Research Article - Der Pharma Chemica ( 2021) Volume 13, Issue 7
Chemical constituents of Garcinia mannii (Glusiaceae)
Hasan M.H. Muhaisen*Hasan M.H. Muhaisen, Department of Chemistry, Faculty of Science and Arts, Najran University, Sharurah, Kingdom of Saudi Arabia, Email: orgchem2001@yahoo.com
Received: 20-Jun-2021 Accepted Date: Jul 05, 2021 ; Published: 12-Jul-2021
Abstract
Biflavonoid named as I-3, II-3, I-5, II-5, I-7, II-7, I-40 , II-40 -octahydroxy [I-20 , II-20] biflavone, quercetin, apigenin along with dimethylether of phloroacetophenone named as 2,4- dimethoxy-6-hydroxy acetophenone, have been isolated from leaves of Garcinia mannii. Their structures were elucidated by chemical and physical data (IR, UV, H-NMR, C-NMR and Mass spectra).
Keywords
Garcinia mannii, Leaves, Biflavone, Flavonoids, acetophenone
Introduction
The genus Garcinia includes more than 300 species and belongs to the family Clusiaceae. The plants of the genus have multiple applications in culinary, pharmaceutical, and industrial fields. It is also ornamental, with a dense canopy of green leaves and red-tinged tender emerging leaves. The genus is native to Asia and Africa. About 35 species are common in India and are endemic to the evergreen forests of Western Ghats, Gujarat, Andaman and Nicobar Islands and North-Eastern region of India. The tree is large, having elliptic, oblong with deep-green glossy leaves up to 5–8 cm long and 2–3 cm broad. The flowers are fleshy, dark pink, solitary or in spreading cluster. The fruit is brownish or purple about the size of an orange, marbled with yellow, and is crowned by the 4-parted, stalk less stigma. The fruit pulp is juicy, white, and delicious in taste and odor, consists 6–8 seeds. Commonly, the plants in this genus are called kokum, saptrees, mangosteens, garciniasor, and ambiguously ‘‘monkey fruit’ [1].
Garcinia mannii is a rain forest tree of West Africa of Cameroon. The twigs and small adventitious roots have been observed to be used as chew-sticks and the dried, powdered root-bark is reported to be a cure for severe diarrhoea and dysentery [2]. Earlier investigations on this plant reported the isolation of lactone (3-α-hydroxy-5-(heptadec-8′-enyl)-tetrahydro- furan-2-one), biflavanone (1-3’-11-3,3’-1-4’-11-4’-1-5-11-5-1-7-11-7-nonahydroxy-1-3-11-8-biflavanone) [3] and xanthone derivative xanthochymol [4] from stem bark. This led to our interest in carrying out a comprehensive investigation of the leaves of G. mannii. The present article deals with the isolation and characterization of a biflavonoid, I-3, II-3, I-5, II-5, I-7, II-7, I-40 , II-40 -octahydroxy [I-20 , II-20 ] biflavone (I), along with three known compounds, 2,4- dimethoxy-6-hydroxy acetophenone, quercetin, apigenin.
Material and Methods
The leaves of G. mannii (2.5 kg) procured from Nigeria, were exhaustively extracted with acetone. The acetone extract was concentrated under reduced pressure. The acetone concentrate was successively extracted with light petroleum ether (60-80°C), benzene and ethylacetate. The ethylacetate extract gave positive test for flavonoids [5]. On TLC examination, the ethylacetate extract showed four major spots in TEF and BPF solvent systems. Repeated column chromatography over silica gel followed by fractional crystallization and preparative TLC, afforded four crystalline TLC homogenous substances marked as compounds (1), (2), (3) and (4).
Result and Discussion
Compound (1)
Compound (1) was obtained from the column by benzene-ethylacetate (9:1) mixture, and was crystallized from benzene-chloroform as white shining crystals (140 mg). m.p. 149 ° C. It gave greenish colour with alcoholic solution of ferric chloride. The elemental analysis agreed with molecular formula C10H12O4. The IR spectrum showed the characteristic bands at 1460 cm-1 (C=C), 1660 cm-1 (C=O) and 3300 cm-1 (OH) and uv spectrum gave λmax at 312 nm.
