Method
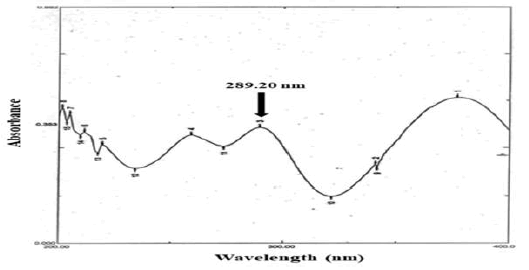
Lornoxicam in phosphate buffer solution, pH 7.4 showed maximum absorption at 289.20 nm and this wavelength was selected as the analytical wavelength. Beer’s law was obeyed in the range between 5 to 30 µg/ml. Regression equation for standard curve was y=0.0329x+0.0015. Correlation coefficient was found to be 0.9999 signifying that the linear relationship between concentration of the drug and absorbance.
Spectral interference with analytical wavelength of lornoxicam
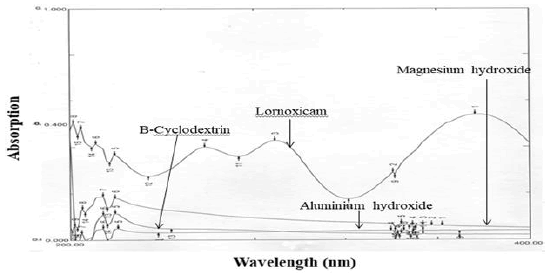
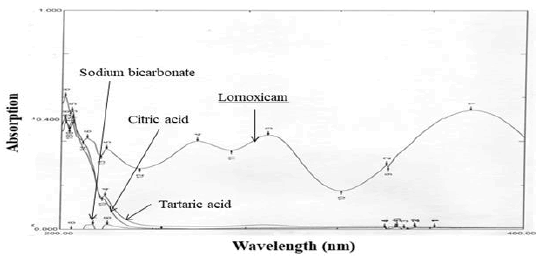
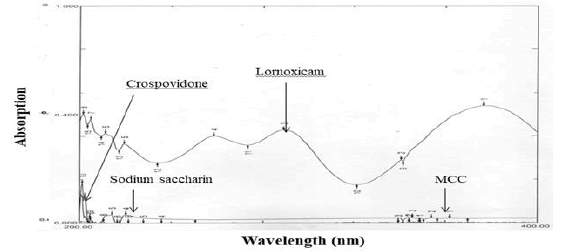
Excipients, if not all, used were organic substances and were suspected to absorb UV radiation interfering lornoxicam estimation. Therefore, interference of excipients with the estimation of lornoxicam was studied. The spectra for pure lornoxicam shown in Figure 5. The spectra for lornoxicam and excipients are shown in Figures 7 to 9. Perusal to the Figures 6 to 8 indicated that excipients did not interfere with the estimation of lornoxicam. Thus, the selected λmax 289.20 nm was suitable as an analytical wavelength [9].
Content estimation of lornoxicam in inclusion complexes
Lornoxicam content was determined in all the complexes prepared by different methods. The results are given in the Table 4 [10].
| Method | Absorbance* Mean ± SD |
|---|---|
| Microwave method | 0.376 ± 0.03 |
| Co-evaporation method | 0.346 ± 0.027 |
| Mixing at high speed (15000 rpm) | 0.763 ± 0.007 |
| Physicalmixing method | 0.304 ± 0.017 |
| *Each value is an average of six determinations | |
Table 4: Data for content estimation of lornoxicam in inclusion complexes.
The results revealed that, the complexes developed using the method ‘mixing at high speed (15000 rpm) have shown maximum lornoxicam content. Therefore, these complexes were used further to develop the formulation.