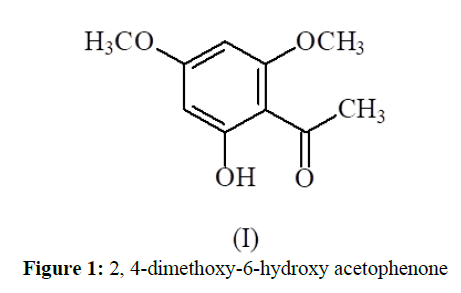
The H-NMR spectrum (Table-1), exhibited a sharp singlet integrating for three protons at δ 2.0 assigned to CH3. Two independent singlets of one proton each at δ 6.38 and δ 7.10 were ascribed to H-3 and H-5 protons respectively. Two singlets at δ 3.16 and δ 3.41 integrating for three protons each indicated the presence of two methoxyl groups. On the basis of elemental analysis, ir, uv and 1H-NMR spectroscopy, the compound (1) was identified as dimethyl ether of phloroacetophenone (I).
| Assignment | No. of protons | Signals |
|---|---|---|
| CH3 | 3 | 2.0 (s) |
| OCH3 | 3 | 3.16 (s) |
| OCH3 | 3 | 3.41 (s) |
| H-3 | 1 | 6.38 (s) |
| H-5 | 1 | 7.10 (s) |
| OH | 1 | 12.5 (s) |
s= singlet, spectrum run in CDCl3 at 300 MHz, using TMS as internal standard.
Table 1: 1H-nmr data of (1), values on δ- scale
The assigned structure was further supported by C-NMR spectrum (Table-2) which showed the presence of three oxygenated carbons at 166.2, 165.6 and 163.4, a carbonyl carbon at 180.1 and a methyl group at 16.5 ppm. The mass spectrum showed the molecular ion peak at m/z 196.
| Carbon No. | Assignment |
|---|---|
| C-1 | 104.8 |
| C-2 | 166.2 |
| C-3 | 91.2 |
| C-4 | 165.6 |
| C-5 | 94.1 |
| C-6 | 163.4 |
| C=O | 180.1 |
| CH3 | 16.5 |
| OCH3 | 55.3 |
| OCH3 | 56.1 |
Table 2: 13C-nmr data of (1), values on δ- scale
In the light of above results, Figure (1) was characterized as 2, 4-dimethoxy-6-hydroxy acetophenone (dimethylether of phloroacetophenone) [6](I).
Compound (2)
Compound (2) was eluted from the column by benzene-ethyl acetate (8:2) mixture. It was crystallized from chloroform-methanol as yellow shining crystals (100 mg), m.p. 311-12 °C. It was identified as quercetin (IIa) by m.p., m.m.p. and co-chromatography with an authentic sample of quercetin. It gave an acetate m.p. 194-95 °C and penta methyl ether m.p. 151-52 °C. Its identity as quercetin was further confirmed by comparing its spectral data with those of an authentic sample of quercetin [7].
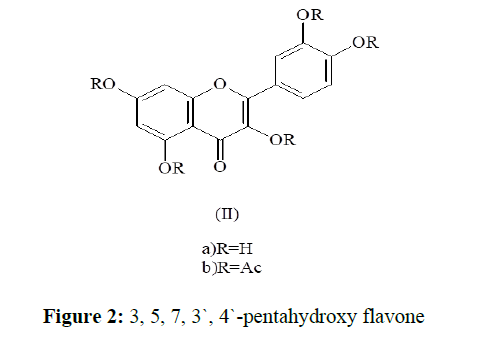
The 1H-nmr spectrum of its acetate (2) (Ac) (IIb) showed the signals due to five acetoxyl groups in the range of δ 2.35-2.38. The meta-coupled doublets at δ 6.79 (J=2.5 Hz) and δ 7.21 (J=2.5 Hz) were assigned to C-6 and C-8 protons of A-ring respectively. The B-ring protons showed the ABX pattern, two doublets at δ 7.73 (J=2.5 Hz) for H-2` and δ 7.27 (J=9.0 Hz) for H-5` and quartet at δ 7.67 (J1= 2.5 Hz, J2=9.0 Hz) for H-6`.