Design of formulations: Masking the bitter taste of the lornoxicam drug and formulating it with the antacids was the heart of the experiment and was very important to formulate the effervescent tablets which are able to produce in vitro disintegration within 5 minutes as per IP standards (Tables 5 and 6) [11].
| Ingredients (mg) | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|
| Lornoxicam complex (equivalent to 8 mg drug) | 57 | 57 | 57 | 57 | 57 | 57 | 57 | 57 | 57 |
| Aluminium hydroxide gel | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 |
| Magnesium hydroxide | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 | 250 |
| Citric acid | 35 | - | 35 | 30 | 25 | 20 | 15 | 10 | 5 |
| Tartaric acid | 70 | - | 70 | 60 | 50 | 40 | 30 | 20 | 10 |
| Sodium bicarbonate | 119 | - | 119 | 102 | 85 | 68 | 51 | 34 | 17 |
| Crosspovidone (5%) | - | 42.5 | 42.5 | 42.5 | 42.5 | 42.5 | 42.5 | 42.5 | 42.5 |
| Sodium saccharin | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Talc (2%) | 17 | 17 | 17 | 17 | 17 | 17 | 17 | 17 | 17 |
| MCC | 47 | 228.5 | 4.5 | 36.5 | 68.5 | 100.5 | 132.5 | 164.5 | 196.5 |
| Total weight of tablet | 850 | 850 | 850 | 850 | 850 | 850 | 850 | 850 | 850 |
Table 5: Formulations of lornoxicam effervescent tablets.
| Parameter | F-1 | F-2 | F-3 | F-4 | F-5 | F-6 | F-7 | F-8 | F-9 |
|---|---|---|---|---|---|---|---|---|---|
| Loose bulk density (gm/cc) | 0.378 | 0.378 | 0.366 | 0.356 | 0.38 | 0.39 | 0.367 | 0.384 | 0.396 |
| Tapped bulk density (gm/cc) | 0.491 | 0.504 | 0.485 | 0.475 | 0.5 | 0.52 | 0.488 | 0.51 | 0.52 |
| Angle of repose (°) | 39.7 | 37.35 | 39 | 38.89 | 39.2 | 41.01 | 39 | 38.8 | 39.5 |
| Compressibility index (%) | 23 | 25 | 24.5 | 24.2 | 24 | 25 | 24.7 | 24.7 | 23.84 |
| Hausner ratio | 1.298 | 1.33 | 1.325 | 1.342 | 1.32 | 1.33 | 1.329 | 1.328 | 1.313 |
Table 6: Physical properties of the powder-blends for the batches F-1 to F-9.
The tablets were prepared, after getting all physical properties of the powder-blend satisfactory. The tablets were subjected to evaluation for the post compression parameters. The results are given in the Tables 7 [12].
| Parameters | F-1 | F-2 | F-3 | F-4 | F-5 | F-6 | F-7 | F-8 | F-9 |
|---|---|---|---|---|---|---|---|---|---|
| Hardness (kg/cm2)* | 1.86 | 2.36 | 2.16 | 3.4 | 2.5 | 2.166 | 5.33 | 5.53 | 3.966 |
| ± 0.32 | ± 0.23 | ± 0.28 | ± 0.5 | ± 0.5 | ± 0.28 | ± 0.57 | ± 0.5 | ± 0.5 | |
| Friability (%)# | 1.4 | 0.8 | 1.11 | 1.1 | 1.12 | 1.01 | 0.87 | 0.58 | 0.21 |
| Uniformity of weight (mg)# | 848.9 | 850.8 | 848.5 | 849.3 | 849.4 | 848.7 | 850.6 | 849.5 | 849.2 |
| ± 0.89 | ± 0.2 | ± 0.4 | ± 0.7 | ± 0.4 | ± 0.9 | ± 0.1 | ± 0.4 | ± 0.7 | |
| Drug content (%)** | 98.62 | 99.98 ± 0.57 | 101.1 | 99.8 | 98.3 | 98.73 | 99.9 | 100.1 | 99.9 |
| ± 1.2 | ± 1.4 | ± 1.65 | ± 1.9 | ± 1.8 | ±1.67 | ±1.4 | ± 1.67 | ||
| Thickness (mm)* | 8.7 | 8.1 | 8.11 | 8.13 | 8.85 | 8.86 | 8.11 | 8.18 | 8.12 |
| ±0.01 | ±0.05 | ±0.13 | ±0.05 | ±0.01 | ±0.03 | ±0.04 | ±0.11 | ±0.04 | |
| Diameter (mm)* | 12.08 | 12.01 | 12.1 | 12.1 | 12.09 | 12.1 | 12.09 | 12.09 | 12.1 |
| ±0.03 | ±0.04 | ±0.04 | ±0.08 | ±0.01 | ±0.02 | ±0.01 | ±0.03 | ±0.008 | |
| Wetting time (seconds)** | 133 | 50.8 | 274 | 200 | 125 | 121 | 43.3 | 50 | 35 |
| ±0.04 | ±0.02 | ±0.07 | ±0.01 | ±0.2 | ±0.3 | ±0.13 | ±0.2 | ±0.01 | |
| disintegraion time (seconds)* | 59.3 | 150 | 300 | 240 | 140 | 130 | 50 | 60 | 40 |
| ±0.01 | ±0.13 | .±0.2 | ±0.01 | ±0.12 | ±0.07 | ±0.2 | ±0.1 | ±0.1 | |
| pH | 8 | 8.24 | 8.06 | 8.16 | 8.17 | 8.18 | 8.16 | 8.17 | 8.1 |
| *Each value was an average of six determinations **Each value was an average of three determinations #Results of one batch |
|||||||||
Table 7: Properties of lornoxicam effervescent tablets for the batch F-1 to F-9.
Dissolution Profiles
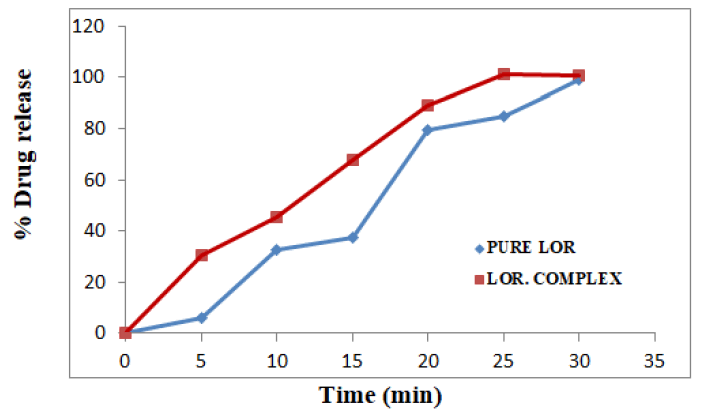
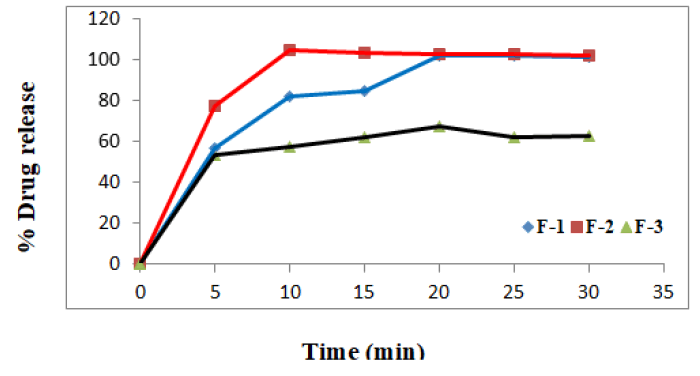
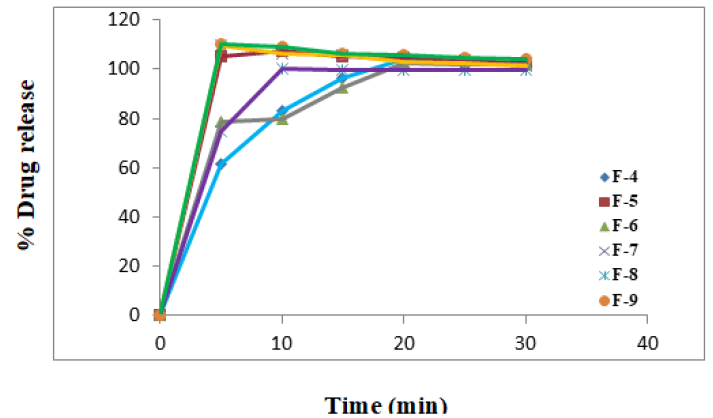
All the batches F-1 to F-9 were showing satisfactory results for all the physical parameters, the dissolution profiles of these batches were studied along with the pure lornoxicam (API) and lornoxicam complexs [13].
Tablets of the batches F-1 and F-2 were formulated for the comparison purpose and from the drug release profiles, it was observed that, 100% lornoxicam was released from the tablets of F-1 in 20 minutes which contains only effervescent mixture. Thus the use of crospovidone at a concentration of 5% was attempted in the tablets of F-2 vomiting effervescent mixture. Surprisingly the dissolution rate was increased with 100% release in 10 minutes. So, both the techniques were adopted in the subsequent development process. But there was no complete release of lornoxicam even after one hour. This behaviour was disappointing. Efforts continued by following randomly selected method of decreasing the concentration of effervescent mixture in the subsequent batches. It was observed inverse proportionality between the concentration of effervescent mixture and percent drug release [14].