In the light of the above results, the compound (2) was assigned the structure as 3, 5, 7, 3`, 4`-pentahydroxy flavone (quercetin) (IIa), supported by the mass spectrum which showed molecular ion peak at m/z 302. (Figure 2)
Compound (3)
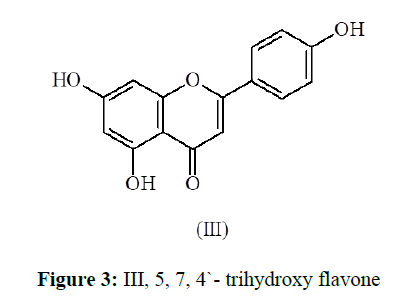
Compound (3) was eluted from the column by benzene-ethyl acetate (7:3) mixture. It was characterized as apigenin [8,9] (III), by comparison with an authentic sample (Rf-value, m.p., m.m.p. and co-chromatography). The identity of the compound (3), as apigenin (III, 5, 7, 4`- trihydroxy flavone) was further confirmed by 1H-nmr studies of its acetate, m.p. 183-84 °C (Table-3).
| Assignment | No. of protons | Signals |
|---|---|---|
| H-2`,6` | 2 | 7.76 (d, J= 8.6 Hz) |
| H-3`,5` | 2 | 7.20 (d, J= 8.6 Hz) |
| H-8 | 1 | 6.89 (d, J= 2.5 Hz) |
| H-6 | 1 | 6.74 (d, J= 2.4 Hz) |
| H-3 | 1 | 6.58 (s) |
| OAc-5 | 3 | 2.44 (s) |
| OAc-4`,7 | 6 | 2.35 (s) |
s= singlet, d= doublet, spectrum run in CDCl3 at 300 MHz, using TMS as internal standard.
Table 3: 1H-NMR data of (3) (Ac), values on δ- scale
Compound (4)
The fraction obtained by the elution of the column by benzene-ethyalacetate (1:1), on TLC examination was found to be an intricate mixture of several compounds, with one of them as the major one. Repeated column chromatography followed by preparative-TLC failed to separate the mixture. However, by PTLC, most of the minor fractions were removed. The major fraction along with some very minor impurities was analysed by 1H-NMR spectrum. As the compound was not absolutely pure, the spectrum could not be successfully interpreted. However, the presence of °CH3 group was ruled out by the absence of any peak in the 1H-NMR spectrum.
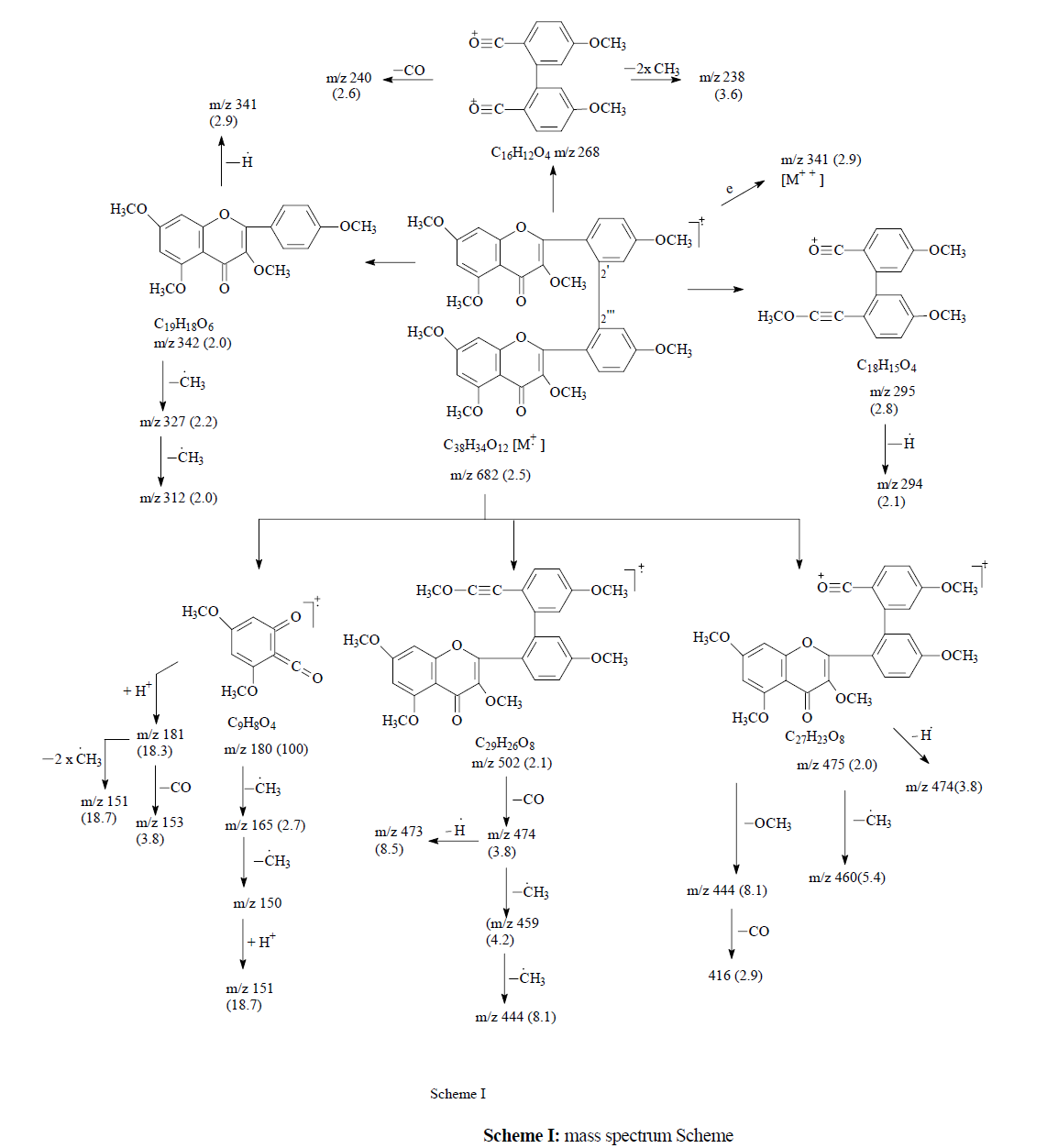
The mixture was methylated with dimethyl sulphate and major band was separated by PTLC. It was purified by crystallization (165 mg), m.p. 189-90 °C. The uv spectrum and colour test [10] showed it to be a flavone. Elemental analysis gave the molecular formula as C38H34O12, corresponding to the molecular ion peak at m/z 682. The molecular weight indicated it to be methyl ether of biflavone. The structure of the methyl ether was established by H-NMR, C-nmr and mass spectroscopy.
The H-nmr spectrum of (4) (Me) (Table-4), showed the presence of eight methoxyl groups by four independent singlets of six protons each at δ 3.68, 3.85, 3.91 and 3.94 respectively. The A-ring protons appeared as two singlets for two protons each at δ 6.16 ascribed to H-I-6 and H-II-6 and at δ 6.20 attributed to H-I-8 and H-II-8. The B-ring protons showed typical ABX pattern consisting of an ortho-coupled doublet at δ 7.32 (J= 8.1 Hz) assigned to H-I-6` and H-II-6` and a double doublet for four protons in the range of δ 6.09-6.17 (J1= 8.0 Hz, J2= 2.0 Hz) corresponded to H-I-3`, 5` and H-II-3`, 5` protons. Hence, it indicated that H-I-2` and H-II-2` are involved in inter flavonoid linkage which was further proved by the fragment ions at m/z 475, 460, 444, 416, 295, 268 in the mass spectrum Scheme-1). The RDA fragments appeared at m/z 180 and 502. Other fragments are rationalized from the Scheme-1.