The tablets of all the batches were showing good dissolution profile. It was noted that 100% lornoxicam was released in 5 minutes from the tablets of F-9, which contain least amount of effervescence mixture. The tablets of F-7 and F-5 have shown similar release profile when compared to the tablets of F-9. But the tablets of F-9 were preferred, since these were possessing comparatively less friability and fast disintegration as shown in the Table 7 which are good features of the tablets.
Acid Neutralizing Capacity of Tablets (ANC)
Antacid tablets are administered usually after occurrence of acidity for any reason. Therefore, more antacid is required. But in the proposed formulation, antacid is added to neutralize the released acid which could be due to the co-administration of NSAID (Table 8). Therefore, relatively lesser concentration of antacid is required in the tablet [15].
| Formulation code | Acid neutralization capacity (ANC) in mEq/dose | |||
|---|---|---|---|---|
| Trial 1 | Trial 2 | Trial 3 | Mean ± S. D. | |
| F-1 | 12.97 | 12.63 | 12.91 | 12.83 ± 0.180 |
| F-2 | 12.73 | 12.61 | 12.51 | 12.61 ± 0.110 |
| F-3 | 12.86 | 12.3 | 12.7 | 12.62 ± 0.200 |
| F-4 | 12.3 | 12.4 | 12.1 | 12.26 ± 0.120 |
| F-5 | 12.89 | 12.78 | 12.84 | 12.83 ± 0.044 |
| F-6 | 12.83 | 12.81 | 12.79 | 12.81 ± 0.016 |
| F-7 | 12.78 | 12.78 | 12.75 | 12.77 ± 0.014 |
| F-8 | 12 | 12.11 | 12.2 | 12.10 ± 0.081 |
| F-9 | 12.86 | 12.62 | 12.47 | 12.65 ± 0.160 |
Table 8: Acid neutralization capacity of the tablets of the batches F-1 to F-9.
Perusal of the Table 8 indicates that, ANC of all formulated batches falls within the range of 12-13 mEq/dose which is sufficient to neutralize the acid which could be released by the co-administration of lornoxicam.
Comparison of dissolution profile of optimized lornoxicam tablet with marketed lornoxicam 8 mg tablets
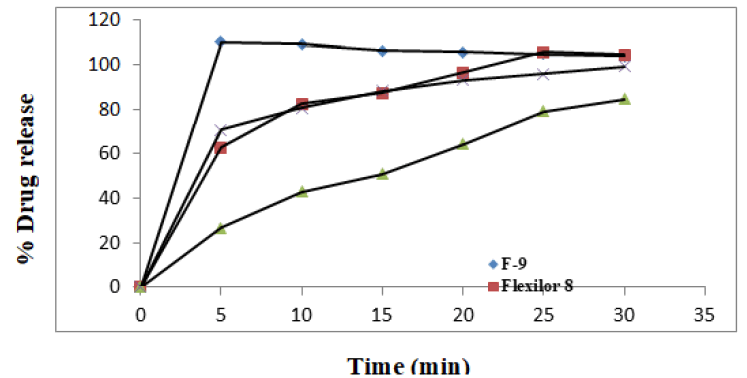
Based on the accessibility, 3 different lornoxicam 8 mg tablets and 2 antacid tablets were purchased from the local chemists’ shop Davangere, India on 2019. The in vitro drug release of the optimized tablets was compared with the lornoxicam 8 mg marketed products, namely “Flexilor 8, Lofecam 8 and Lornicam 8”, where the ANC was compared with Gelusil MPS and Digene, the widely used antacids.
In vitro drug release of the marketed tablets in phosphate buffer solution, pH 7.4 was determined and compared with the in vitro drug release of the optimized tablets. The results are given in Figure 12 [16].
Perusal of the Figure 12 indicates that marketed products need more than 25 minutes for the complete drug release whereas, developed tablets were showing 100 % drug release within 5 minutes. Thus prepared formulations are superior than marketed tablets.