| Assignment | No. of protons | Signals |
|---|---|---|
| 2 x OMe | 6 | 3.68 (s) |
| 2 x OMe | 6 | 3.85 (s) |
| 2 x OMe | 6 | 3.91 (s) |
| 2 x OMe | 6 | 3.94 (s) |
| H-I-6, H-II-6 | 2 | 6.16 (s) |
| H-I-8, H-II-8 | 2 | 6.20 (s) |
| H-I-3`,5` and H-II-3`, 5` | 4 | 7.09-7.17 (dd, J1= 8.0 Hz, J2= 2.0 Hz) |
| H-I-6`, H-II-6` | 2 | 7.32 (d, J= 8.1 Hz) |
s= singlet, d= doublet, dd= double doublet, spectrum run in CDCl3 at 300 MHz, using TMS as internal standard.
Table 4: 1H-NMR data of (1) (Me), values on δ- scale
The above assigned structure was also supported by the C-nmr spectrum (Table-5) of (4) (Me) in which both the carbonyl groups were observed at 188.8 ppm. It also showed a downfield shift for I and II-C-2`, further confirming the inter flavonoid linkage between I-C-2` and II-C-2`.
| Carbon No. | Assignment |
|---|---|
| 8 x OCH3 | 55.64 55.99 56.10 56.28 |
| I, II-C-2 | 160.6 |
| I, II-C-3 | 105.8 |
| I, II-C-4 | 188.8 |
| I, II-C-5 | 159.6 |
| I, II-C-8 | 98.4 |
| I, II-C-7 | 162.6 |
| I, II-C-6 | 98.6 |
| I, II-C-9 | 151.6 |
| I, II-C-10 | 108.8 |
| I, II-C-1` | 119.9 |
| I, II-C-2` | 129.8 |
| I, II-C-3`, 5` | 113.4 |
| I, II-C-4` | 165.0 |
| I, II-C-6` | 128.0 |
Table 5: C-nmr data of (4) (Me), values on δ- scale
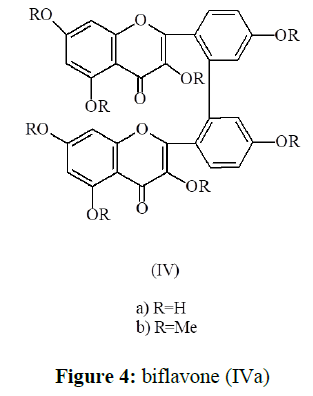
In view of the above facts (4) (Me) was assigned the structure I-3, II-3, I-5, II-5, I-7, II-7, I-4`, II-4`-Octamethoxy [I-2`, II-2`] biflavone (IVb). The parent compound (4), was therefore, assigned the structure as I-3, II-3, I-5, II-5, I-7, II-7, I-4`, II-4`-Octahydroxy [I-2`, II-2`] biflavone (IVa) which is being reported for the first time. (Figure 4).
Experimental
The melting points were taken on a Kofler block and are uncorrected. All ultraviolet spectra were measured on Beckmann Model DU and Pye Unicam PU-8800 spectrophotometers in methanol / ethanol. Infrared spectra were taken on Shimadzu IR-408 Perkin Elmer 1800 (FTIR). The mass and 1H-nmr spectra were obtained from different institutes in the country and outside. The mass spectra were mostly measured in E.I. mode on Jeol D-300 while, the 1H-nmr spectra were usually recorded on Varian EM-360 L (60 MHz), 270 MHz, JEOL 4H-100 MHz, Perkin Elmer R-32 (90 MHz), Brucker dpx 200 MHz, DRX 300 MHz and WM 400 MHz in CDCI3 / DMSO-d6 using TMS as internal standard.
The silica gel used for different chromatographic purposes, was obtained from E. Merck (India), E. Merck (Germany) and SRL (India). TLC solvent systems used were benzene-pyridine-formic acid (BPF, 36:9:5), toluene – ethylformate - formic acid (TEF, 5:4:1), ethylacetale-ethylmethylketone-acetic acid-water (EtOAc-EtMeCO-AcOH-H2O, 5:3:1:1; 20:3:1:1; 30:3:1:1), ethylacetate-methanol-water (EtOAc-MeO -H2O, 8:1:1), petrol-benzene (2:8) n-butanol-acetic acid-water (BAW, 4:1:5), n-butanol-water-ethanol (BEW, 60:28.5:16.5).
Alcoholic ferric chloride, iodine vapours and aniline hydrogen phthalate solutions were used as spray reagents for visualization of spots on TLC and on paper chromatograms.
Plant material
Leaves of G. mannii were collected from Nigeria. It was identified by Prof. Wazahat Hussain, Department of Botany, A.M.U., Aligarh, India. The voucher specimen has been deposited at Department of Botany, A.M.U., Aligarh.
Extraction and isolation
The leaves of G. mannii (2.5 kg) procured from Nigeria, were exhaustively extracted with acetone. The acetone extract was concentrated under reduced pressure. The acetone concentrate was successively extracted with light petroleum ether (60-80 °C), benzene and ethylacetate. The ethylacetate extract gave Positive test for flavonoids6. On TLC examination, the ethylacetate extract showed four major spots in TEF and BPF solvent systems. Repeated column chromatography over silica gel followed by fractional crystallization and preparative TLC, afforded four crystalline TLC homogenous substances marked as compounds (1), (2), (3) and (4).
Compound (1)
Compound (1) was obtained from the column by benzene-ethylacetate (9:1) mixture. It was crystallized from benzene-chloroform as white shining crystals (140 mg). m.p.149 oC. It gave greenish colour with alcoholic solution of ferric chloride.
Analysed for C10H12O4.
Calcd: C, 61.22; H, 6.12%
Found: C, 61.19; H, 6.09%
IR, νKBrmax cm-1
3300 (OH), 1660 (C=O), 1460 (C=C).
UV λmax (MeOH) nm 312
1H-NMR (300 MHz, CDCl3) on δ-scale
2.0 (3H, s, CH3), 3.16 (3H, s, OMe), 3.41 (3H, s, OMe), 6.38 (1H, s, H-3), 7.10 (1H, s, H-5), 12.5 (1H, s, OH).
13C-NMR (300 MHz, CDCl3) on δ-scale
16.5 (CH3), 91.2 (C-3), 94.1 (C-5), 104.8 (C-1), 163.4 (C-6), 165.6 (C-4), 166.2 (C-2), 180.1 (C=O), 55.3 (°CH3), 56.1 (°CH3).
Mass, m/z
196 [M+] (38.0), 181 [M+ -CH3] (15.1), 166 [181-CH3] (10.0), 168 [M+-CO] (20.2), 153 [M+-C°CH3] (26.0).
Compound (2)
Compound (2) was eluted from the column by benzene-ethyl acetate (8:2) mixture. It was crystallized from chloroform-methanol as yellow shining crystals (100 mg), m.p. 311-12 °C.
Analysed for C15H10O7.
Calcd: C, 59.60; H, 3.31 %
Found: C, 59.64; H, 3.33 %
UV data with shift reagents λmax nm
| MeOH | 258, 272 sh, 300 sh, 371 |
| NaOAc | 258 sh, 275, 390 |
| NaOAc/H3BO3 | 263, 301, 389 |
| AlCl3 | 273, 304 sh, 335, 457 |
| AlCl3/HCl | 264, 358, 429 |
| NaOMe | 248 sh, 321 |
Acetylation of compound (2)
Compound (2) (20 mg) was heated with pyridine (1 ml) and acetic anhydride (2 ml) on a water bath for about two hours. After cooling, the mixture was poured on crushed ice and left-over night. The solid obtained was collected, washed with water and dried. On crystallization from ethylacetate, it gave cream-coloured crystals (15 mg), m.p.194-95 °C.