Comparison of acid neutralizing capacity of effervescent tablets with marketed tablets
In an intention to standardize the manufactured tablets, the ANC of the marketed products were compared. ANC of marketed tablets (Digene and Gelusil MPS) was carried out similar to the procedure given for ANC of the developed formulations and compared. The data are given in the Table 9 [17].
| Name of marketed product | Acid Neutralization Capacity (ANC) in mEq/dose | |||
|---|---|---|---|---|
| Trial 1 | Trial 2 | Trial 3 | Mean ± S.D. | |
| Gelusil MPS | 17.4 | 17.3 | 17.6 | 17.4 ± 0.12 |
| Digene | 12.4 | 12.5 | 12.3 | 12.41 ± 0.08 |
| F-9 | 12.86 | 12.62 | 12.47 | 12.65 ± 0.160 |
Table 9: Data for acid neutralization capacity of marketed products.
Perusal to the Table 9, clears that the acid neutralizing capacity of the tablets is in the order of Gelusil MPS>F-1 to F-9>Digene. It shows that the prepared tablets are more effective than the Digene in neutralizing acidity present in the stomach of the patients (Table 10) [18].
| Parameters | F-9 | Reproducible batch |
|---|---|---|
| Hardness (kg/cm2)* | 3.966 ± 0.5 | 4.1 ± 07 |
| Friability (%)# | 0.21 | 0.3 |
| Uniformity of weight (mg)# | 849.2 ± 0.7 | 850 ± 0.1 |
| Drug content (%)** | 99.90 ± 1.67 | 99.8 ± 1.1 |
| Thickness (mm)* | 8.12 ± 0.04 | 8.1 ± 0.08 |
| Diameter (mm)* | 12.10 ± 0.008 | 12.1 ± 0.007 |
| Wetting time (seconds)** | 35 ± 0.01 | 42.1 ± 0.01 |
| Disintegration time (seconds)* | 40 ± 0.1 | 50 ± 1.01 |
| ANC (mEq) | 12.65 ± 0.160 | 12.5 ± 0.12 |
| *Each value was an average of six determinations **Each value was an average of three determinations #Results of one batch |
||
Table 10: Properties of lornoxicam effervescent tablets for the batches F-9 and reproducible batch.
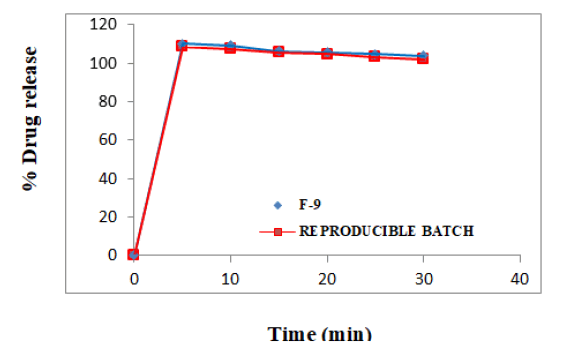
There was no significant difference in the results for the tablets batches F-9 and reproducible batch (Figure 13).
Figure 13 shows that, both are producing overlapped graphs, proving the reproducibility.
Stability study
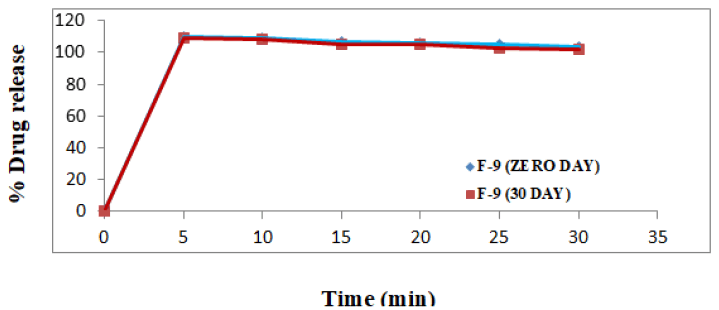
The tablets of optimised batch F-9 were selected for stability studies (Tables 11 and 12). The stability studies were needed to be carried out at 40 ± 2°C and 75 ± 5% RH for 6 months. At the last, tablets were analysed [19].
| Formula | Days | Color |
|---|---|---|
| F-9 | Tablets of zero days | Pale yellow |
| Tablets of six month | Pale yellow |
Table 11: Evaluation for physical appearance after stability studies of lornoxicam effervescent tablets of batch F-9.
| Time | %Drug content** | Hardness* (kg/cm2) | disintegration time** (seconds) | ANC |
|---|---|---|---|---|
| Tablets of zero days | 99.64 ± 1.309 | 3.966 ± 0.5 | 40.0 ± 0.1 | 12.65 ± 0.160 |
| Tablets of 6 months | 99.41 ± 1.014 | 4.1± 0.00 | 41.00 ± 1.000 | 12.5 ± 0.11 |
| *Each value is an average of five determinations **Each value is an average of three determinations | ||||
Table 12: Evaluation lornoxicam effervescent tablets of batch F-9 after stability studies.