1H-NMR (60 MHz, CDCl3), values on δ-scale
2.38 (3H, s, OAc), 2.35 (12H, m, 4x OAc), 6.79 (1H, d, J=2.5 Hz, H-6), 7.21 (1H, d, J=2.5 Hz, H-8), 7.27 (1H, d, J=9.0 Hz, H-5`), 7.67 (1H, q, J1=2.5 Hz, J2=9.0 Hz, H-6`), 7.73 (1H, d, J=2.5 Hz, H-2`).
Methylation of compound (2)
Crystalline (2) (25 mg), dry acetone (30 ml), dimethylsulphate (1 ml) and anhydrous potassium carbonate (0.5 g) were refluxed for 24 hours. The reaction mixture was filtered and the inorganic residue washed several times with hot acetone. On distilling off the solvent, a colourless semi-solid mass was left behind. It was washed with hot petroleum ether to remove the excess of dimethyl sulphate. The solid residue on crystallization from methanol-ethylacetate gave colourless needles (20 mg), m.p. 151-52 °C.
Compound (3)
The fraction obtained from the elution of the column with benzene-ethylacetate (7:3) mixture gave a yellow solid mass which was crystallized from benzene-acetone as yellow needles (180 mg), m.p. 352 oC.
Analysed for C15H10O5.
Calcd: C, 66.66; H, 3.70 %
Found: C, 66.78, H, 3.74 %
UV data with shift reagents λmax nm
| MeOH | 266, 298 sh, 339 |
| NaOAc | 279, 304, 377 |
| NaOAc/H3BO3 | 268, 301 sh, 338 |
| AlCl3 | 280, 302, 342,392 |
| AlCl3/HCl | 280, 301, 342, 391 |
Acetylation of compound (3)
Compound (3) (40 mg) was heated with pyridine (1 ml) and acetic anhydride (2 ml) on water bath for about two hours. On usual work-up, the solid obtained was crystallized from chloroform-methanol as colourless needles (35 mg), m.p. 183-84 °C.
1H-NMR (300 MHz, CDCl3), values on δ-scale
2.35 (6H, s, OAc-4`,7), 2.44 (3H, s, OAc-5), 6.58 (1H, s, H-3), 6.74 (1H, d, J=2.4 Hz, H-6), 6.89 (1H, d, J=2.5 Hz, H-8), 7.20 (2H, d, J=8.6 Hz, H-3`,5`), 7.76 (2H, d, J=8.6 Hz, H-2`,6`).
Compound (4)
The fraction obtained by the elution of the column by benzene-ethylacetate (1:1), on TLC examination was found to be an intricate mixture of several compounds; one of them was the major one. Repeated column chromatography followed by preparative-TLC failed to separate the mixture. However, by PTLC using TEF (5: 4: 1) system as the developing solvent most of the minor fractions were removed. The mixture was methylated with dimethyl sulphate and major band was separated. It was purified by crystallization (160 mg), m.p.189-90 °C. The uv spectrum and colour test showed it to be a flavone. Elemental analysis gave the molecular formula as C38H34O12. Further confirmed by the molecular ion peak at m/z 682.
Methylation of compound (4)
Crystalline (4) (150 mg), dry acetone (30 ml), dimethylsulphate (2 ml) and anhydrous potassium carbonate (0.5 g) were refluxed for 24 hours. After usual work-up the solid obtained was crystallized from chloroform-methanol as white crystals (165 mg), m.p.189-90 °C.
Analysed for C38H34O12
Calcd: C, 66.86; H, 4.98%
Found: C, 66.90; H, 5.01%
IR, νKBrmax cm-1
3200-3400 (br, OH), 1680 (C=O), 1600, 1510, 1440
UV data with shift reagent λmax nm
| MeOH | 258 sh, 268, 294 sh, 323 sh, 368 |
| NaOAc | 276, 304, 387 |
| NaOAc/H3BO3 | 277, 304, 388 |
| AlCl3 | 262 sh, 278, 303 sh, 351, 424 |
| AlCl3/HCl | 263 sh, 279, 303 sh, 351, 424 |
| NaOMe | 279, 316, 416 (dec.) |
1H-NMR (300 MHz, CDCl3), values of δ-scale
3.68 (6H, s, 2 x OMe), 3.85 (6H, s, 2 x OMe), 3.91 (6H, s, 2 x OMe), 3.94 (6H, s, 2 x OMe), 6.16 (2H, s, H-I-6, H-II-6), 6.20 (2H, s, H-I-8, H-II-8), 7.09-7.17 (4H, dd, J1=8.0 Hz, J2=2.0 Hz, H-I-3`,5`, H-II-3`, 5`), 7.32 (2H, d, J=8.1 Hz, H-I-6`, H-II-6`).
13C-NMR (300 MHz, CDCl3), values of δ-scale
55.64, 55.99, 56.10, 56.28 (8 x OMe), 160.6 (I-II-C-2), 105.8 (I-II-C-3), 188.8 (I-II-C-4), 159.6 (I-II-C-5), 98.4 (I-II-C-8), 162.6, (I-II-C-7). 98.6 (I- II-C-6), 151.6 (I-II-C-9), 108.8 (I-II-C-10), 119.9 (I-II-C-1`), 129.8 (I-II-C-2`), 113.4 (I-II-C-3`,5`), 165.0 (I-II-C-4`), 128.0 (I-II-C-6`).
Mass m/z (rel. int.)
682 [M+•] (2.5), 502 (2.1), 475 (2.0), 474 (3.8), 473 (8.5), 460 (5.4), 459 (4.2), 444 (8.1), 416 (2.9), 342 (2.0), 341 (2.9), 327 (2.2), 312 (2.0), 295 (2.8), 268, 240 (2.6), 238 (3.6), 181 (18.3), 180 (100), 165 (2.7), 153 (3.8), 151 (18.7), 150 (2.6).
Conclusion
Known constituents have been isolated from leaves of Garcinia mannii for the first time. In the present study can be categorized under the biflavonoid. The constituents named as I-3, II-3, I-5, II-5, I-7, II-7, I-40, II-40 -octahydroxy [I-20, II-20] biflavone. The compounds and their derivatived were prepared and subjected elemental analysis. Their structure was elucidated by chemical and physical data (IR, UV, 1H-NMR and Mass spectra).
Acknowledgements
The authors would like to express their gratitude's to the ministry of education and the deanship of scientific research - Najran University - Kingdom of Saudi Arabia for their financial and technical support.
References
- Hemshekhar M., Sunitha K, Sebastin Santhosh M et al., Phytochem Rev. 2011, 10: p. 325–351.
- BEENTJE HJ and GHAZANEAR SA, Flora of Tropical East Africa-Apocynaceae: BYEA Omino, 2002.
- Raouf A. Hussain and Peter G. Phytochemistry. 1982, 21(6): p. 1393-1396.
- Binh TD Trinh, Tam TT Quach, Dung N Bui et al., Fitoterapia, 2017, (118): p. 126-131.
- Sang H J, Soo Y P, Youngmi K P et al., Chem. Pharm. Bull. 2006, 54(3): p. 368-371.
- Ahluwalia V.K, Bhagat P, Aggarwal R. and Chandra R, Intermediates for organic synthesis. 2010.
- Iris Erlund, Nutri Res, 2004, 24(10): p. 851-874.
- Hasan MH and Muhaisen. IOSR J Pharm, 2021, 3(1): p. 10-21.
- Hasan MH Muhaisen. Advance Science, Engineering and Medicine, 2014, 12(16): p. 1235-1250.
- Hasan MH Muhaisen. Merit Res J Medicine and Medical Sci, 2020, 8(10): p. 575-580.
- Hasan MH Muhaisen, Ilyas M, Mushfiq M et al., J Chem Res, 2002, p. 276-278.
- Mabry TJ, Markham KR and Thomas MB. The Systematic Identification of Flavonoids. 2012,
- Basudan OA, Ilyas M, Mushfiq M et al., Natural Product Letters, 2004,18(3): p. 269-275.