By comparison it was found that, there were no changes in the physical chemical as well as drug release properties of the tablets of the batch F-9, after a period of 6 months of storage [20].
Conclusion
The study was undertaken with an aim to formulate a combine effervescent tablet of lornoxicam with antacids. The results revealed that the amounts of crosspovidone and effervescent material significantly affect the disintegration time. It was proved that, by following the randomly selected method of decreasing the concentration of effervescent mixture approach, optimized release mechanism can be reached in the shortest time with minimum efforts. It is thus concluded that the prepared tablets of lornoxicam with crosspovidone and sodium saccharin can be chewed and alternatively used as fast dissolving tablets, as it undergoes disintegration within 40 seconds and 100% drug release shown within 5 minutes with increased patient compliance.
Acknowledgement
We the authors thank Bapuji Pharmacy College, Davanagere for providing the necessary chemicals, glassware and equipment’s to perform this research work.
Conflict of Interest
Authors do not have any conflict of interest.
References
- Siddiqui MN, Garg G, Sharma PK. Int J Pharm Sci Rev Res. 2010; 4(2): p.87-96.
- Fardous J, Perveen FF, Islam MZ, et al. JMCDD. 2017; 3(5): p.67-70.
- Dhikav V, Singh S, Pande S, et al. J Indian Acad Clin Med. 2003; 4(4): p.315-322.
- Ahmed MAM, Ibrahim AM. Int J Res Pharm Sci. 2017; 7(2): p. 1-6.
- Warrington SJ, Debbas NM, Farthing M, et al. Postgrad Med J. 1990; 66(778): p.622-626.
[Crossref] [Google Scholar] [PubMed]
- Sheth SK, Patel SJ, Shukla JB. Int J of Pharma Bio Sci. 2010; 1(2): p.1-9.
- Anjankumar PB, Nazmuddin M, Kulkarni U, et al. Int Res J Pharm. 2011; 2(4):130-133.
- Gupta VR, Mutalik S, Patel MM, et al. Acta Pharm. 2007; 57(2):173-184.
[Crossref] [Google Scholar] [PubMed]
- Mutalik S, Naha A, Usha AN, et al. Arch Pharm Res. 2007; 30: p.222-234.
[Crossref] [Google Scholar] [PubMed]
- Yatsu FK, Koester LS, Lula I, et al. Carbohydr Polym. 2013; 98(1):726-735.
[Crossref] [Google Scholar] [PubMed]
- Sarfraz RM, Ahmad M, Mahmood A, et al. Trop J Pharm Res. 2015; 14(11):1961-1968.
- Lindgren S, Janzon L. Dysphagia. 1991; 6: p.187-192.
[Crossref] [Google Scholar] [PubMed]
- Shah S, Patel JK, Patel N. Der Pharmacia Sinica. 2010; 1 (3): p. 232-244.
- Ashutosh M, Rajesh KP, Mukesh CG. Asian J Pharm 2008; p.177-181.
- Ulla SN, Roy AK. Int J Drug Dev Res 2011; 3(1): p.31-44.
- Debjit B, Chiranjib B, Krishnakanth P, et al. JOCPR. 2009; 1(1): p.163-77.
- Subrahmanyam CVS. 2nd,Vallabh Prakashan, Delhi,India 2005.
- Gadhve MV, Garje VN, Narad VR, et al. Int J Recent Sci Res. 2016; 7(10):13802-13806.
- Anumolu PD, Sunitha G, Bindu SH, et al. Indian J Pharm Sci. 2015; 77(3):p. 249-366.
[Google Scholar] [PubMed]
- Gadade DD, Kulkarni DA, Rathi PB, et al. Indian J Pharm Sci. 2017; 79(2): p.277-286.